Abstract
Purpose of Review
Interstitial lung disease (ILD) is the leading cause of mortality in systemic sclerosis, a rare autoimmune disease characterised by fibrosis and vasculopathy. The variety of phenotypes in SSc-ILD have inspired multiple studies aimed at the identification of biomarkers which can provide disease-specific information but due to the complex pathogenesis of SSc-ILD, it has been challenging to validate such markers. We provide a comprehensive update on those most studied along with emerging biomarkers.
Recent Findings
We review the up-to-date findings with regard to the use of well-studied molecular biomarkers in SSc-ILD along with novel biomarkers offering promise as prognostic markers such as IGFBP-2 and IGFBP-7, the adipokine CTRP9, endothelial progenitor cells, and cellular markers such as CD21lo/neg B cells. Expression profiling data is being used in SSc patients to determine genetic and epigenetic clusters which shed further light on mechanisms involved in the pathogenesis of SSc-ILD and are likely to uncover novel biomarkers.
Summary
With the exception of autoantibodies, there are no routinely measured biomarkers in SSc-ILD and reliable validation of the many potential biomarkers is lacking. Identifying biomarkers which can offer diagnostic and prognostic certainty may help patients to receive preventative treatment as part of a personalised medicine approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic sclerosis (SSc) is a rare, heterogeneous autoimmune disease which affects 3 to 24 per 100,000 globally. With regard to disease-specific pathology, the hallmarks of SSc are skin fibrosis, vasculopathy, and Raynaud’s phenomenon. SSc also involves organs such as the lung, heart, renal system, and gastrointestinal tract [1]. The extent of organ involvement varies widely and is influenced by the primary autoantibody signature of the disease [2••].
Interstitial lung disease (ILD) affects 80% of patients with SSc [1]. A proportion of those with ILD will maintain stable disease with little progression; however, 25–30% will go on to develop progressive ILD [1]. ILD remains the leading cause of mortality amongst the population with SSc, accounting for 35% of deaths [3]. This clearly demonstrates that there is much to be gained from examining the mechanisms by which SSc-ILD becomes progressive in order to improve patient outcome.
In this article, we will review the pathogenesis of SSc-ILD with a focus on emerging biomarkers of SSc-ILD. We will also discuss recent advances in therapies including the recently licensed antifibrotic medication, nintedanib.
A biomarker in terms of SSc-ILD could include genetic polymorphism or biochemical molecules that can be identified in either serum or BALF or skin of the individual. This could be used alongside recognised markers of disease such as forced vital capacity (FVC) or extent of disease on HRCT and ideally input into an algorithm to facilitate prompt recognition of prognosis and suitable treatment.
Multiple biomarkers exist which represents the complex pathogenesis of diseases such as SSc; however, a useful exercise would be to identify those biomarkers which can further define the disease subsets and ultimately provide prognostic information which might in turn affect treatment options.
Epidemiology
EUSTAR database studying 6004 in prospective analysis observed progression of ILD in 23–27% of the cohort [4]. A large retrospective observational study found that in terms of variation between ethnic groups, lung fibrosis was more prevalent amongst Afro-Caribbean patients with SSc (31% vs. 53%, p = 0.007) [5]. A study examining sex differences in SSc also found that male patients with SSc are at increased risk of ILD (52% vs. 39%, p < 0.0001) which is also more likely to be severe [6].
The incidence of SSc-ILD development is highest within the first 5 years following diagnosis with reducing incidence thereafter [2••]. Those with diffuse disease are also at increased risk compared to those with limited disease.
Unlike its counterpart idiopathic pulmonary fibrosis which results in inevitable progression of fibrosis, SSc-ILD can follow multiple trajectories. A proportion of patients (10–20%) will never be diagnosed with ILD or it will be described at < 5% on HRCT without any impact on the pulmonary function tests. Some patients develop limited disease which is < 20% extent on HRCT but from this starting point, a proportion remain stable with minimal progression of their ILD and the remainder of the group develop a progressive disease with high mortality.
Whilst the detection of ILD has improved with the availability of HRCT and the advent of surveillance pulmonary function tests (PFTs) at regular interval, the anticipation of progressive disease remains a challenge. It has been shown that a meaningful decline in lung function is predictive of mortality [7]. In practice, this is measured as FVC > 10% decline, or 5–9% decline with an associated 15% diffusing capacity for carbon monoxide (DLCO) decline. By this point of detection, irreversible lung damage is likely to have occurred.
Multiple histopathological patterns of ILD are described, and these patterns are seen across other conditions such as idiopathic pulmonary fibrosis. Non-specific interstitial pneumonia is the most common pattern observed and describes temporally uniform, diffuse patchy inflammatory change which is most commonly fibrotic as opposed to cellular [8] (Fig. 1). This differs from IPF, in which the common histological pattern seen is usual interstitial pneumonia (UIP) pattern. UIP is observed in SSc-ILD, but does not confer a worse prognosis, and indeed, outcomes are more tightly linked to severity at diagnosis and serial reduction in DLCO than the histopathological group [9].
Cycle of Fibrosis
Pathogenesis
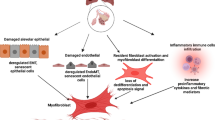
The overarching process leading to SSC-ILD is believed to be altered cellular biology as a result of repeat epithelial injury leading to architectural distortion and extracellular matrix (ECM) deposition.
The first step in this process is repeat injury to alveolar epithelial cells (AEC) which activates both the innate and adaptive immune response causing an influx of inflammatory medicators which results in recruitment of fibroblasts and transformation into myofibroblasts [10]. Myofibroblast cells play an important part in SSc biology, they can arise from resident fibroblasts or epithelial mesenchymal transition and are characterised by highly expressed anti-apoptotic mediators and deposit large amounts of ECM. In response to these inflammatory changes, abhorrent healing processes occur forcing some epithelial cells to undergo apoptosis whereas a proportion are believe to undergo epithelial-mesenchymal transition (EMT) [11].
EMT is a phenomenon also harnessed by metastatic malignant cells. In the context of AEC and fibrotic disease, it is the process by which epithelial cells gain mesenchymal function including that of increased resistance to apoptosis, increased migratory function, and increase production of ECM in response to medicators including transforming growth factor-beta (TGF-β) [11]. EMT is recognised as a response to injury in many adult tissues including lung, kidney, and eye but has been observed in SSc-ILD specifically and has also been observed in bleomycin mouse models [12]. This process may allow well differentiated AEC to lose polarity and give rise to fibroblasts and myofibroblasts, driving the fibrotic process following epithelial injury. EMT results in the loss of epithelial cell markers and gain of mesenchymal cell markers including matrix metalloproteins (MMPs) [11]. MMPs are likely to play a central role in various aspects of ILD along with EMT, including ECM remodelling and basement membrane breakdown along with playing a role in proteinase cascades [13].
