Abstract
Purpose of Review
Multiple classes of medications have been studied for the treatment of Raynaud’s phenomenon (RP) with or without digital ischemia. The goal of this review is to discuss the outcomes of recent studies and to report on our approach to the management of RP in light of the available evidence.
Recent Findings
Comparing treatments for RP remains a challenge as efficacy endpoint vary widely among trials. While calcium channel blockers (CCB) are used first line in the pharmacologic management of RP, phosphodiesterase 5 inhibitors (PDE5i) have also been shown to be beneficial in reducing symptoms. In the setting of digital ischemia, administration of intravenous prostanoids is the standard of care. Bosentan an endothelin receptor antagonist (ERA), has shown benefit in the prevention of future digital ulcers in patients with scleroderma. Botulinum toxin (Btx) therapy was ineffective in a clinical trial involving scleroderma patients; more controlled studies are needed in other subsets of patients. Digital sympathectomy may be beneficial in cases of critical digital ischemia, though recurrence of symptoms is common.
Summary
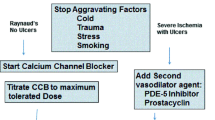
Comparative effectiveness studies are needed to determine which therapeutic interventions are most beneficial in patients with RP. Based on the available evidence, we start with a CCB and add a PDE5i if symptoms are not controlled, or an intravenous prostacyclin in the setting of severe critical digital ischemia. We may additionally add an ERA in cases of recurrent digital ulcers. A surgical sympathectomy may be used in refractory cases of digital ischemia. A digital block may also be a less invasive, but temporary, intervention allowing for titration of medical therapy.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Freedman RR, Ianni P. Effects of general and thematically relevant stressors in Raynaud’s disease. J Psychosom Res. 1985;29:275–80.
Freedman RR, Ianni P. Role of cold and emotional stress in Raynaud’s disease and scleroderma. Br Med J (Clin Res Ed) [Internet]. 1983;287:1499–502. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=6416474.
Freedman RR, Mayes MD. Familial aggregation of primary Raynaud’s disease. Arthritis Rheum. 1996;39:1189–91.
Walker UA, Tyndall A, Czirják L, Denton C. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials And Research group database. Ann Rheum Dis. 2007;66:754–63.
Kwakkenbos L, Thombs BD. Non-drug approaches to treating Raynaud’s phenomenon. In: Wigley FM, Herrick AL, Flavahan NA, editors. Raynaud’s Phenom. A Guid. to Pathog. Treat. Springer; 2015. p. 299–313.
Gladue H, Maranian P, Paulus HE, Khanna D. Evaluation of test characteristics for outcome measures used in Raynaud’s phenomenon clinical trials. Arthritis Care Res. 2014;65:630–6.
Merkel PA, Herlyn K, Martin RW, Anderson JJ, Mayes MD, Bell P, et al. Measuring disease activity and functional status in patients with scleroderma and Raynaud’s phenomenon. Arthritis Rheum. 2002;46:2410–20.
Khanna PP, Maranian P, Gregory J, Khanna D. The minimally important difference and patient acceptable symptom state for the Raynaud’s condition score in patients with Raynaud’s phenomenon in a large randomised controlled clinical trial. Ann Rheum Dis. 2010;69:588–91.
Pauling JD, Saketkoo LA, Domsic RT. Patient perceptions of the Raynaud’s Condition Score diary provide insight into its performance in clinical trials of Raynaud’s phenomenon. Arthritis Rheumatol [Internet]. 2018; Available from: http://doi.wiley.com/10.1002/art.40481.
Pauling JD, Shipley JA, Harris ND, McHugh NJ. Use of infrared thermography as an endpoint in therapeutic trials of Raynaud’s phenomenon and systemic sclerosis. Clin Exp Rheumatol [Internet]. 2012;30:S103–15. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22691218.
Murray A, Pauling JD. Non-invasive methods of assessing Raynaud’s phenomenon. In: Wigley FM, Herrick AL, Flavahan NA, editors. Raynaud’s Phenom. A Guid. to Pathog. Treat. Springer; 2015. p. 199–242.
