Abstract
Currently, allergen-specific immunotherapy (AIT) with active ingredients derived from the causative allergen source is the only disease-modifying treatment for allergic patients. However, compared to, e.g., live-attenuated vaccines for the prevention of infectious diseases, purified allergens for AIT in many cases display only a low immunogenicity. This reduces treatment efficacy and prolongs treatment duration. Here, adjuvants may be a promising tool, allowing for dose reduction of the respective allergen while increasing immunogenicity of co-applied allergens and/or modulating allergen-specific immune responses toward T helper 1 (Th1) or regulatory phenotypes or the production of blocking antibody isotypes. Currently available adjuvants can be distinguished into first-generation adjuvants (promoting immune responses via aggregation and controlled release of co-applied allergens from a depot) and second-generation adjuvants (triggering immune responses via the activation of pattern recognition receptors expressed by immune cells). This review summarizes the mechanisms and effects of adjuvants currently or previously used for AIT (aluminum hydroxide, calcium phosphate, microcrystalline tyrosine, and monophosphoryl lipid A [MPLA]) and focuses on novel developments using mannan-, virus-like particle (VLP)-, and flagellin-based adjuvants and therapeutics for the treatment of allergic diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type I allergies pose a serious health concern with a high prevalence in Western countries and an overall increasing prevalence worldwide ([1] and reviewed in [2,3,4]). Immune responses to otherwise harmless, exogenous antigens (allergens) are elicited upon allergen contact and lead to allergic symptoms in affected individuals (reviewed in [5]). Allergic symptoms may range from conjunctivitis, rhinitis, and asthma to eczema, urticaria, and severe, life-threatening allergic reactions (anaphylaxis; reviewed in [2, 5, 6]). In type I allergies, an imbalance in T helper (Th) 1/2 differentiation (with a pathological shift toward Th2) and a resulting increase in allergen-specific immunoglobulin E (IgE) antibodies are reported to be the main underlying mechanism (reviewed in [5,6,7]). Upon allergen contact, allergen-specific IgE antibodies lead to the degranulation of sensitized mast cells, the release of inflammatory mediators, and the onset of allergy (reviewed in [5,6,7]).
Regarding allergy treatment, current strategies include allergen avoidance, symptom management, and allergen-specific immunotherapy (AIT). Thereof, AIT is currently the only disease-modifying treatment (reviewed in [7,8,9]). Currently, AIT is performed by repeatedly administering allergens either orally (oral immunotherapy [OIT]), subcutaneously (SCIT), or sublingually (SLIT), allowing for hyposensitization to the respective allergen(s) and symptom reduction after successful treatment (reviewed in [10, 11]). In AIT, allergen(s) are often initially administered in gradually increasing amounts in the dose-escalation phase until an effective maintenance dose is reached, which is continuously administered in the maintenance phase (reviewed in [11, 12]). Herein, the dose-escalation phase can be accelerated dependent on the treatment scheme (reviewed in [11, 12]). Mechanistically, AIT induces an allergen-specific tolerance by shifting immune responses from Th2- to more Th1-biased responses and by the induction of blocking IgG antibodies as well as regulatory T‑ and B‑cell responses (reviewed in [8, 9, 11, 13]). In addition to T and B cells, innate immune cells also contribute to immune modulation observed in AIT (reviewed in [9, 14]). Here, interleukin (IL)-10-producing innate lymphocyte cells (ILC2s) were shown to be induced during AIT [15], while the frequency of Th2-promoting ILC2s was reduced [16, 17]. Furthermore, successful AIT was paralleled by an induction of tolerogenic dendritic cells (DCs; suppressing Th2 responses) and a reduction in both mast cell and basophil number and function (reviewed in [9, 14]). Despite its effectiveness, a major challenge of AIT is patient adherence due to long treatment regimens as well as potential adverse events caused by the allergen(s) (reviewed in [8, 13]). To reduce treatment duration and doses (linked to the risk of adverse events), adjuvants can be co-administered along with the allergen(s). Adjuvants are immune potentiators or immunomodulators that increase the immunogenicity of the accompanying allergen(s), leading to an overall more potent and long-lasting immune response (reviewed in [7, 8, 13, 18]). Overall, adjuvants can be grouped into first- and second-generation adjuvants, depending on their mechanism of action.