Fibroblasts also play a key role in driving fibrosis in the lung. Fibrosis is initiated by activation of resident fibroblasts. Under circumstances of normal wound healing, the activated fibroblasts are at some points deactivated or undergo apoptosis; however, in SSc, there is persistent activation and production of ECM and growth factors such as fibroblast growth factor and connective tissue growth factor (CTGF:CCN2) [14]. Fibroblasts comprise a large part of the connective tissue, they deposit ECM proteins including collagen types I and III. Fibroblasts regulate the deposition of ECM by producing MMP and their inhibitors tissue inhibitors of metalloproteinases (TIMPs) [14]. Fibroblasts can be activated by multiple mechanisms including activation of tissue resident cells, EMT, endothelial-mesenchymal transition, pericyte to mesenchymal transition, and smooth muscle cell differentiation [14]. Persistent production of these myofibroblasts allows then to congregate leading to overproduction of the ECM, the hallmark feature of SSc. Along with fibroblasts, there is also influx of other immune cells including B and T cells which express inflammatory cytokines, the Th2 T-cell plays a role in driving fibrotic response [15].
Fibroblasts are a heterogeneous group and can display various phenotypes of which some are more pro-fibrotic. The response displayed by a fibroblast varies depending on the stimulus the cell receives. Alongside this, SSc-ILD fibroblasts have been shown to behave differently to healthy populations by expressing anti-apoptotic protein B-cell lymphoma-2 (Bcl-2) in response to interleukin-6 (IL-6) [16], whereas the healthy fibroblasts displayed pro-apoptotic Bcl-2-associated X protein (BAX) (Fig. 2).
TGF-β is a well-studied participant in the pathogenesis of lung fibrosis. It exerts its effects by binding to its cell surface receptor and produce downstream activation of the transcription activator SMAD4 which leads to increase production of ECM such as collagen, plasminogen activator inhibitor-1, and CTGF: CCN2 [11]. In addition to cell surface binding, TGF-β also influences lymphocyte proliferation and supresses anti-inflammatory cytokines [10]. Forming a pro-fibrotic cycle, the injured epithelial cells recruit immune cells including macrophages which in turn release more TGF-β [17]. In vivo, deletion of TGF-β was found to be protective against bleomycin-induced pulmonary fibrosis [18].
Epithelial injury is also recognised in the pathogenesis of SSc-ILD. Increased 99mTc-DTPA clearance, a marker of epithelial injury, has been shown within the SSc-ILD cohort to predict a shorter time to FVC decline [19].
Thrombin levels have also been found to be significantly raised in SSc-ILD compared to healthy controls when measured at bronchiolar lavage (BAL) [20]. Thrombin increases fibroblast proliferation including the apoptosis-resistant myofibroblast lineage [21]. Thrombin also induced a variety of pro-fibrotic cytokines including TGF-β and ECM proteins [10]. Thrombin acts through the g-coupled receptor PAR-1 which is upregulated in SSc-ILD patients.
The Wnt pathway occurs in AEC type-2 cells which are activated during epithelial injury along with AEC type-1 apoptosis. The Wnt pathways act to upregulate WISP1 which in turn leads to increased induction of pro-fibrotic cytokines such as SPP1, MMP-7, MMP-9, and PAI-1 from AEC [22].
Risk Factors for SSc-ILD
Disease stratification is extremely important so that those with likely indolent disease can avoid chemo-toxic drugs and those with multiple risk factors for progression or high levels of established biomarkers can receive appropriate therapy at an early juncture.
The EUSTAR database which followed up 6004 European patients with SSC-ILD over a 5-year period identified male sex, high modified Rodnan Skin Score (mRSS), and presences of reflux as independent risk factors for progressive ILD [4] in patients who had 3 or more serial PFT readings over the 5-year follow up period [4].
Other risk factors for ILD also reported in large retrospective studies include older age, male sex, extent of disease on HRCT, lower FVC, and DLCO [23] (Table 1).
Perhaps the most widely used determinator of risk for SSC-ILD used is the autoantibody profile of a patient. It is well established that anti topoisomerase (ATA/Scl-70) antibodies confer an increased risk of ILD, and a large retrospect cohort analyses by Niytanova et al. found that 80.3% of ATA positive patients had developed meaningful ILD after 5 years. This study elegantly demonstrated that anti-Scl-70 positivity was strongly predictive of development of ILD, independent of skin involvement (LcSSc 86.1% and DcSSc 84% at 15 years). Interestingly, the presence of ACA antibodies reduced the hazard of developing SSC-ILD significantly compared to other antibody types (hazard ratio 0.048 when compared to ATA) [2••].
Whilst it is clinically helpful to identify those patients at risk of progressive SSc-ILD, the risk factors do not offer a reliable prediction of mortality. Several models including the composite physiological index, the du Bois, and modified du Bois index have been reported to help predict mortality with the modified du Bois showing good discrimination in prediction of 1 year mortality in SSc-ILD [25].
Genome wide studies (GWAS) have identified loci associated with SSc development with specific HLA alleles conferring risk of pulmonary fibrosis in small studies (HLA-B*62 and HLA-Cw*0602) [26]. Multiple epigenetic mechanisms have been implicated which may be affected by environmental factors. DNA methylation analysis of SSc fibroblasts methyl-CpG-binding domain protein 2 (MBD2) mediates fibrosis via polarisation of M2 macrophages and intratracheal administration of MBD2 small interfering RNA loaded liposomes siRNA protected mice from BLM-induced lung injuries and fibrosis [27]. Histone deacetyl transferases (HDACs) have been implicated in fibrosis, interestingly either pro- or antifibrotic depending on the subgroup [28, 29]. Noncoding RNAs have also been implicated in the development SSc [30••]. Genome studies in China using the Gene Expression Omnibus (GEO) have formed a competing endogenous RNA (ceRNA) network which enable the identification of three core subnetworks (SNHG16, LIN01128, RP11-834C11.4(LINC02381)/hsa-let-7f-5p/IL6, LINC01128/has-miR-21-5p/PTX3, and LINC00665/hsa-miR-155-5p/PLS1) which are involved in immune regulation and proliferation of some cancers. The identification of these networks could lead to further novel biomarker identification [31].
Biomarkers of Disease
A useful biomarker should be easily measurable, widely applicable, and offer diagnostic and/or prognostic information about a disease. Due to the complex processes and triggers involved not only in the development of SSc but also SSc-ILD, a single and widely applicable biomarker has proved difficult to elicit, and there is currently no validated biomarker in SSc-ILD.
In order to obtain biologic sample representative of SSc-ILD, logic dictates that it might be preferable to obtain this from the lung; however, this raises the issue of multiple invasive tests. Lung biopsies are now rarely performed in patients with scleroderma so information regarding local inflammation comes from animal studies [32]. BALF again is also not routinely used to stage SSc-ILD but if found to show distinct information from serum with regard to prognostic indicators, this could change. At present, studies have shown that the neutrophilia observed is related to disease activity but not specific to lung involvement hence not routinely used in practice [24••]. Small prospective studies comparing the induced sputum and serum of SSc and SSc-ILD patients did see increased levels of markers known to be associated with inflammation and fibrosis (IGFBP-1, TGF-β, IL-8, YKL-40, and MMP-7) compared to healthy controls but there was no significant difference between the SSc groups [33], indicating this is not a sensitive method of detecting biomarkers for SSc-ILD.