Lambrecht V, Cutolo M, De Keyser F, Decuman S, Ruaro B, Sulli A, et al. Reliability of the quantitative assessment of peripheral blood perfusion by laser speckle contrast analysis in a systemic sclerosis cohort. Ann Rheum Dis. 2016;75:1263–4.
Pauling JD, Shipley JA, Hart DJ, McGrogan A, McHugh NJ. Use of laser speckle contrast imaging to assess digital microvascular function in primary Raynaud phenomenon and systemic sclerosis: a comparison using the Raynaud condition score diary. J Rheumatol. 2015;42:1163–8.
Franks AG. Topical glyceryl trinitrate as adjunctive treatment in Raynaud’s disease. Lancet. 1982;1:76–7.
Hughes M, Moore T, Manning J, Wilkinson J, Dinsdale G, Roberts C, et al. Reduced perfusion in systemic sclerosis digital ulcers (both fingertip and extensor) can be increased by topical application of glyceryl trinitrate. Microvasc. Res. [Internet]. The Authors; 2017;111:32–6. Available from: https://doi.org/10.1016/j.mvr.2016.12.008.
Curtiss P, Schwager Z, Cobos G, Lo Sicco K, Franks AG. A systematic review and meta-analysis of the effects of topical nitrates in the treatment of primary and secondary Raynaud’s phenomenon. J Am Acad Dermatol [Internet]. Elsevier Inc; 2018; Available from: https://doi.org/10.1016/j.jaad.2018.01.043.
Wortsman X, Del Barrio-Díaz P, Meza-Romero R, Poehls-Risco C, Vera-Kellet C. Nifedipine cream versus sildenafil cream for patients with secondary Raynaud phenomenon: a randomized, double-blind, controlled pilot study. J Am Acad Dermatol. 2018;78:189–90.
Rodeheffer RJ, Rommer JA, Wigley FM, Smith CR. Controlled double-blind trial of nifedipine in the treatment of Raynaud’s phenomenon. N Engl J Med. 1983;308:880–3.
Smith CD, Mckendry JR. Controlled trial of nifedipine in the treatment of Raynaud’s phenomenon. Lancet. 1982;5:1299–301.
• Rirash F, Tingey P, Harding S, Lj M, Tanjong Ghogomu E, Wells G, et al. Calcium channel blockers for primary and secondary Raynaud’s phenomenon (review). Cochrane Database Syst Rev. 2017. Systematic review and meta-analysis demonstrating the benefits of calcium channel blocker therapy in the treatment of Raynaud's phenomenon.
Kowal-Bielecka O, Fransen J, Avouac J, Becker M, Kulak A, Allanore Y, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis. 2017;76:1327–39.
Varga J, Denton CP, Wigley FM, Allanore Y, Kuwana M. Scleroderma: from pathogenesis to comprehensive management. Second. Varga J, Denton CP, Wigley FM, Allanore Y, Kuwana M, editors. Springer Science; 2017.
Fries R, Shariat K, Von Wilmowsky H, Böhm M. Sildenafil in the treatment of Raynaud’s phenomenon resistant to vasodilatory therapy. Circulation. 2005;112:2980–5.
Brueckner CS, Becker MO, Kroencke T, Huscher D, Scherer HU, Worm M, et al. Effect of sildenafil on digital ulcers in systemic sclerosis: analysis from a single centre pilot study. Ann Rheum Dis. 2010;69:1475–8.
Andrigueti F V, Arismendi MI, Ebbing PCC, Division R. Evaluation of the effect of sildenafil on the microvascular blood flow and on the endothelial progenitor cells in patients with early systemic sclerosis: a randomized, double-blind, placebo controlled study F. Clin Exp Rheumatol. 2017;35.
Herrick AL, Van Den Hoogen F, Gabrielli A, Tamimi N, Reid C, O’Connell D, et al. Modified-release sildenafil reduces Raynaud’s phenomenon attack frequency in limited cutaneous systemic sclerosis. Arthritis Rheum. 2011;63:775–82.
