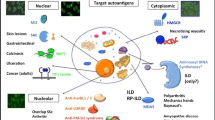
Abstract
Purpose of Review
Autoimmune myopathies (IMs) are a group of diseases characterized by muscle weakness and inflammatory infiltrates on the muscle biopsy. `However, muscle involvement is not always present and other organs and tissues (lung, skin, and joints) are commonly affected. The most accepted classification for IM includes dermatomyositis, sporadic inclusion body myositis, immune-mediated necrotizing myopathy, polymyositis, and overlap myositis. Seventy percent of the autoantibodies that IM patients have are myositis-specific autoantibodies (MSAs). Importantly, MSAs are associated with specific clinical phenotypes in IM patients. The therapy of IMs consists of glucocorticoids, immunosuppressants, biologic, and immunomodulatory drugs, but there is recent evidence that patients with some types of MSAs respond better to certain treatments. The purpose of this review is to summarize the IM treatment from an autoantibody perspective.
Recent Findings
Each MSA is maybe associated with the activation of specific pathogenic pathways which can be effectively targeted by specific drugs. The last few years have shown multiple examples of successful treatments for each MSA group of patients as we will describe in the following paragraphs.
Summary
The adverse effects of the IM therapeutic agents and some patients’ refractoriness call for a continued search for better and more targeted therapies. MSA groups should be considered for a treatment decision.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Selva-O’Callaghan A, et al. Classification and management of adult inflammatory myopathies. Lancet Neurol. 2018;17(9):816–28.
Mulcahy KP, Langdon PC, Mastaglia F. Dysphagia in inflammatory myopathy: self-report, incidence, and prevalence. Dysphagia. 2012;27(1):64–9.
Oh TH, et al. Dysphagia in inflammatory myopathy: clinical characteristics, treatment strategies, and outcome in 62 patients. Mayo Clin Proc. 2007;82(4):441–7.
Oddis CV, Aggarwal R. Treatment in myositis. Nat Rev Rheumatol. 2018;14(5):279–89.
Ghirardello A, et al. Myositis autoantibodies and clinical phenotypes. Auto Immun Highlights. 2014;5(3):69–75.
• McHugh NJ, Tansley SL. Autoantibodies in myositis. Nat Rev Rheumatol. 2018;14(5):290–302. A review on MSA and their association with a distinctive pattern of disease, which has implications for diagnosis and a more personalized approach to therapy.
Ghirardello A, et al. Autoantibodies in polymyositis and dermatomyositis. Curr Rheumatol Rep. 2013;15(6):335.
•• Casal-Dominguez M, et al. Performance of the 2017 EULAR/ACR classification criteria for inflammatory myopathies in patients with myositis-specific autoantibodies. Arthritis Rheumatol. 2022;74:508–17. This article from one of the largest cohorts of IM includes 524 patients with MSA concludes that although the EULAR/ACR criteria successfully classified 91% of MSA-positive myositis patients, certain MSA-defined subgroups, are frequently misclassified (those with anti-HMGCR, -SRP, and -PL-7 autoantibodies). Moreover, they show that in myositis patients with MSAs, autoantibodies outperform the EULAR/ACR-defined myositis subgroups in predicting the clinical phenotypes of patients.
Lundberg IE, et al. Diagnosis and classification of idiopathic inflammatory myopathies. J Intern Med. 2016;280(1):39–51.
• Aggarwal R, et al. Predictors of clinical improvement in rituximab-treated refractory adult and juvenile dermatomyositis and adult polymyositis. Arthritis Rheumatol. 2014;66(3):740–9. This study intended to identify predictors of IM improvement (clinical and laboratory) in a cohort of patients with IM treated with rituximab. They concluded that the presence of antisynthetase and anti-Mi-2 autoantibodies, juvenile DM subset, and lower disease damage strongly predict clinical improvement in patients with refractory myositis.
Aggarwal R, et al. Autoantibody levels in myositis patients correlate with clinical response during B cell depletion with rituximab. Rheumatology (Oxford). 2016;55(6):991–9.
Cavagna L, et al. Cyclosporine in anti-Jo1-positive patients with corticosteroid-refractory interstitial lung disease. J Rheumatol. 2013;40(4):484–92.
• Huapaya JA, et al. Long-term treatment with human immunoglobulin for antisynthetase syndrome-associated interstitial lung disease. Respir Med. 2019;154:6–11. This retrospective analysis of AS-ILD patients shows that IVIg may be a useful complementary therapy in active progressive AS-ILD but is associated with potential side effects.
Kato M, et al. Successful treatment for refractory interstitial lung disease and pneumomediastinum with multidisciplinary therapy including tofacitinib in a patient with anti-MDA5 antibody-positive dermatomyositis. J Clin Rheumatol. 2021;27(8S):S574–7.