ATA antibodies, whilst strongly associated with SSc-ILD, cannot serve as an independent biomarker. Multiple proteins involved in SSc pathogenesis have been studied, many showing clear associations with the presence of ILD but large-scale studies demonstrating diagnostic and prognostic power are lacking. Amongst the most studied are Krebs von Lungen-6, surfactant protein-D, and chemokine ligand-18 but other candidate markers have shown response to therapy in more recent studies as described below.
Alveolar Epithelial Injury
Krebs von den Lungen-6 (KL-6)
KL-6 is a transmembrane mucoprotein which is secreted by injured type-2 alveolar cells [19, 34]. KL-6 has been investigated as a potential biomarker in multiple studies and found to correlate with extent of fibrosis on HRCT and was negatively correlated with FVC and DLCO indicating that it reflects the extent of SSc-ILD [35, 36]. A Chinese study recording KL-6 levels in CTD-associated ILD found that KL-6 levels did reduce in those patients who saw improvement in the extent of their disease after cyclophosphamide [37].
In Japan, where KL-6 levels can be routinely measured, a prospective study of 110 SSc-ILD patients found that KL-6 levels did correlate with extensive disease but there was no correlations between the trend of KL-6 in the 6 months following diagnosis and ILD progression in the first 2 years after diagnosis [38].
Patient enrolled in the Scleroderma Lung Study (SLS) II had serum KL-6 and CCL18 levels measured at baseline and 12 months, both patients receiving cyclophosphamide and MMF showed reduced levels in response the treatment but importantly, those with higher baselines levels were more likely to experience progressive ILD, indicating a role for KL-6 as a prognostic biomarker for progressive disease. Recent studies combining retrospective and prospective data and using linear mixed effect models to compare change over time have continued to offer good evidence that KL-6 is predictive of decline in DLCO in both mild and severe diseases [39•].
Surfactant Protein-D (SPD)
SPD is released by alveolar type-2 pneumocytes to reduce surface tension over alveoli and prevent airway collapse. A cohort study by the Scleroderma Lung Study Research Group found that SPD levels were highly sensitive (97%) for ILD, but less specific (69%) [40] making SDP a relevant biomarker for ILD diagnosis (OR 3.15, 95% CI 1.81–5.48 [p < 0.001]) but it does not predict disease progression [40].
Platelet Factor 4 (CXCL4)
The chemokine CXCL4 is a chemotactic agent and increases expression of pro-fibrotic cytokines whilst also suppressing interferon-γ. CXCL4 was measured as part of the SLS II which found that despite a lack or correlation with ILD at baseline, change in CXCL4 at 12 months was predictive of progression in ILD at 12–24 months. A reduction in CXCL4 was also observed in response to immunosuppression therapy [41•].
Carbohydrate-antigen 125 (CA-125)
CA-125 is the most widely used biomarker in ovarian cancer, mainly with regard to response to chemotherapy and prognosis [42]. It also has potential as a biomarker of epithelial injury, originally recognised in IPF [43]. In the SENSCIS trial, CA-125 demonstrated a fold decrease in the nintedanib arm, compared to placebo [44•]. Carbohydrate antigen 15.3 (CA 15.3), another marker of epithelial damage which is encoded by the same MUC1 gene which encodes KL-6, has been shown to correlate with SSc-ILD severity and when used in conjunction with HRCT had prognostic significance [45].
Immune Mediated Damage or Response to Injury
Interleukin-6
The acute phase inflammatory cytokine, IL-6, is recognised to play a role in the pathogenesis of SSc likely via its effects on both the janus kinase signal transducer of transcription factor 3 (JAK STAT3) pathway and AK-SH2 domain tyrosine phosphatase 2 (SHP2)-mitogen-activated protein (MAP) kinase pathway [46, 47]. In an exploratory cohort of SSc-ILD and IPF patients, IL-6 was found to be independently predictive of DLco decline and predictive of mortality in cases of mild ILD within the first year of diagnosis. The level of IL-6 is also closely correlated to CRP levels [48]. The Canadian Scleroderma research group found raised CRP levels were associated with early disease, DcSSc, and worse pulmonary function parameters (total lung capacity < 80% predicted) [49]. Multiple factors, including infection, can cause an elevated CRP and it is only raised in one-quarter of SSc patients. These properties reduce its strength as a candidate biomarker; however, it remains a useful tool for identifying patients with an inflammatory phenotype that may respond well to therapies such as anti-IL-6.
Chemokine Ligand 1/Fractaline (CX3CL1)
CX3CL1 has shown potential as a biomarker and therapeutic target in recent studies. CX3CL is a chemokine with a unique receptor which can be expressed on multiple immune cells. A large cohort study combining two independent cohorts from Norway and California explored CX3CL in the serum and lung tissue of SSc patients with matched control. Serum levels of CX3CL correlated with DLCO and lung fibrosis. Immunostaining of SSc-ILD lungs demonstrated CX3CL is expressed from epithelium and infiltrating interstitial leucocytes whereas its receptor CX3CR1 is expressed on infiltrating interstitial monocytes, particularly plasma cells. Smaller studies which preceded this work, however, had not found a clear association between CX3CL and decline in lung function [46].
Aberrant Fibrogenesis and Matrix Remodelling
Chemokine Ligand-18 (CCL18)
CCL18, formerly known as pulmonary and activated-regulation chemokine (PARC), is associated with M2-macrophage cells and is directly related to pulmonary inflammation, and high levels have been identified in the serum and BAL of SSc-ILD patients [50•, 51, 52]. A Scleroderma Lung Study Research Group cohort study also found that CCL18 levels were independently predictive of a > 10% decline in FVC and for de novo development of extensive disease [40]. After 3 months of treatment with tocilizumab in the faSSinate trial, there was a significant reduction in CCL18 levels compare to the placebo arm, and this reduction was sustained over the 18-month trial period [53].
Matrix Metalloproteinase-9 (MMP-9)
MMP-9 (C3M) and propeptide of type VI collagen (pro-C6), both markers of ECM turnover, demonstrated fold change decreases in the arms treated with the antifibrotic nintedanib as part of the SENSCIS trial [44•]. Similar trends in biomarkers of ILD in non-IPF cases were observed in the INBUILD study [43].
Matrix Metalloproteinase-7 (MMP-7)
MMP-7 is another proteolytic enzyme which plays a role in regulating the turnover of ECM and has been shown to be significantly elevated in SSc and significantly associated with ILD [54]. A study exploring the approach of induced sputum to compare components in SSc patients vs. healthy control found that MMP-7 levels were increased but there was no difference between SSc and SSc-ILD groups [33]. MMP-12 has also been found at increased concentrations in sera, skin, and lung biopsies in SSc-ILD compared to healthy controls [55].