Hachulla E, Hatron PY, Carpentier P, Agard C, Chatelus E, Jego P, et al. Efficacy of sildenafil on ischaemic digital ulcer healing in systemic sclerosis: the placebo-controlled SEDUCE study. Ann Rheum Dis. 2016;75:1009–15.
Tadalafil Package Insert [Internet]. 2009. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022332lbl.pdf.
Schiopu E, Hsu VM, Impens AJ, Rothman JA, McCloskey DA, Wilson JE, et al. Randomized placebo-controlled crossover trial of tadalafil in Raynaud’s phenomenon secondary to systemic sclerosis. J Rheumatol [Internet]. 2009;36:2264–8. Available from: http://www.jrheum.org/cgi/doi/10.3899/jrheum.090270.
Shenoy PD, Kumar S, Jha LK, Choudhary SK, Singh U, Misra R, et al. Efficacy of tadalafil in secondary Raynaud’s phenomenon resistant to vasodilator therapy: a double-blind randomized cross-over trial. Rheumatology. 2010;49:2420–8.
Caglayan E, Axmann S, Hellmich M, Moinzadeh P, Rosenkranz S. Vardenafil for the treatment of Raynaud phenomenon: a randomized, double-blind, placebo-controlled crossover study. Arch Intern Med. 2012;172:1182–4.
FDA. Veletri (epoprostenol) for Injection. Packag. Inser. [Internet]. 2012; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022260s005lbl.pdf.
FDA. Orenitram (TM) (treprostinil) extended release. Packag. Inser. [Internet]. 2013; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/203496s000lbl.pdf.
Denton CP, Hachulla É, Riemekasten G, Schwarting A, Frenoux JM, Frey A, et al. Efficacy and safety of selexipag in adults with Raynaud’s phenomenon secondary to systemic sclerosis: a randomized, placebo-controlled, phase II study. Arthritis Rheumatol. 2017;69:2370–9.
Colaci M, Sebastiani M, Giuggioli D, Manfredi A, Rossi R, Modena MG, et al. Cardiovascular risk and prostanoids in systemic sclerosis. Clin Exp Rheumatol. 2008;26:333–6.
Cruz JE, Ward A, Anthony S, Chang S, Bae H(B), Hermes-DeSantis ER. Evidence for the use of epoprostenol to treat Raynaud’s phenomenon with or without digital ulcers. Ann Pharmacother. 2016;50:1060–7.
Belch JJ, Drury JK, Capell H, Forbes CD, Newman P, Mckenzie F, et al. Intermittent epoprostenol (prostacyclin) infusion in patients with Raynaud’s syndrome: a double-blind controlled trial. Lancet. 1983:313–5.
Kingma K, Wollersheim H, Thien T. Double-blind, placebo-controlled study of intravenous prostacyclin on hemodynamics in severe Raynaud’s phenomenon: the acute vasodilatory effect is not sustained. J Cardiovasc Pharmacol. 1995;26:388–93.
Pope J, Fenlon D, Thompson A, Shea B, Furst D, Wells GA, et al. Iloprost and cisaprost for Raynaud’s phenomenon in progressive systemic sclerosis. Cochrane Database Syst Rev [Internet]. 1998; Available from: http://doi.wiley.com/10.1002/14651858.CD000953.
Wigley FM, Korn JH, Csuka ME, Medsger TA, Rothfield NF, Ellman M, et al. Oral iloprost treatment in patients with Raynaud’s phenomenon secondary to systemic sclerosis: a multicenter, placebo-controlled, double-blind study. Arthritis Rheum. 1998;41:670–7.
Black CM, Halkier-Sørensen L, Belch JJ, Ullman S, Madhok R, Smit AJ, et al. Oral iloprost in Raynaud’s phenomenon secondary to systemic sclerosis: a multicentre, placebo-controlled, dose-comparison study. Br J Rheumatol [Internet]. 1998;37:952–60. Available from: http://rheumatology.oxfordjournals.org/cgi/reprint/37/9/952%5Cnpapers://454c05cc-adc5-4015-bf6b-be819de563dc/Paper/p2432.