First-generation adjuvants are particulate carriers that function by adsorbing the antigens due to physical properties such as electric charge and surface area (reviewed in [19,20,21]). Through adsorption of the antigen, first-generation adjuvants enhance co-delivery and antigen stability, and they may enable a slow, systemic antigen release resulting in a prolonged immune response, the so-called depot effect (reviewed in [7, 19, 21, 22]). Besides functioning as a delivery system, first-generation adjuvants can further elicit immune responses by inflammasome activation and induce balanced Th1/Th2 immune responses (reviewed in [21]). First-generation adjuvants used in AIT include aluminum salts, calcium phosphate (CP), and microcrystalline tyrosine (MCT®).
Second-generation adjuvants are immune stimulants that activate antigen-presenting cells (APCs) by targeting pattern recognition receptors (PRRs), triggering innate immune responses (reviewed in [20, 21]). Second-generation adjuvants are either pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) that can be recognized by APCs. Depending on the PRR activated by the second-generation adjuvant, activated and matured APCs express different co-stimulatory molecules and secrete different cytokines, thereby enhancing and modulating the adaptive immune response to the allergen. So far, the Toll-like receptor (TLR) 4‑ligand monophosphoryl lipid A (MPLA) is the only second-generation adjuvant used in AIT. Further examples of second-generation adjuvants include polysaccharides, flagellin, and CpG DNA motifs.
Novel adjuvant systems can further include both first- and second-generation adjuvants, e.g., immune stimulants formulated in oil-in-water emulsions, virus-like particles (VLPs), immune-stimulating complexes, or liposomes [23].
Adjuvants currently used in AIT
The adjuvants that were either previously used or are still used in AIT include aluminum hydroxide, CP, microcrystalline tyrosine, and MPLA.
Aluminum hydroxide
Introduced as early as in 1920 by Glenny et al. [24], aluminum hydroxide is the most commonly used adjuvant in humans. It is frequently but incorrectly designated as “alum”, which is the name for the composition AlK(SO4)2*12 H2O, a precipitate of aluminum salts with a monovalent cation (e.g., potassium) forming under basic conditions [25].
While aluminum hydroxide was shown to skew mouse CD4+ T cell responses toward prostaglandin E2-dependent Th2 responses (reviewed in [26, 27]), in humans, depending on its formulation and the co-applied antigen, aluminum hydroxide seems to induce more balanced Th1/Th2 responses [28, 29]. In line with this, aluminum hydroxide was demonstrated to extensively modify immune cell activation induced by mannan (see “Mannan” section for more information). Here, aluminum hydroxide both decreased PD-L1 expression on human DCs and the secretion of anti-inflammatory IL-10 while increasing the secretion of the proinflammatory cytokine IL-23 [30]. Consequently, human DCs co-stimulated with mannan and aluminum hydroxide had a reduced capacity to induce Forkhead-Box-Protein P3 (FOXP3)+ regulatory T cells (Tregs) but promoted the induction of mixed Th1/Th2/Th17 responses both in vitro in human cells and in mice in vivo [30]. These effects of aluminum hydroxide were shown to be mediated by a suppression of mammalian target of rapamycin (mTOR) activation preventing Warburg metabolism in DCs [30].