Emerging Biomarkers
Discussed above are some of the biomarkers which have been most scrutinised for their potential as biomarkers for SSc-ILD. There are numerous other pathways and proteins which remain under investigation.
Proteomic analysis of bronchiolar lavage fluid (BAF) in 7 patients with UIP SSc-ILD has provided fresh insight to local proteostasis. Increased protein expression in SSc-ILD BAF, particularly mannose receptor C1 (MRC1), suggests increased activity of type-2 macrophages which are involved in MMP9 mediated tissue repair and fibrosis [56•]. This analysis also identified novel potential biomarkers C3a, apolipoprotein AI (APOAI), protein S100A6, and protein 14–3-3e which affects regulation of the pulmonary surfactant-associated proteinA2 (SPFA2) gene which together may play a role in inflammatory IL-6 signalling [56•].
Altered adipose tissue metabolism has more recently been recognised as a pathogenic pathway to fibrosis in SSc and previous cross-sectional studies have demonstrated that specific adipokines are associated with SSc-ILD [57•]. Preliminary retrospective work inspecting the adipokine C1q/TNF-related protein 9 (CTRP9) found that high levels are associated with SSC-ILD whereas low levels are associated with pulmonary stability [58], whether this can offer more specific prognostic information will require further investigation.
Insulin-like growth factor binding protein-2 (IGFBP-2) has recently been shown to directly correlate with low KCO (< 70% predicted) after 2 years in a cohort study comparing SSc both with and without ILD [59]. IGFBPs are transport proteins for IGF, and increased levels in SSc-ILD could indicate increase availability of IGF which could further contribute towards the fibrotic process. A genomic study using a weighted correlation network analysis method was able to identify key module and hub genes associated with SSc and found that IGFBP-7 is upregulated in SSc-ILD patients [60].
As discussed above, a key step in the pathogenesis of SSc-ILD is endothelial injury and dysfunctional repair. It is possible to count the number of endothelial progenitor cells which can then be used as a surrogate for endothelial regeneration or repair. In a small study, the frequency of EPCs was significantly higher in SSC-ILD patients compared to healthy controls and SSc patient with no ILD. EPC frequency was also higher in early compared to late disease [61].
Measuring dysregulated proteins as biomarkers may offer useful clinical information in SSc-ILD but it does not necessarily represent the cellular mechanisms or abnormality which is driving the process. To this end, various cell lines including monocytes and T cells have been investigated. A recently published study looking at B cells found that auto-immune-disposed subset CD21lo/neg B cell measured from PBMCs were significantly increased in SSc-ILD patients compared to both healthy controls and SSc no ILD patients [62•].
The complement pathway has been implicated in the process of SSc-ILD in various studies. Through activation of the classical, lectin, and alternative pathways, C3a and C5a anaphylatoxins are produced which work to recruit inflammatory cells including neutrophils, mast cells, and monocytes. C3a and C5a are observed at elevated levels in IPF and interact with TGF-β in vitro to augment epithelial injury [63, 64]. The complement pathway has been implicated in other forms of lung damage such as emphysema related to alpha-1 anti-trypsin deficiency [65•] and smoking related lung damage [66]. Emphysema is an increasingly recognised pattern of lung damage in SSc-ILD patients so there may be mechanistic parallels to be drawn from these similar patterns of lung destruction.
Current Treatment Options
Patients may first present with ILD reporting exertional or non-exertional dyspnoea or cough but in many cases, the patient may be asymptomatic. Up-to-date consensus recommends baseline PFTs and HRCT to screen for ILD at the earliest opportunity. Pulmonary manifestations of SSc can affect the parenchyma, vasculature, or musculature so it is important to incorporate these diagnoses into the differential.
Patients with SSC-ILD to the extent which impacts on PFTs typically demonstrate a restrictive pattern with low FVC and a normal or increase FVC/FEV1 ratio. The DLCO will typically be reduced, and the trend in FVC and DLCO is a particularly useful tool when calculating the progression of ILD [24••].
As previously mentioned, there is no consensus on thresholds for treatment in SSc-ILD. The ‘Brompton UK-RSA’ or ‘Goh’ classification can be utilised by clinicians during consultations to estimate the extent of fibrosis. CT imaging is assessed at 5 levels based on extent of grounds glass, reticulation, and cystic change. The resulting measure of extent defines the patient as limited (clearly < 20%), extensive (clearly > 20%), or indeterminate. Indeterminate cases are sorted into the limited or extensive category using the FVC threshold of 70% predicted [7].
An international panel of expert rheumatologists and pulmonologists collaborated to propose the following guidance to identify the change in PFT that correlates with disease progression [24••] (Fig. 3).
Proposed algorithm to define a meaningful progression of SSc-ILD. Reproduced with kind permission from Distler et al. [24••]
Once SSc-ILD has been confirmed and deemed clinically significant, several immunosuppressive therapies exist in the clinician’s armamentarium.
Disease-modifying Anti-rheumatic Drugs (DMARDs)
DMARDs have been favoured as a first-line option for many years for a number of reasons including the route of delivery, cost, and side effect profile. They are effective in treating ILD, in fact the SLS II study demonstrated a significant improvement in FVC% after treatment with either mycophenolate or oral cyclophosphamide, the groups were not significantly different, but the mycophenolate was better tolerated [67]. A pooled analysis of the SLS I and II trials allowed an estimation for minimally important clinical difference (MCID) in FVC of FVC% improvement ranged from 3.0 to 5.3% and for worsening from − 3.0 to − 3.3% which will be helpful when determining treatment effects in future trials [68]. When applying the FVC MCID to results from the SENSCIS trial receiving either nintedanib or placebo, 34.5% and 43.8% had a decrease in FVC of ≥ 3.3% predicted (proposed MCID for reduction of FVC), and 23.0% and 14.9% had an increase in FVC of ≥ 3.0% predicted (proposed MCID for improvement in FVC) [69].
Nintedanib
The SENSCIS trial for nintedanib met its primary endpoint, showing a reduction in the rate of progression of ILD. As of late 2021, nintedanib has been licensed in the UK for use in SSc-ILD demonstrating progression despite immunosuppression by the National Institute for Health and Care Excellence (NICE) [70] and can be used safely in combination with MMF [71].
The latest analysis of the SENSCIS data focussed on the reduction in decline in FVC (mL/year) in patients with risk factors for progressive ILD. Patients receiving nintedanib with elevated CRP (> 6 mg/L) or thrombophilia (> 330 × 109/L) saw a difference of 52.5 mL/year in rate of decline in the nintedanib arm. A reduction in rate of FVC decline was also seen in patients with DcSSc and those with high mRSS (> 15) [72••]. The change in FVC for the nintedanib was also sustained, as observed in the SENSCIS-ON open arm extension trial [73]. Patients in these high-risk groups might typically receive immunosuppression but would not normally be treated with nintedanib, particularly early in their disease course. This analysis demonstrates that the addition of antifibrotic therapy can salvage FVC decline which may lead to better long-term outcomes.