Shah AA, Schiopu E, Hummers LK, Wade M, Phillips K, Anderson C, Wise R, Boin F, Seibold JR, Wigley F, Rollins KD Open label study of escalating doses of oral treprostinil diethanolamine in patients with systemic sclerosis and digital ischemia: pharmacokinetics and correlation with digital perfusion. Arthritis Res Ther [Internet]. 2013;15:1–10. Available from.
Seibold JR, Wigley FM, Schiopu E, Denton CP, Silver RM, Steen VD, et al. Digital ischemic ulcers in scleroderma treated with oral treprostinil diethanolamine: a randomized, double-blind, placebo-controlled, multicenter study. Arthritis Rheum. 2011;63
Shah AA, Schiopu E, Chatterjee S, Csuka ME, Frech T, Goldberg A, et al. The recurrence of digital ulcers in patients with systemic sclerosis after discontinuation of oral treprostinil. J Rheumatol. 2016;43:1665–71.
Sitbon O, Channick R, Chin KM, Frey A, Gaine S, Galiè N, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med [Internet]. 2015;373:2522–33. Available from: http://www.nejm.org/doi/10.1056/NEJMoa1503184.
Houde M, Desbiens L, D’Orléans-Juste P. Endothelin-1: biosynthesis, signaling and vasoreactivity. Adv Pharmacol. 2016;77:143–75.
Boss C, Bolli MH, Gatfield J. From bosentan (Tracleer®) to macitentan (Opsumit®): the medicinal chemistry perspective. Bioorg Med Chem Lett. 2016;26:3381–94.
Actelion Pharmaceuticals US. Tracleer® (Bosentan). Prescribing information. Packag. Inser. [Internet]. 2009; Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021290s012lbl.pdf.
Selenko-Gebauer N, Duschek N, Minimair G, Stingl G, Karlhofer F. Successful treatment of patients with severe secondary Raynaud’s phenomenon with the endothelin receptor antagonist bosentan. Rheumatology. 2006;45:45–8.
Giordano N, Puccetti L, Papakostas P, Di Pietra N, Bruni F, Pasqui AL, et al. Bosentan treatment for Raynauds phenomenon and skin fibrosis in patients with systemic sclerosis and pulmonary arterial hypertension: an open-label, observational, retrospective study. Int J Immunopathol Pharmacol. 2010;23:1185–94.
Funauchi M, Kishimoto K, Shimazu H, Nagare Y, Hino S, Yano T, et al. Effects of bosentan on the skin lesions: an observational study from a single center in Japan. Rheumatol Int. 2009;29:769–75.
Hettema ME, Zhang D, Bootsma H, Kallenberg CGM. Bosentan therapy for patients with severe Raynaud’s phenomenon in systemic sclerosis. Ann Rheum Dis. 2007;66:1398–9.
Nguyen VA, Eisendle K, Gruber I, Hugl B, Reider D, Reider N. Effect of the dual endothelin receptor antagonist bosentan on Raynaud’s phenomenon secondary to systemic sclerosis: a double-blind prospective, randomized, placebo-controlled pilot study. Rheumatology. 2009;49:583–7.
Rosato E, Molinaro I, Borghese F, Rossi C, Pisarri S, Salsano F. Bosentan improves skin perfusion of hands in patients with systemic sclerosis with pulmonary arterial hypertension. J Rheumatol. 2010;37:2531–9.
Korn JH, Mayes M, Matucci Cerinic M, Rainisio M, Pope J, Hachulla E, et al. Digital ulcers in systemic sclerosis: prevention by treatment with bosentan, an oral endothelin receptor antagonist. Arthritis Rheum. 2004;50:3985–93.
• Matucci-Cerinic M, Denton CP, Furst DE, Mayes MD, Hsu VM, Carpentier P, et al. Bosentan treatment of digital ulcers related to systemic sclerosis: results from the RAPIDS-2 randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2011;70:32–8.Study demonstrated that subjects treated with bosentan had a reduction in new digital ulcers compared with placebo.