Despite being used for more than a century, its mechanism of action is not fully understood. It is well established that aluminum derivates increase the immunogenicity of co-applied antigens through the recruitment of innate immune cells (eosinophils, neutrophils, monocytes, NK cells, NKT cells, and DCs) at the injection site as well as secretion of proinflammatory cytokines and chemokines [31]. Furthermore, aluminum hydroxide enhances CD4+ T cell responses and antibody production as well as CD8+ T cell priming [31]. These effects are likely mediated by the formation of an insoluble depot displaying repetitively arranged allergen molecules adsorbed to aluminum hydroxide as well as the local release of uric acid and other molecules from cells that act as DAMPs [32]. High-molecular aggregates are more easily phagocytized by APCs and result in MHC II-dependent induction of long-lasting memory responses ([33,34,35]; reviewed in [36]). In addition, using nucleotide binding oligomerization domain (NOD)-like receptor (NLR) protein 3 (NLRP3) inflammasome-deficient mice, aluminum hydroxide was shown to induce secretion of the strongly proinflammatory and pyrogenic cytokines IL-1β and IL-18 in an NLRP3-dependent manner [37]. Although the involvement of the NLRP3 inflammasome in aluminum hydroxide-induced immune responses was confirmed in independent studies, different studies reported that deficiency in either NLRP3 or capase‑1 did not decrease, but instead unexpectedly increased levels of antigen-specific antibody responses such as IgE and IgG2c [31, 38, 39]. Furthermore, the absence of NLRP3 had no significant effect on T‑ and B‑cell responses in mice [40, 41]. Therefore, the genuine function of the NLRP3 inflammasome in aluminum hydroxide-mediated immune activation remains controversial.
However, the pronounced immune system activation by aluminum hydroxide is not without disadvantages, as it frequently induces acute and chronic inflammation at the injection site [27, 42]. Moreover, even though the amounts of aluminum hydroxide in AIT products in Germany do not exceed 1.25 mg per human dose, as specified in the Monograph on Allergen Products [25, 43], its low biodegradability leads to its accumulation upon repeated application [27, 42]. Consequently, the application of aluminum hydroxide as an adjuvant for type I allergy treatment is under ongoing investigation [44,45,46,47].
Microcrystalline tyrosine
Implemented in AIT in 1970 [43], MCT® is used as an authorized adjuvant in Germany [48]. It consists of the crystalline, needle-like form of the non-essential amino acid L‑tyrosine [22], which exists ubiquitously in the human body [49]. In terms of application, both co-precipitation of allergens with MCT® and adsorption of the allergens to preformed MCT® crystals are reported [22]. Here, allergens can either be native or modified (in most cases glutaraldehyde-modified; [50, 51]). Due to its high biodegradability and short half-life of 48 h [52] combined with a lack of toxicity [49], MCT® is often suggested as a substitute for aluminum hydroxide [22, 49].
As first-generation adjuvant, MCT® forms a depot and activates the NLRP3 inflammasome, leading to caspase-dependent IL-1β secretion as shown in vitro for T cells [22]. Furthermore, it leads to rapid exudation of neutrophils and eosinophils [22]. Compared with aluminum hydroxide, application of egg white allergen ovalbumin (OVA) adsorbed to MCT® resulted in similar levels of IgG1 and IgG2a production in mice while at the same time significantly lowering levels of IL‑4 and IgE and increasing interferon (IFN)-γ production [22].
Clinical studies performed between 1976 and 2001 demonstrated the tolerability of MCT® with enhanced IgG immune responses (reviewed in [49]). As side effects, reversible local reactions at the injection side were reported that disappeared within a few days (reviewed in [49]).
Calcium phosphate
The mineral salt CP was used as an authorized adjuvant in some European countries in grass pollen or mite extracts [43]. Favorable adjuvant properties are its good biocompatibility and biodegradability due to its natural occurrence in the human body coupled with only minor inflammatory reactions at the site of administration [53]. Compared with aluminum hydroxide, CP induces more balanced Th1/Th2 immune responses, as seen in increased allergen-specific IgG1/IgG2a ratios in mice [54] and an increase in allergen-specific serum levels of IgG4 in a placebo-controlled trial with 29 grass pollen-allergic patients upon adsorbing CP to grass pollen extract [55]. Postulated mechanisms of immune activation include activation of the NLRP3 inflammasome [56, 57], depot function, and enhanced uptake through phagocytic cells [54]. Despite its favorable adjuvant properties, CP is no longer used in commercially available AIT products in Europe (Stallergenes Greer, Baar, Switzerland, personal communication).