Kurasawa K, et al. Tofacitinib for refractory interstitial lung diseases in anti-melanoma differentiation-associated 5 gene antibody-positive dermatomyositis. Rheumatology (Oxford). 2018;57(12):2114–9.
•• Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med. 2015;373(17):1680–2. In this study, 3 out of 82 patients with autoimmune myopathy triggered by statins were treated with IVIG in monotherapy due to rejection of corticosteroids for concerns about potential side effects. After two or three rounds of IVIG, they experienced a decline in the mean creatine kinase level and the strength improved by dynamometry. Notwithstanding this, 2 patients continued having elevated creatine kinase levels and all of them had persistently positive anti-HMG-CoA reductase autoantibody-positive. Those findings may suggest that IVIG may attenuate statin-treated autoimmune myopathy but may not eliminate the cause of muscle damage completely.
Lundberg IE, et al. Idiopathic inflammatory myopathies. Nat Rev Dis Primers. 2021;7(1):86.
•• Greenberg SA, et al. Interferon-alpha/beta-mediated innate immune mechanisms in dermatomyositis. Ann Neurol. 2005;57(5):664–78. Comprehensive study demonstrating the importance of the interferon pathway in DM.
• Pinal-Fernandez I, et al. Identification of distinctive interferon gene signatures in different types of myositis. Neurology. 2019;93(12):e1193–204. Study comparing the expression of interferon 1 (IFN1) and interferon 2 (IFN2) inducible genes between the different IM groups. They conclude that the different types of IM have differences in the activation of the IFN1 and IFN2 pathways. That fact may have therapeutic implications because different immunosuppressive drugs target different IFN pathway.
Zhang Y, et al. The dermatomyositis-specific autoantigen Mi2 is a component of a complex containing histone deacetylase and nucleosome remodeling activities. Cell. 1998;95(2):279–89.
Ahringer J. NuRD and SIN3 histone deacetylase complexes in development. Trends Genet. 2000;16(8):351–6.
Kashiwagi M, Morgan BA, Georgopoulos K. The chromatin remodeler Mi-2beta is required for establishment of the basal epidermis and normal differentiation of its progeny. Development. 2007;134(8):1571–82.
Rider LG, et al. The myositis autoantibody phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine (Baltimore). 2013;92(4):223–43.
Pinal-Fernandez I, et al. More prominent muscle involvement in patients with dermatomyositis with anti-Mi2 autoantibodies. Neurology. 2019;93(19):e1768–77.
Ghirardello A, et al. Anti-Mi-2 antibodies. Autoimmunity. 2005;38(1):79–83.
Hengstman GJ, et al. Clinical characteristics of patients with myositis and autoantibodies to different fragments of the Mi-2 beta antigen. Ann Rheum Dis. 2006;65(2):242–5.
Love LA, et al. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore). 1991;70(6):360–74.
•• Paik JJ, et al. Study of tofacitinib in refractory dermatomyositis: an open-label pilot study of ten patients. Arthritis Rheumatol. 2021;73(5):858–65. In this prospective, open-label clinical trial of tofacitinib, it is reported the clinical efficacy of the JAK-inhibitor tofacitinib in patients with refractory DM.
Takahashi K, et al. Dynamic regulation of p53 subnuclear localization and senescence by MORC3. Mol Biol Cell. 2007;18(5):1701–9.
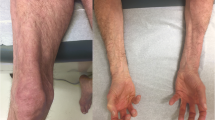
Albayda J, et al. Antinuclear matrix protein 2 Autoantibodies and edema, muscle disease, and malignancy risk in dermatomyositis patients. Arthritis Care Res (Hoboken). 2017;69(11):1771–6.
Shneyderman M, et al. Calcinosis in refractory dermatomyositis improves with tofacitinib monotherapy: a case series. Rheumatology (Oxford). 2021;60(11):e387–8.
Fujimoto M, et al. Myositis-specific anti-155/140 autoantibodies target transcription intermediary factor 1 family proteins. Arthritis Rheum. 2012;64(2):513–22.
Sozeri B, Demir F. A striking treatment option for recalcitrant calcinosis in juvenile dermatomyositis: tofacitinib citrate. Rheumatology (Oxford). 2020;59(12):e140–1.
•• Kim, H., et al., Janus kinase (JAK) inhibition with baricitinib in refractory juvenile dermatomyositis. Ann Rheum Dis, 2020. In this study, 4 patients with chronically active juvenile dermatomyositis, who did not respond to 3–6 immunomodulatory drugs, were enrolled in a compassionate study of the use of baricitinib. They reported that baricitinib is safe and beneficial for those patients.
Fiorentino DF, et al. Distinctive cutaneous and systemic features associated with antitranscriptional intermediary factor-1gamma antibodies in adults with dermatomyositis. J Am Acad Dermatol. 2015;72(3):449–55.