Chung L, Ball K, Yaqub A, Lingala B, Fiorentino D. Effect of the endothelin type A-selective endothelin receptor antagonist ambrisentan on digital ulcers in patients with systemic sclerosis: results of a prospective pilot study. J Am Acad Dermatol. 2014;71:400–1.
Bose N, Bena J, Chatterjee S. Evaluation of the effect of ambrisentan on digital microvascular flow in patients with systemic sclerosis using laser Doppler perfusion imaging: a 12-week randomized double-blind placebo controlled trial. Arthritis Res Ther [Internet]. 2015;17:44. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4384235&tool=pmcentrez&rendertype=abstract.
Gatfield J, Mueller Grandjean C, Sasse T, Clozel M, Nayler O. Slow receptor dissociation kinetics differentiate macitentan from other endothelin receptor antagonists in pulmonary arterial smooth muscle cells. PLoS One. 2012;7:e47662.
Khanna D, Denton CP, Merkel PA, Krieg TM, Le Brun FO, Marr A, et al. Effect of macitentan on the development of new ischemic digital ulcers in patients with systemic sclerosis: dual-1 and dual-2 randomized clinical trials. JAMA. 2016;315:1975–88.
Cutolo M, Ruaro B, Pizzorni C, Ravera F, Smith V, Zampogna G, et al. Long term treatment with endothelin receptor antagonist bosentan and iloprost improves fingertip blood perfusion in systemic sclerosis. J Rheumatol. 2014;41:881–6.
Uppal L, Dhaliwal K, Butler PE. A prospective study of the use of botulinum toxin injections in the treatment of Raynaud’s syndrome associated with scleroderma. J Hand Surg Eur Vol. 2014;39:876–80.
Bello RJ, Cooney CM, Melamed E, Follmar K, Yenokyan G, Leatherman G, et al. The therapeutic efficacy of botulinum toxin in treating scleroderma-associated Raynaud’s phenomenon: a randomized, double-blind, placebo-controlled clinical trial. Arthritis Rheumatol [Internet]. 2017;69:1661–9. Available from: http://doi.wiley.com/10.1002/art.40123.
Thune TH, Ladegaard L, Licht PB. Thoracoscopic sympathectomy for Raynaud’s phenomenon-a long term follow-up study. Eur J Vasc Endovasc Surg. 2006;32:198–202.
Maga P, Kuzdzał J, Nizankowski R, Szczeklik A, Sładek K. Long-term effects of thoracic sympathectomy on microcirculation in the hands of patients with primary Raynaud disease. J Thorac Cardiovasc Surg. 2007;133:1428–33.
Karapolat S, Turkyilmaz A, Tekinbas C. Effects of endoscopic thoracic sympathectomy on Raynaud’s disease. J Laparoendosc Adv Surg Tech [Internet]. 2018;0:lap.2017.0634. Available from: http://online.liebertpub.com/doi/10.1089/lap.2017.0634.
Hartzell TL, Makhni EC, Sampson C. Long-term results of periarterial sympathectomy. J Hand Surg Am [Internet]. Elsevier Inc.; 2009;34:1454–60. Available from: https://doi.org/10.1016/j.jhsa.2009.05.003.
Soberon JR, Tuxillo TM, Gethers CC, Smith TA, Davis WE. Axillary block-induced chemical sympathectomy in the setting of digital ischemia. Ochsner J. 2016;16:450–6.
Funding
Dr. Wigley reports grants from the Scleroderma Research Foundation and support from the Martha McCrory Professorship during the conduct of the study.
Dr. Hinze reports grants from National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number T32AR048522 during the conduct of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Alicia M. Hinze declares that she has no conflict of interest. Fredrick M. Wigley declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Additional information
This article is part of the Topical Collection on Pain in Rheumatology
Rights and permissions
About this article
Cite this article
Hinze, A.M., Wigley, F.M. Pharmacotherapy Options in the Management of Raynaud’s Phenomenon. Curr Treat Options in Rheum 4, 235–254 (2018). https://doi.org/10.1007/s40674-018-0102-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40674-018-0102-6