Monophosphoryl lipid A
The adjuvant 3‑O-deactylated MPLA is a detoxified lipid A derived from the lipopolysaccharide of the gram-negative bacterium Salmonella minnesota R595 and activates the PRR TLR4 [58, 59]. Due to the detoxification and the adjuvant activity of MPLA, it is employed in multiple vaccines against infectious diseases. Herein, MPLA is either adsorbed to aluminum phosphate in the adjuvant system 04 (AS04) used, e.g., in the hepatitis B vaccine Fendrix® [60] or formulated within the liposomal formulation AS01 which is, e.g., used in the malaria vaccine Mosquirix® [61]. However, in AIT, MPLA is currently only included in one AIT product line for the treatment of allergic rhinitis (Pollinex Quattro® by Allergy Therapeutics Limited, Worthing, UK / Bencard Allergie GmbH, Munich, Germany), which is currently in a marketing authorization procedure according to the Therapy Allergen Ordinance in Germany ([62]; and is marketable until the authorization procedure is completed). It was shown that MPLA elicited Th1-biased immunity by inducing IL-12 cytokine secretion from APCs [63]. In the Pollinex Quattro® AIT products, both first- (MCT®) and second-generation (MPLA) adjuvants are co-formulated with allergoids [64]. With four to six pre-seasonal injections, Pollinex Quattro® aims for a short-course SCIT [64,65,66].
In mouse models, MPLA in combination with MCT® was shown to synergistically shift immune responses to Th1 and to enhance IgG (especially IgG2) immune responses without enhancing antigen-specific IgE antibodies [67].
Treatment with modified allergen tyrosine adsorbed (MATA) MPLA was evaluated in grass-, birch-, ragweed-, and tree-MATA short-course SCITs in recent phase II clinical trials and was shown to reduce clinical symptoms in allergic patients and to have good tolerability [65, 68, 69].
In a company-sponsored, multicenter, randomized, double-blind placebo-controlled (DBPC) clinical phase III study including 141 grass pollen-sensitive patients, grassMATA MPLA treatment was shown to not only increase allergen-specific IgG (especially IgG1 and IgG4) responses but to decrease nasal and eye-related clinical symptoms [64, 70]. Furthermore, the verum group showed a reduction in IgE antibodies after seasonal allergen exposure compared with the placebo control groups [64, 70].
Novel adjuvants in AIT
Currently, many different adjuvants are investigated for application in AIT. Among others, these include liposomes, oil-in-water emulsions, VLPs, viral components, carbohydrate-based adjuvants, flagellin, and CpG oligodeoxynucleotide (reviewed in [23]). For this article we will focus on the current developments on mannan, VLPs, and flagellin.
Mannan
The polysaccharide mannan consists of linked mannose residues and is abundant in nature, e.g., in fungi, bacteria, or yeast [71]. Aside from its presence in various pathogens, mannan is also a principal structural component of frequently consumed commensal microbes such as the yeast S. cerevisiae, (e.g., contained in bread, beer, etc.), which might contribute to its tolerogenic potential. Indeed, upon co-application with peanut extract on human monocyte-derived DCs, mannan was recently shown to promote the induction of human FOXP3+ Tregs [72].
Wide commercial availability and low toxicity enable its testing as an adjuvant in therapeutics in biomedicine, e.g., for antitumor therapy or immune-system modulation. In addition, mannan is tested as an adjuvant for AIT.
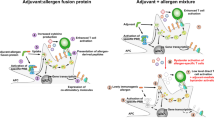
The most clinically advanced application of mannan as an adjuvant in AIT studies is the usage of allergoid–mannan conjugates that were pre-clinically shown to induce allergen tolerance (Fig. 1a). For this purpose, allergoids were polymerized and conjugated to non-oxidized mannan using glutaraldehyde. Those allergoid–mannan conjugates are captured by DCs via the mannose receptor (Mannose R, CD206), the DC-specific intracellular adhesion molecule-3-grabbing non-integrin (DC-SIGN, CD209), and Dectin‑2, leading to a significantly increased internalization of allergens (Fig. 1a). Changes in epigenetic and metabolic state led to the induction of blocking antibodies and functional FOXP3+ Treg cells in mice and humans (Fig. 1a; [30, 73]). It was also shown that monocytes from non-atopic and from allergic patients can be reprogrammed into tolerogenic DCs or macrophages when treated with allergoid–mannan conjugates (Fig. 1a; [30, 74]).