Gunawardena H, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology (Oxford). 2008;47(3):324–8.
Pinal-Fernandez I, et al. Tumour TIF1 mutations and loss of heterozygosity related to cancer-associated myositis. Rheumatology (Oxford). 2018;57(2):388–96.
Ge Y, et al. Anti-melanoma differentiation-associated gene 5 (MDA5) antibody-positive dermatomyositis responds to rituximab therapy. Clin Rheumatol. 2021;40(6):2311–7.
Fiorentino D, et al. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol. 2011;65(1):25–34.
Sato S, et al. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: Association with rapidly progressive interstitial lung disease. Arthritis Rheum. 2009;60(7):2193–200.
Fujisawa T, et al. Prognostic factors for myositis-associated interstitial lung disease. PLoS ONE. 2014;9(6):e98824.
Paik JJ, Christopher-Stine L. A case of refractory dermatomyositis responsive to tofacitinib. Semin Arthritis Rheum. 2017;46(4):e19.
•• Chen Z, Wang X, Ye S. Tofacitinib in Amyopathic Dermatomyositis-Associated Interstitial Lung Disease. N Engl J Med. 2019;381(3):291–3. The objective of this single-center, open-label clinical study was to evaluate the efficacy of tofacitinib in patients with early-stage anti-MDA5–positive AMD-ILD. Eighteen patients that fulfilled the inclusion criteria received a combination of glucocorticoids with tofacitinib. They included 32 patients who met the same criteria and received conventional treatment as historical controls. They demonstrate a higher survival and improvement on the ferritin level, FVC (percent of predicted value), single-breath carbon monoxide diffusing capacity, and findings on high-resolution CT in the group of patients treated with tofacitinib.
Sabbagh S, et al. Treatment of anti-MDA5 autoantibody-positive juvenile dermatomyositis using tofacitinib. Brain. 2019;142(11):e59.
Ogawa Y, et al. Effective administration of rituximab in anti-MDA5 antibody-positive dermatomyositis with rapidly progressive interstitial lung disease and refractory cutaneous involvement: a case report and literature review. Case Rep Rheumatol. 2017;2017:5386797.
Mao MM, et al. Ultra-low dose rituximab as add-on therapy in anti-MDA5-positive patients with polymyositis /dermatomyositis associated ILD. Respir Med. 2020;172:105983.
Marguerie C, et al. Polymyositis, pulmonary fibrosis and autoantibodies to aminoacyl-tRNA synthetase enzymes. Q J Med. 1990;77(282):1019–38.
Connors GR, et al. Interstitial lung disease associated with the idiopathic inflammatory myopathies: what progress has been made in the past 35 years? Chest. 2010;138(6):1464–74.
Nishikai M, Reichlin M. Heterogeneity of precipitating antibodies in polymyositis and dermatomyositis Characterization of the Jo-1 antibody system. Arthritis Rheum. 1980;23(8):881–8.
Kalluri M, et al. Clinical profile of anti-PL-12 autoantibody Cohort study and review of the literature. Chest. 2009;135(6):1550–6.
Pinal-Fernandez I, et al. A longitudinal cohort study of the anti-synthetase syndrome: increased severity of interstitial lung disease in black patients and patients with anti-PL7 and anti-PL12 autoantibodies. Rheumatology (Oxford). 2017;56(6):999–1007.
Marie I, et al. Comparison of long-term outcome between anti-Jo1- and anti-PL7/PL12 positive patients with antisynthetase syndrome. Autoimmun Rev. 2012;11(10):739–45.
Hervier B, et al. Hierarchical cluster and survival analyses of antisynthetase syndrome: phenotype and outcome are correlated with anti-tRNA synthetase antibody specificity. Autoimmun Rev. 2012;12(2):210–7.
Oddis CV, et al. Tacrolimus in refractory polymyositis with interstitial lung disease. Lancet. 1999;353(9166):1762–3.
Wilkes MR, et al. Treatment of antisynthetase-associated interstitial lung disease with tacrolimus. Arthritis Rheum. 2005;52(8):2439–46.
Guglielmi S, et al. Acute respiratory distress syndrome secondary to antisynthetase syndrome is reversible with tacrolimus. Eur Respir J. 2008;31(1):213–7.
Tellus MM, Buchanan RR. Effective treatment of anti Jo-1 antibody-positive polymyositis with cyclosporine. Br J Rheumatol. 1995;34(12):1187–8.
Dawson JK, Abernethy VE, Lynch MP. Effective treatment of anti Jo-1 antibody-positive polymyositis with cyclosporin. Br J Rheumatol. 1997;36(1):144–5.
Sauty A, et al. Pulmonary fibrosis with predominant CD8 lymphocytic alveolitis and anti-Jo-1 antibodies. Eur Respir J. 1997;10(12):2907–12.