Rituximab and Cyclophosphamide
Findings from the DESIRES trial suggest that rituximab is an effective therapy for reducing fibrosis particularly in the skin and in ILD, administered at 375 mg/m2 weekly for 4 weeks [74••]. In double-blind, randomised study, FVC improved in rituximab group vs. placebo (0.09% increase versus a 2.87% decrease), inflammatory ground glass findings on HRCT also reduced in the rituximab group compared to placebo (0.32% reduction in ILD areas on CT compared with a 2.39% increase in the placebo group). DLCO decreased in both groups during follow up with no statistical difference between groups (1.32% and 3.56% in rituximab and placebo) but the DLCO in the rituximab group did stabilise from 4 weeks onwards [74••].
An open label randomised trial in India involving 60 patients with DcSSc saw an increase in FVC 61.30 (11.28) to 67.52 (13.59) in the rituximab arm compared to the cyclophosphamide arm 59.25 (12.96) to 58.06 (11.23) at 6 months [75]. There were also more adverse reactions in the cyclophosphamide arm including pneumonia and herpes zoster. A Russian open label prospective study involving 107 patients with SSc-ILD over a period of 13 months found an increase in FVC in each treatment arm (cyclophosphamide p = 0.034 and rituximab 0.000045) and again, the therapy was better tolerated in the rituximab arm [76].
Retrospective comparisons have also been conducted [77], and these, along with the studies outlined above, have shown efficacy of both cyclophosphamide and rituximab therapy, and head to head randomised control trials such as the RECITAL trial [78] are needed to help inform decision-making for treatment in SSc-ILD.
Tocilizumab (TCZ)
The FocuSSed trial, a large phase III randomised control trial comparing TCZ (anti-IL-6) to placebo in combination with its predecessor, FaSSinate, demonstrated a clear stabilisation in lung function particularly within the DcSSc subgroup who have fibrosis present at treatment initiation [79, 80].
Transplant
Autologous stem cell transplant (SCT) has been studied in three randomised controlled trials which have demonstrated that they are an effective treatment stabilising pulmonary disease, improving lung volumes, and reducing the extent of ILD observed on HRCT. There is significant morbidity associated with undergoing SCT, and extensive ILD has been associated with poor outcomes [81•]. It has been suggested that the optimal patient groups for SCT are early diffuse patients with rapidly progressive disease who have not yet developed significant visceral involvement [82].
Solid organ transplant such as heart and lung transplant is a therapeutic option for a small number of patients with SSc-ILD. Unfortunately, transplant does not exclude recurrence, and gastro-oesophageal reflux presents a significant barrier to transplant for SSc patients.
Conclusion
At present in clinical practice, immunosuppressive treatment is initiated after fall in FVC which implies a certain degree of ILD has already occurred. By combining biomarkers of disease alongside risk factors such as male age, high mRSS, and reflux, there may be scope to develop a reliable algorithm by which allows targeted therapy to be initiated earlier in these individuals with the goal to prevent rather than stabilise ILD.
The introduction of platforms such as GWAS, proteomics, and metabolomics is deepening our understanding of the pathophysiology and architecture of systemic sclerosis. With these developments, a breadth of candidate biomarkers are being studied but the challenge lies in finding readily measurable biomarker which offer specific diagnostic and prognostic value above that of PFTs and imaging. The numerous environmental triggers and epigenetic mechanisms involved in the pathogenesis of SSc make finding a single biomarker which can accurately represent SSc-ILD a further challenge. Large randomised controlled trials which have facilitated new licensed treatments in SSc-ILD have also offered valuable insight into the response of candidate biomarkers but further large-scale studies focussing on biomarkers are needed to validate these in order to incorporate them into routine disease stratification.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390(10103):1685–99.
•• Nihtyanova SI, Sari A, Harvey JC, Leslie A, Derrett-Smith EC, Fonseca C, et al. Using autoantibodies and cutaneous subset to develop outcome-based disease classification in systemic sclerosis. Arthritis Rheumatol. 2020;72(3):465–76. (A landmark paper in SSc defining the antibody phenotypes in systemic sclerosis including important information about complication risk and survival).
Tyndall AJ, Bannert B, Vonk M, Airò P, Cozzi F, Carreira PE, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69(10):1809–15.
Hoffmann-Vold AM, Allanore Y, Alves M, Brunborg C, Airó P, Ananieva LP, et al. Progressive interstitial lung disease in patients with systemic sclerosis-associated interstitial lung disease in the EUSTAR database. Ann Rheum Dis. 2021;80(2):219–27.
Al-Sheikh H, Ahmad Z, Johnson SR. Ethnic variations in systemic sclerosis disease manifestations, internal organ involvement, and mortality. J Rheumatol. 2019;46(9):1103–8.
Peoples C, Medsger TA Jr, Lucas M, Rosario BL, Feghali-Bostwick CA. Gender differences in systemic sclerosis: relationship to clinical features, serologic status and outcomes. Journal of scleroderma and related disorders. 2016;1(2):204–12.
Goh NS, Desai SR, Veeraraghavan S, Hansell DM, Copley SJ, Maher TM, et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med. 2008;177(11):1248–54.
Katzenstein AL, Fiorelli RF. Nonspecific interstitial pneumonia/fibrosis. Histologic features and clinical significance. Am J Surg Pathol. 1994;18(2):136–47.
Bouros D, Wells AU, Nicholson AG, Colby TV, Polychronopoulos V, Pantelidis P, et al. Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am J Respir Crit Care Med. 2002;165(12):1581–6.
Akter T, Silver RM, Bogatkevich GS. Recent advances in understanding the pathogenesis of scleroderma-interstitial lung disease. Curr Rheumatol Rep. 2014;16(4):411.
Willis BC, Borok Z. TGF-β-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol-Lung Cell Mol Physiol. 2007;293(3):L525–34.
Kim KK, Wei Y, Szekeres C, Kugler MC, Wolters PJ, Hill ML, et al. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest. 2009;119(1):213–24.
Pardo A, Selman M. Matrix metalloproteases in aberrant fibrotic tissue remodeling. Proc Am Thorac Soc. 2006;3(4):383–8.
Gilbane AJ, Denton CP, Holmes AM. Scleroderma pathogenesis: a pivotal role for fibroblasts as effector cells. Arthritis Res Ther. 2013;15(3):215.
Bagnato G, Harari S. Cellular interactions in the pathogenesis of interstitial lung diseases. Eur Respir Rev. 2015;24(135):102–14.
Moodley YP, Misso NL, Scaffidi AK, Fogel-Petrovic M, McAnulty RJ, Laurent GJ, et al. Inverse effects of interleukin-6 on apoptosis of fibroblasts from pulmonary fibrosis and normal lungs. Am J Respir Cell Mol Biol. 2003;29(4):490–8.
Khalil N, Bereznay O, Sporn M, Greenberg AH. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med. 1989;170(3):727–37.
Li M, Krishnaveni MS, Li C, Zhou B, Xing Y, Banfalvi A, et al. Epithelium-specific deletion of TGF-β receptor type II protects mice from bleomycin-induced pulmonary fibrosis. J Clin Investig. 2011;121(1):277–87.