Immunologic effects of mannan:allergoid conjugates. Effects are differentiated into pre-clinical results (a) and results from clinical trials (b). Mannan:allergoid conjugates were shown to be taken up efficiently by mouse and human monocytes and dendritic cells (DCs), resulting in the differentiation of tolerogenic monocyte-derived DCs (moDC) that promoted the differentiation of Forkhead-Box-Protein P3 (FOXP3)+ regulatory T cells (Tregs) and the production of allergen-specific IgG2a antibodies. In clinical trials, mannan:allergoid conjugates either administered subcutaneously or sublingually promoted allergen-specific IgG4 antibody production while also improving allergic symptoms, medication use, and local allergic reactions. For more information, see the text. CSMS combined symptom medication score, DC-SIGN specific intracellular adhesion molecule-3-grabbing non-integrin, GM-CSF granulocyte macrophage-colony stimulating factor, Mannose R mannose receptor, NPT nasal provocation test, SCIT subcutaneous immunotherapy, SLIT sublingual immunotherapy
Several phase II studies (all sponsored by Inmunotek S.L., Madrid, Spain), with these allergoid–mannan conjugates, have been completed (grass pollen SCIT & SLIT [2014-005471-88], house dust mite [HDM] SCIT & SLIT [2015-000820-27], and birch pollen SCIT [2018-002522-23]). In a multicenter, randomized, double dummy DBPC phase II study with 196 individuals with HDM allergic rhinitis, patients treated with mannan-conjugated HDM allergoids, the primary (improvements in nasal provocation tests [NPT]) and secondary outcomes (improved combined symptom and medication scores [CSMS] and immunogenicity) were met for subcutaneous (s.c.) and sublingual (s.l.) application of either 3000 or 5000 mannan–conjugated therapeutic units (mTU)/mL [75]. Herein, 56–62% of actively treated participants showed improved NPT results, compared with 16% in the placebo group (Fig. 1b). Active treatment further led to a 62–70% and 35–40% CSMS reduction compared with placebo-treated patients for s.c. and s.l. application, respectively (Fig. 1b; [75]). Immunogenicity increased with enhanced IgG4 response to Dermatophagoides pteronyssinus and Dermatophagoides farinae upon s.c. application (Fig. 1b; [75]). For mannan-conjugated birch pollen allergoids, 246 birch pollen-allergic individuals participated in a prospective, randomized, DBPC, dose-finding phase II study [76]. The highest tested concentration of 10,000 mTU/mL led to the most promising results, reducing the CSMS by 24.7% compared with placebo as well as increasing production of Bet v 1-specific IgG4 (up to 4.5-fold) and reducing IgE/IgG4 ratios (up to 70%; [76]). On the basis of the mannan-conjugated birch pollen concentration of 10,000 mTU/mL, a follow-up phase II study (2020-004126-32) was completed in June 2023. Study results for this study, as well as for the grass pollen phase II study, have not been published yet.
Mannan-conjugated allergoids for the treatment of birch pollen and HDM allergy are currently further investigated in phase III studies (sponsored by Inmunotek S.L.). A prospective, randomized, DBPC multicenter study for birch pollen SCIT (2021-002252-36) with a concentration of 10,000 mTU/mL, and an open-phase III follow-up study (2022-004082-20), were completed in June 2022. A phase III clinical trial for SCIT and SLIT of HDM allergy (2021-003014-39) prematurely ended in May 2023 (reasons for this have not been published yet), but in November 2023 a new phase III prospective, randomized, DBPC clinical trial for HDM (NCT05641272) was started, containing only the SLIT branch. Its estimated completion date is in 2026. Results for the phase III studies on birch pollen and HDM allergy have not been published yet.