Koreeda Y, et al. Clinical and pathological findings of interstitial lung disease patients with anti-aminoacyl-tRNA synthetase autoantibodies. Intern Med. 2010;49(5):361–9.
Lambotte O, et al. Efficacy of rituximab in refractory polymyositis. J Rheumatol. 2005;32(7):1369–70.
Levine TD. Rituximab in the treatment of dermatomyositis: an open-label pilot study. Arthritis Rheum. 2005;52(2):601–7.
Brulhart L, Waldburger JM, Gabay C. Rituximab in the treatment of antisynthetase syndrome. Ann Rheum Dis. 2006;65(7):974–5.
Tokunaga K, Hagino N. Dermatomyositis with rapidly progressive interstitial lung disease treated with rituximab: a report of 3 Cases in Japan. Intern Med. 2017;56(11):1399–403.
Leclair V, et al. Efficacy and safety of rituximab in anti-synthetase antibody positive and negative subjects with idiopathic inflammatory myopathy: a registry-based study. Rheumatology (Oxford). 2019;58(7):1214–20.
Andersson H, et al. Long-term experience with rituximab in anti-synthetase syndrome-related interstitial lung disease. Rheumatology (Oxford). 2015;54(8):1420–8.
Tanaka T, Ogata A, Narazaki M. Tocilizumab for the treatment of rheumatoid arthritis. Expert Rev Clin Immunol. 2010;6(6):843–54.
Narazaki M, et al. Therapeutic effect of tocilizumab on two patients with polymyositis. Rheumatology (Oxford). 2011;50(7):1344–6.
Murphy SM, et al. The successful use of tocilizumab as third-line biologic therapy in a case of refractory anti-synthetase syndrome. Rheumatology (Oxford). 2016;55(12):2277–8.
Pinal-Fernandez, I. and A.L. Mammen, On using machine learning algorithms to define clinically meaningful patient subgroups. Ann Rheum Dis, 2019:
Christopher-Stine L, et al. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum. 2010;62(9):2757–66.
Mammen AL, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63(3):713–21.
Tiniakou E, et al. More severe disease and slower recovery in younger patients with anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Rheumatology (Oxford). 2017;56(5):787–94.
Ramanathan S, et al. Clinical course and treatment of anti-HMGCR antibody-associated necrotizing autoimmune myopathy. Neurol Neuroimmunol Neuroinflamm. 2015;2(3):e96.
Meyer A, et al. Statin-induced anti-HMGCR myopathy: successful therapeutic strategies for corticosteroid-free remission in 55 patients. Arthritis Res Ther. 2020;22(1):5.
Bergua C, et al. In vivo pathogenicity of IgG from patients with anti-SRP or anti-HMGCR autoantibodies in immune-mediated necrotising myopathy. Ann Rheum Dis. 2019;78(1):131–9.
Allenbach Y, et al. Necrosis in anti-SRP(+) and anti-HMGCR(+)myopathies: role of autoantibodies and complement. Neurology. 2018;90(6):e507–17.
Walter P, Ibrahimi I, Blobel G. Translocation of proteins across the endoplasmic reticulum I Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981;91(2 Pt 1):545–50.
Miller T, et al. Myopathy with antibodies to the signal recognition particle: clinical and pathological features. J Neurol Neurosurg Psychiatry. 2002;73(4):420–8.
Targoff IN, Johnson AE, Miller FW. Antibody to signal recognition particle in polymyositis. Arthritis Rheum. 1990;33(9):1361–70.
Pinal-Fernandez I, et al. Longitudinal course of disease in a large cohort of myositis patients with autoantibodies recognizing the signal recognition particle. Arthritis Care Res (Hoboken). 2017;69(2):263–70.
Valiyil R, et al. Rituximab therapy for myopathy associated with anti-signal recognition particle antibodies: a case series. Arthritis Care Res (Hoboken). 2010;62(9):1328–34.
Allenbach Y, et al. 224th ENMC International Workshop: Clinico-sero-pathological classification of immune-mediated necrotizing myopathies Zandvoort, The Netherlands, 14–16 October 2016. Neuromuscul Disord. 2018;28(1):87–99.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
Maria Casal-Dominguez declares that she has no conflict of interest. Iago Pinal-Fernandez declares that he has no conflict of interest. Andrew L. Mammen declares that he has no conflict of interest
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Special thanks to Dr. Josep Maria Grau for reviewing
This article is part of the Topical Collection on Other CTD: Inflammatory Myopathies and Sjogren’s.
Rights and permissions
About this article
Cite this article
Casal-Dominguez, M., Pinal-Fernández, I. & Mammen, A.L. Utility of Myositis-Specific Autoantibodies for Treatment Selection in Myositis. Curr Treat Options in Rheum 8, 105–116 (2022). https://doi.org/10.1007/s40674-022-00198-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40674-022-00198-1