Goh NS, Desai SR, Anagnostopoulos C, Hansell DM, Hoyles RK, Sato H, et al. Increased epithelial permeability in pulmonary fibrosis in relation to disease progression. Eur Respir J. 2011;38(1):184–90.
Ohba T, McDonald JK, Silver RM, Strange C, LeRoy EC, Ludwicka A. Scleroderma bronchoalveolar lavage fluid contains thrombin, a mediator of human lung fibroblast proliferation via induction of platelet-derived growth factor alpha-receptor. Am J Respir Cell Mol Biol. 1994;10(4):405–12.
Bogatkevich GS, Tourkina E, Silver RM, Ludwicka-Bradley A. Thrombin differentiates normal lung fibroblasts to a myofibroblast phenotype via the proteolytically activated receptor-1 and a protein kinase C-dependent pathway. J Biol Chem. 2001;276(48):45184–92.
Königshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009;119(4):772–87.
Winstone TA, Assayag D, Wilcox PG, Dunne JV, Hague CJ, Leipsic J, et al. Predictors of mortality and progression in scleroderma-associated interstitial lung disease: a systematic review. Chest. 2014;146(2):422–36.
•• Distler O, Assassi S, Cottin V, Cutolo M, Danoff SK, Denton CP, et al. Predictors of progression in systemic sclerosis patients with interstitial lung disease. Eur Respir J. 2020;55(5):1902026. (An expert consensus allowing definition of the thresholds by which we can diagnose meaningful progression of SSc-ILD. A thorough review of risk factors for progression and monitoring advice).
Ryerson CJ, O’Connor D, Dunne JV, Schooley F, Hague CJ, Murphy D, et al. Predicting mortality in systemic sclerosis-associated interstitial lung disease using risk prediction models derived from idiopathic pulmonary fibrosis. Chest. 2015;148(5):1268–75.
Gladman DD, Kung TN, Siannis F, Pellett F, Farewell VT, Lee P. HLA markers for susceptibility and expression in scleroderma. J Rheumatol. 2005;32(8):1481–7.
Wang Y, Zhang L, Wu GR, Zhou Q, Yue H, Rao LZ, Yuan T, Mo B, Wang FX, Chen LM, Sun F, Song J, Xiong F, Zhang S, Yu Q, Yang P, Xu Y, Zhao J, Zhang H, Xiong W, Wang CY. MBD2 serves as a viable target against pulmonary fibrosis by inhibiting macrophage M2 program. Sci Adv. 2021;7(1):eabb6075. https://doi.org/10.1126/sciadv.abb6075.
Palumbo-Zerr K, Zerr P, Distler A, Fliehr J, Mancuso R, Huang J, et al. Orphan nuclear receptor NR4A1 regulates transforming growth factor-β signaling and fibrosis. Nat Med. 2015;21(2):150–8.
Chu H, Jiang S, Liu Q, Ma Y, Zhu X, Liang M, et al. Sirtuin1 protects against systemic sclerosis-related pulmonary fibrosis by decreasing proinflammatory and profibrotic processes. Am J Respir Cell Mol Biol. 2018;58(1):28–39.
•• Tsou P-S, Varga J, O’Reilly S. Advances in epigenetics in systemic sclerosis: molecular mechanisms and therapeutic potential. Nat Rev Rheumatol. 2021;17(10):596–607. (An exciting review about recent advances and understanding of epigenetics related to SSc with particular focus on the role of DNA methylation and its effects on fibroblast function. The paper also discusses the possibility of targeting these enzymes therapeutically).
Yan YM, Zheng JN, Wu LW, Rao QW, Yang QR, Gao D, et al. Prediction of a competing endogenous RNA co-expression network by comprehensive methods in systemic sclerosis-related interstitial lung disease. Front Genet. 2021;12:633059.
Marangoni RG, Varga J, Tourtellotte WG. Animal models of scleroderma: recent progress. Curr Opin Rheumatol. 2016;28(6):561–70.
Jacquerie P, Henket M, André B, Moermans C, de Seny D, Gester F, et al. Inflammatory profile of induced sputum composition in systemic sclerosis and comparison with healthy volunteers. Sci Rep. 2021;11(1):10679.
Hant FN, Silver RM. Biomarkers of scleroderma lung disease: recent progress. Curr Rheumatol Rep. 2011;13(1):44–50.
Lee JS, Lee EY, Ha YJ, Kang EH, Lee YJ, Song YW. Serum KL-6 levels reflect the severity of interstitial lung disease associated with connective tissue disease. Arthritis Res Ther. 2019;21(1):58.
Zhong D, Wu C, Bai J, Hu C, Xu D, Wang Q, et al. Comparative diagnostic efficacy of serum Krebs von den Lungen-6 and surfactant D for connective tissue disease-associated interstitial lung diseases: a meta-analysis. Med (Baltimore). 2020;99(16): e19695.
Ma H, Lu J, Song Y, Wang H, Yin S. The value of serum Krebs von den Lungen-6 as a diagnostic marker in connective tissue disease associated with interstitial lung disease. BMC Pulm Med. 2020;20(1):6.
Shirai Y, Fukue R, Kaneko Y, Kuwana M. Clinical relevance of the serial measurement of Krebs von den Lungen-6 levels in patients with systemic sclerosis-associated interstitial lung disease. Diagnostics (Basel). 2021;11(11):2007. https://doi.org/10.3390/diagnostics11112007.
• Stock CJW, Hoyles RK, Daccord C, Kokosi M, Visca D, De Lauretis A, et al. Serum markers of pulmonary epithelial damage in systemic sclerosis-associated interstitial lung disease and disease progression. Respirology. 2021;26(5):461–8. (A recently published paper adding to the body of evidence that highlights KL-6 levels provide prognostic information about DLCOLCO decline in moderate and severe ILD when corrected for age, gender, ethnicity, smoking history, and the MUC1 allele.)
Elhai M, Hoffmann-Vold AM, Avouac J, Pezet S, Cauvet A, Leblond A, et al. Performance of candidate serum biomarkers for systemic sclerosis–associated interstitial lung disease. Arthritis Rheumatol. 2019;71(6):972–82.
• Volkmann ER, Tashkin DP, Roth MD, Clements PJ, Khanna D, Furst DE, et al. Changes in plasma CXCL4 levels are associated with improvements in lung function in patients receiving immunosuppressive therapy for systemic sclerosis-related interstitial lung disease. Arthritis Res Ther. 2016;18(1):305. (This up-to-date prospective study, performed in the context of a randomised control trial in SSc patient receiving MMF or oral cyclophosphamide, measured the response of CXCL4. The study demonstrates CXCL levels decrease in response to immunosuppression and the levels of CXCL4 correspond to future progression of ILD, indicating CXCL4 may hold a role as a prognostic biomarker.)
Zhang M, Cheng S, Jin Y, Zhao Y, Wang Y. Roles of CA125 in diagnosis, prediction, and oncogenesis of ovarian cancer. Biochim Biophys Acta Rev Cancer. 2021;1875(2):188503.