A different strategy published recently uses mannan as the surface structure of a carrier system: mannan-coated allergen particles. Instead of glutaraldehyde crosslinking, as used for the allergoid–mannan conjugates described earlier, mannose residues containing proteins and the allergen are assembled via heating and H2O2 disulfide bond formation in a one-pot reaction [77]. The addition of human serum albumin as a matrix protein can reduce the amount of allergen needed [78]. After the uptake by immune cells, these nanoparticles disassemble due to a reduction of the disulfide bonds in the endosomes, leading to the intracellular release of the allergens [78]. This type of crosslinking was hypothesized to keep T cell epitopes of the allergen and the mannan part of the mannoprotein as intact as possible [78]. In in vitro tests with the allergen OVA, these nanoparticles induced allergen-specific Treg cells while showing a minimized reactivity with anti-OVA antibodies (IgE, IgG2a and IgG1), compared with either isolated OVA protein or mannan–OVA conjugates [77], lowering the risk for anaphylaxis during treatment. In a mouse model of allergic asthma, the mannan-coated OVA particles had both a preventative and therapeutic effect when administered either s.c. or orally. Therefore, this novel concept could be a promising additional application for mannan-conjugated allergoids in AIT.
Over the past few years, mannan–allergoid conjugates have progressed from purely pre-clinical application into clinical studies with initial promising results in phase II clinical trials and currently ongoing phase III studies. Also, mannan-coated allergen particles were investigated pre-clinically. Both strategies succeeded in demonstrating the induction of regulatory responses and modulation of antibody production by mannan in mice and mannan-allergoid conjugates in humans. Therefore, mannan bears potential as an adjuvant in AIT, but more studies are needed to characterize its benefits and risks for use in AIT.
Virus-like particles
The VLPs are virus-derived structures 25–100 nm in size consisting of one or more different viral proteins with the ability to self-assemble [79]. Their size makes them optimal for drainage to lymph nodes and promotes their uptake by APCs. These VLPs mimic the form and size of a viral particle, but lack the genetic material, and thus have no ability to infect host cells.
Virus-like particles were repeatedly described to induce Th1-biased immune responses while lacking the potential to activate mast cells and to induce IgE production [80,81,82,83,84], making them interesting adjuvants for the treatment of Th2-mediated allergies.
The biggest number of clinical studies in the field of allergology have been performed with VLPs based on the bacteriophage QβG10 (all sponsored by Cytos Biotechnology AG, Schlieren, Switzerland). While initially showing promising results in clinical studies [85,86,87], clinical development of QβG10 VLPs was stopped after no differences between QβG10- and placebo-treated patients were observed in a phase IIb study investigating patients with persistent moderate-to-severe allergic asthma [88].
While Cytos stopped their clinical development program on VLPs, some of the scientists involved (under participation of Allergy Therapeutics Limited) continued research using a cucumber mosaic virus-derived VLPs containing the tetanus toxin-derived universal T cell epitope tt830-843 (CuMVTT). These VLPs activate the immune system via their highly ordered repetitive structure, E. coli RNA packaged into the particles, and the induction of recall responses in previously tetanus-vaccinated individuals (Fig. 2; [89]).
Pre-clinical immunologic effects of virus-like particles (VLPs) in allergen-specific immunotherapy. Virus-like particles displaying allergen(s) on their surface or packaging it into the particles are efficiently drained to lymph nodes where they are taken up by plasmacytoid dendritic cells (DCs). DCs are activated by the highly ordered, repetitive structure of the VLPs and the contained immune-stimulating RNA. Activated DCs promote the induction of T helper 1 (Th1) and regulatory T cell (Treg) responses, while a potential induction of Th2 responses is currently unclear. Th1 cells promote the differentiation of B cells producing allergen-specific, blocking IgG antibodies that can suppress IgE-mediated mast cell degranulation via a FcγRIIB-dependent mechanism. Together, these effects contribute to the VLP-mediated protection against allergic reactions. ICOS(-L) inducible co-stimulator (ligand), TLR Toll-like receptor
In a mouse peanut allergy model, prophylactic application of CuMVTT VLPs displaying either one of the major peanut allergens Ara h 1, Ara h 2, or peanut extract were able to protect sensitized animals from peanut-induced anaphylaxis, suggesting that “vaccination” against a single major allergen can protect against challenge with the complete extract [89]. Mechanistically, this protection was shown to depend on inhibitory FcγRIIB-signaling in mast cells mediated by the induced peanut-specific IgG2a/b antibodies (Fig. 2; [89]). In a follow-up study, Sobczak et al. demonstrated that the CuMVTT VLPs furthermore prevented local allergic reactions in mouse skin prick tests and that antibodies from vaccinated animals conferred passive protection to naïve animals in an FcγRIIB-dependent manner [90].
Krenger et al. further investigated the contribution of TLR7-activation via the RNA packaged inside the CuMVTT VLPs to immune responses induced by CuMVTT-Ara h 2 [91]. In C57BL/6 mice, total production of Ara h 2-specific IgG antibodies by CuMVTT-Ara h 2 was shown to be only slightly impaired in TLR7-deficient animals, while IgG antibody avidity to Ara h 2 was strongly reduced [91]. By contrast, CuMVTT-Ara h 2 preparations with lower RNA content resulted in both diminished total Ara h 2-specific IgG responses and strongly reduced avidity of the induced total IgG and IgG2b/c antibodies to Ara h 2 [91], suggesting that besides TLR7 also TLR3 activation contributed to immune activation by these particles. In conclusion, protection from peanut-induced anaphylaxis in mice was shown to be dependent on packaged RNA. Furthermore, the number of Ara h 2 molecules present on the VLP strongly correlated with VLP immunogenicity and protective capacity [91].
Immunization of cats against the major cat allergen Fel d 1 presented on the surface of a CuMVTT VLP induced strong anti-Fel d 1 IgG responses that resulted in a reduction in the endogenous Fel d 1 levels in cat tear fluid [92]. Cats immunized with Fel d 1-CuMVTT VLPs (termed HypoCatTM) were shown to be associated with reduced allergic symptoms in cat-allergic human individuals (reported via accumulated petting time until induction of allergic symptoms and different symptom scores in their respective owners) in a combined human and animal field study including ten cat-allergic participants living with their cats [93].
In another approach, VLPs derived from the capsid protein of the Acinetobacter bacteriophage AP205 displaying the major HDM allergen Der p 1 on their surface showed lower IgE reactivity and strongly reduced capacity to induce human IgE-mediated rat basophil leukemia cells (RBL)-SX38 degranulation compared to Der p 1 alone [82]. In BALB/c mice, the Der p 1-presenting VLPs induced Th1 responses with increased IgG1, IgG2a, and IFN‑γ production (Fig. 2; [82]). Similar results were reported for AP205 VLPs displaying the major shrimp allergen Pen m 1 [83] and the major HDM allergen Der p 2 [84]. The Der p 2-displaying VLPs also prevented the development of HDM-allergic responses in mice [84]. In a model of allergic contact dermatitis, AP205 VLPs displaying murine IL-1β protein co-adjuvanted with AddaVaxTM (a squalene-based oil-in-water nano-emulsion) resulted in the induction of IL-1β neutralizing antibodies in C57BL/6 mice that reduced IL-1β levels in vivo, which correlated with reduced clinical symptoms upon challenge with 1‑Fluoro‑2,4‑dinitrobenzene ([94]; study performed under participation of AdaptVac Aps, Copenhagen, Denmark).
In contrast to the aforementioned VLPs, immunization of BALB/c mice with vesicular stomatitis virus (VSV)-glycoprotein VLPs delivering hen egg lysozyme (HEL) was shown to predominantly trigger HEL-specific IgE responses and acute allergic responses upon HEL-challenge, suggesting a Th2-promoting effect of the VLPs [95].
However, not all VLPs described induce prominent immune responses. Moloney murine leukemia virus (MoMLV) matrix protein-based VLPs either displaying the major mugwort allergen Art v 1 on their surface or shielding it inside the particles were described by Kratzer et al. [96]. Here, VLPs packaging Art v 1 induced lower levels of RBL degranulation than particles displaying the allergen on their surface [96]. Upon intranasal application, only particles displaying the allergen externally induced allergen-specific antibody production (including IgE; [96]). However, VLPs packaging the allergen conferred protection against subsequent immunization via the induction of Th1/Treg responses in mice expressing a human Art v 1-specific T cell receptor as well as human HLA-DR1 [96].