Maher TM, Oballa E, Simpson JK, Porte J, Habgood A, Fahy WA, et al. An epithelial biomarker signature for idiopathic pulmonary fibrosis: an analysis from the multicentre PROFILE cohort study. Lancet Respir Med. 2017;5(12):946–55.
• Assassi S, Kuwana M, Denton C, Maher T, Diefenbach C, Ittrich C, et al. POS0853 effects of nintedanib on circulating biomarkers in subjects with systemic sclerosis-associated interstitial lung disease (SSc-ILD). BMJ Publishing Group Ltd. 2022;81:719–20. (Analysis from the SENSCIS trial, measuring circulating biomarkers of ECM turnover and epithelial injury in response to nintedanib therapy. A fold reduction in KL-6, CA-125, CRP, and PROC6 was observed suggesting nintedanib reduces circulating markers of ECM turnover and damage.)
Celeste S, Santaniello A, Caronni M, Franchi J, Severino A, Scorza R, et al. Carbohydrate antigen 15.3 as a serum biomarker of interstitial lung disease in systemic sclerosis patients. Eur J Intern Med. 2013;24(7):671–6.
De Lauretis A, Sestini P, Pantelidis P, Hoyles R, Hansell DM, Goh NS, et al. Serum interleukin 6 is predictive of early functional decline and mortality in interstitial lung disease associated with systemic sclerosis. J Rheumatol. 2013;40(4):435–46.
Muangchan C, Pope JE. Interleukin 6 in systemic sclerosis and potential implications for targeted therapy. J Rheumatol. 2012;39(6):1120–4.
Ohtsuka T. Serum interleukin-6 level is reflected in elevated high-sensitivity C-reactive protein level in patients with systemic sclerosis. J Dermatol. 2010;37(9):801–6.
Muangchan C, Harding S, Khimdas S, Bonner A, Baron M, Pope J. Association of C-reactive protein with high disease activity in systemic sclerosis: results from the Canadian Scleroderma Research Group. Arthritis Care Res (Hoboken). 2012;64(9):1405–14.
• Hoffmann-Vold AM, Tennøe AH, Garen T, Midtvedt Ø, Abraityte A, Aaløkken TM, et al. High level of chemokine CCL18 is associated with pulmonary function deterioration, lung fibrosis progression, and reduced survival in systemic sclerosis. Chest. 2016;150(2):299–306. (A large cohort of almost 300 patient from Oslo were compared to healthy controls with CCL18 levels measured. High CCL18 levels were significantly associated with a drop in FVC and ILD progression, seen most notably in early disease (< 3 years) and 5–10 years mortality was also reduced in patients with high CCL18 levels.)
Prasse A, Pechkovsky DV, Toews GB, Jungraithmayr W, Kollert F, Goldmann T, et al. A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am J Respir Crit Care Med. 2006;173(7):781–92.
Kodera M, Hasegawa M, Komura K, Yanaba K, Takehara K, Sato S. Serum pulmonary and activation-regulated chemokine/CCL18 levels in patients with systemic sclerosis: a sensitive indicator of active pulmonary fibrosis. Arthritis Rheum. 2005;52(9):2889–96.
Khanna D, Denton CP, Jahreis A, van Laar JM, Frech TM, Anderson ME, et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet. 2016;387(10038):2630–40.
Moinzadeh P, Krieg T, Hellmich M, Brinckmann J, Neumann E, Müller-Ladner U, et al. Elevated MMP-7 levels in patients with systemic sclerosis: correlation with pulmonary involvement. Exp Dermatol. 2011;20(9):770–3.
Manetti M, Guiducci S, Romano E, Bellando-Randone S, Conforti ML, Ibba-Manneschi L, et al. Increased serum levels and tissue expression of matrix metalloproteinase-12 in patients with systemic sclerosis: correlation with severity of skin and pulmonary fibrosis and vascular damage. Ann Rheum Dis. 2012;71(6):1064–72.
• Landi C, Bargagli E, Carleo A, Refini RM, Bennett D, Bianchi L, et al. Bronchoalveolar lavage proteomic analysis in pulmonary fibrosis associated with systemic sclerosis: S100A6 and 14-3-3ε as potential biomarkers. Rheumatology (Oxford). 2019;58(1):165–78 (Proteomic analysis of BAL samples have shed light on the local inflammatory pathways involved in the pathogenesis of SSc-ILD. Small samples size. (Proteomic analysis of BAL samples have shed light on the local inflammatory pathways involved in the pathogenesis of SSc-ILD. Small samples size (n = 7) with controls selected by smoking history, not including SSc controls.)
• Korman B, Alejo R, Sudhakar D, Hinchcliff M, Agrawal R, Varga J, et al. The novel adipokine C1q-TNF related protein 9 (CTRP9) is elevated in systemic sclerosis-associated interstitial lung disease. Clin Exp Rheumatol. 2018;113(4):184–5. ((This is the first study to explore the role of adipose tissue metabolism in SSc-ILD pathogenesis and the possibility of using adipokines as biomarkers for SSc-ILD, in particular CTRP9. This will no doubt lead to further work in this area to improve our understanding of the interaction of adipose metabolism and inflammation in SSc.))
Yang MM, Balmert LC, Marangoni RG, Carns M, Hinchcliff M, Korman BD, Varga J. Circulating CTRP9 is associated with severity of systemic sclerosis-associated interstitial lung disease. Arthritis Care Res (Hoboken). 2021. https://doi.org/10.1002/acr.24749. Epub ahead of print.
Guiot J, Njock MS, André B, Gester F, Henket M, de Seny D, et al. Serum IGFBP-2 in systemic sclerosis as a prognostic factor of lung dysfunction. Sci Rep. 2021;11(1):10882.
Yan YM, Zheng JN, Li Y, Yang QR, Shao WQ, Wang Q. Insulin-like growth factor binding protein 7 as a candidate biomarker for systemic sclerosis. Clin Exp Rheumatol. 2021;131(4):66–76.
Pulito-Cueto V, Remuzgo-Martínez S, Genre F, Atienza-Mateo B, Mora-Cuesta VM, Iturbe-Fernández D, Lera-Gómez L, Pérez-Fernández R, Prieto-Peña D, Portilla V, Blanco R, Corrales A, Gualillo O, Cifrián JM, López-Mejías R, González-Gay MA. Endothelial progenitor cells: relevant players in the vasculopathy and lung fibrosis associated with the presence of interstitial lung disease in systemic sclerosis patients. Biomedicines. 2021;9(7):847. https://doi.org/10.3390/biomedicines9070847.
• Wilfong EM, Vowell KN, Bunn KE, Rizzi E, Annapureddy N, Dudenhofer RB, et al. CD19 + CD21(lo/neg) cells are increased in systemic sclerosis-associated interstitial lung disease. Clin Exp Med. 2022;22(2):209–20. (This paper is a well-designed study into specific immune cell lines which might be playing a distinct role in SSc-ILD pathology and therefore could be harnessed as biomarkers along with offering further clarity into the cellular mechanisms leading to SSc-ILD.)
Gu H, Fisher AJ, Mickler EA, Duerson F 3rd, Cummings OW, Peters-Golden M, et al. Contribution of the anaphylatoxin receptors, C3aR and C5aR, to the pathogenesis of pulmonary fibrosis. Faseb j. 2016;30(6):2336–50.
Gu H, Mickler EA, Cummings OW, Sandusky GE, Weber DJ, Gracon A, et al. Crosstalk between TGF-β1 and complement activation augments epithelial injury in pulmonary fibrosis. Faseb j. 2014;28(10):4223–34.
• O’Brien ME, Fee L, Browne N, Carroll TP, Meleady P, Henry M, et al. Activation of complement component 3 is associated with airways disease and pulmonary emphysema in alpha-1 antitrypsin deficiency. Thorax. 2020;75(4):321–30. (Although not directly related to ILD or SSc, this paper importantly identifies the potential role of complement activation or dysregulation in emphysema, a pattern that is increasingly recognised in SSc-ILD both smokers and non-smokers. This represents a platform for further study of complement activation in SSc-ILD.)
Yuan X, Chang CY, You R, Shan M, Gu BH, Madison MC, Diehl G, Perusich S, Song LZ, Cornwell L, Rossen RD, Wetsel R, Kimal R, Coarfa C, Eltzschig HK, Corry DB, Kheradmand F. Cigarette smoke-induced reduction of C1q promotes emphysema. JCI Insight. 2019;5(13):e124317. https://doi.org/10.1172/jci.insight.124317.
Tashkin DP, Roth MD, Clements PJ, Furst DE, Khanna D, Kleerup EC, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med. 2016;4(9):708–19.
Kafaja S, Clements PJ, Wilhalme H, Tseng CH, Furst DE, Kim GH, et al. Reliability and minimal clinically important differences of forced vital capacity: results from the Scleroderma Lung Studies (SLS-I and SLS-II). Am J Respir Crit Care Med. 2018;197(5):644–52.
Maher TM, Mayes MD, Kreuter M, Volkmann ER, Aringer M, Castellvi I, et al. Effect of nintedanib on lung function in patients with systemic sclerosis-associated interstitial lung disease: further analyses of a randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 2021;73(4):671–6.
Adler AI, Guo Y, Thiam A, Elliott N, Patel S. Nintedanib for treating progressive fibrosing interstitial lung diseases. Lancet Respir Med. 2021;9(12):e116–7.
Highland KB, Distler O, Kuwana M, Allanore Y, Assassi S, Azuma A, et al. Efficacy and safety of nintedanib in patients with systemic sclerosis-associated interstitial lung disease treated with mycophenolate: a subgroup analysis of the SENSCIS trial. Lancet Respir Med. 2021;9(1):96–106.
Khanna D, Maher T, Volkmann E, Allanore Y, Smith V, Assassi S, et al. OP0157 effect of nintedanib in patients with systemic sclerosis-associated interstitial lung disease (SSc-ILD) and risk factors for rapid decline in forced vital capacity: further analyses of the senscis trial. BMJ Publishing Group Ltd; 2022. (Post hoc analysis of the SENSCIS trial data identifying patients with risk factors for rapid progression of ILD including < 18 months since first non-Raynaud symptom, CRP > 6 mg/L, platelets > 330 × 109/L and high mRSS 15–40 or > 18 at diagnosis. Patients with these characteristics in the placebo arm experienced a greater decline in FVC compared to the treatment arm suggesting nintedanib can slow the rate of fibrotic progression in high risk individuals.)
Allanore Y, Khanna D, Smith V, et al. Continued treatment with nintedanib in patients with limited cutaneous systemic sclerosis and interstitial lung disease. Ann Rheumat Dis 2022;81:251–252.
•• Ebata S, Yoshizaki A, Oba K, Kashiwabara K, Ueda K, Uemura Y, et al. Safety and efficacy of rituximab in systemic sclerosis (DESIRES): a double-blind, investigator-initiated, randomised, placebo-controlled trial. Lancet Rheumatol. 2021;3(7):e489–97. (A randomised control study assigning patients to rituximab or placebo. This is the first RCT to show efficacy of rituximab with skin score (mRSS) as the primary endpoint.)
Sircar G, Goswami RP, Sircar D, Ghosh A, Ghosh P. Intravenous cyclophosphamide vs rituximab for the treatment of early diffuse scleroderma lung disease: open label, randomized, controlled trial. Rheumatol. 2018;57(12):2106–13.
Koneva O, Ananyeva LP, Garzanova L, Desinova O, Ovsyannikova O, Starovoytova M. OP0187 rituximab and cyclophosphamide comparison for efficacy and safety in the patients with systemic sclerosis associated with interstitial lung disease. Ann Rheumat Dis. 2019;78(Suppl 2):169–70.
Yılmaz DD, Borekci S, Musellim B. Comparison of the effectiveness of cyclophosphamide and rituximab treatment in patients with systemic sclerosis-related interstitial lung diseases: a retrospective, observational cohort study. Clin Rheumatol. 2021;40(10):4071–9.
Saunders P, Tsipouri V, Keir GJ, Ashby D, Flather MD, Parfrey H, et al. Rituximab versus cyclophosphamide for the treatment of connective tissue disease-associated interstitial lung disease (RECITAL): study protocol for a randomised controlled trial. Trials. 2017;18(1):275.
Khanna D, Lin CJ, Goldin J, Kim G, Kuwana M, Allanore Y, et al. OP0245 Preservation of lung function observed in a phase 3 randomized controlled trial of tocilizumab for the treatment of early SSc. Ann Rheumat Dis 2019;78:202–203.
Denton CP, Yee P, Ong VH. News and failures from recent treatment trials in systemic sclerosis. Eur J Rheumatol. 2020;7(Suppl 3):S242–8.
• Spierings J, Chiu YH, Voortman M, van Laar JM. Autologous stemcell transplantation in systemic sclerosis-associated interstitial lung disease early action in selected patients rather than escalation therapy for all. Ther Adv Musculoskelet Dis. 2021;13:1759720x211035196. (This expert paper outlines the findings from the three main RCT trials in autologous SCT for SSc with focus on SSc-ILD outcomes. The authors outline patients most likely to benefit from SCT and tolerate the considerable morbidity of the procedure).
Alexander T, Henes J, Distler JHW, Schmalzing M, Blank N, Kötter I, et al. Autologous hematopoietic stem cell transplantation for systemic sclerosis: position statement of the stem cell therapy working party of the German Society of Rheumatology. Z Rheumatol. 2020;79(5):429–36.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Alice Cole declares that she has no conflict of interest. Christopher P. Denton declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cole, A., Denton, C.P. Biomarkers in Systemic Sclerosis Associated Interstitial Lung Disease (SSc-ILD). Curr Treat Options in Rheum 8, 152–170 (2022). https://doi.org/10.1007/s40674-022-00196-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40674-022-00196-3