Finally, Pazos-Castro et al. described turnip mosaic virus-derived VLPs displaying the major peach allergen Pru p 3 for treatment of food allergy [97]. These VLPs induced proliferation of human peripheral blood mononuclear cells (PBMCs), which was further increased when Pru p 3 was complexed with its natural lipid ligand [97]. While having no apparent toxic effects in mice, sublingual administration into C3H mice slightly reduced Pru p 3-specific IgE and serum IgG2a levels [97]. The authors suggested the immune-modulating mechanisms of the tested VLPs to be mediated by reduced immune system activation as opposed to the selective induction of potentially neutralizing IgG subclasses [97].
In summary, currently there are many different VLP-based treatments under pre-clinical investigation. The VLPs combine strongly immune-activating properties (repetitive antigen display, immune-stimulatory RNA sequences, efficient drainage to lymph nodes) with a reduced capacity to activate mast cells (due to steric distance of the allergens on the VLP surface and low effective concentrations of free allergen). However, while most VLPs strongly promote the formation of inhibitory IgG2b/c antibodies, other VLPs (VSV‑g VLPS displaying HEL) were shown to induce IgE-production. Therefore, more research is needed to better understand the immune-modulating properties of different VLPs.
Flagellin
Flagellin is the main component of the bacterial flagellum, a whip-like structure certain bacteria use for locomotion. As bacterial flagella are very different in structure from eukaryotic flagella (these are classic 9 × 2 + 2 microtubule structures), the immune system has developed several receptors to sense the presence of flagellated bacteria. Extracellular flagellin is sensed via the TLR5, while the intracellular presence of flagellated bacteria is sensed via the NLR family caspase activation and recruitment domain (CARD)-containing 4 protein (NLRC4) inflammasome. Also, intracellular TLR11 has been described to recognize flagellin [98]. The activation of these innate PRRs by flagellin molecules results in robust immune system activation with flagellin being a potent mucosal adjuvant that results in the induction of Th1-biased, protective immune responses in mice and monkeys [99,100,101]. Furthermore, experimental vaccines against influenza consisting of influenza antigens fused to flagellin were reported to be immunogenic, safe, and well-tolerated in clinical trials [102, 103].
The currently available literature on flagellin as an adjuvant can be divided into studies that (1) mix flagellin with the antigen of interest and that (2) fuse flagellin to the respective antigens. Fusing flagellin to the antigen of interest with recombinant DNA technologies is meanwhile well-established and allows for reproducible, medium- to large-scale production of pure recombinant fusion proteins. Such flagellin:antigen fusion proteins specifically target the fused antigen to, e.g., TLR5+ APC in vivo by which the antigen is taken up, processed, and presented in the context of the flagellin-induced APC activation. Consequently, flagellin:antigen fusion proteins were repeatedly shown to have superior immune-activating effects compared with the simple mixture of both proteins (see below in this section for more details).
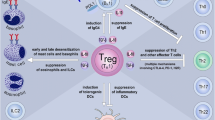
Studies co-applying Vibrio vulnificus flagellin B (FlaB) with OVA into mice reported that intralymphatic injection of flagellin mixed with OVA reduced OVA-induced airway hyperresponsiveness, infiltration of inflammatory cells into the lung, and local secretion of the Th2 cytokines IL‑4 and IL‑5 [104, 105]. In line with flagellin being a mucosal adjuvant, FlaB promoted the production of OVA-specific IgA antibodies in mouse serum [105]. Mechanistically, the allergy-improving effects of FlaB were shown to depend on TLR5 [106] and to be paralleled by a differentiation of IL-10- and transforming growth factor beta (TGF-β)-producing tolerogenic DCs that suppressed both Th1 and Th2 responses while promoting the activation of Tregs (Fig. 3; [106]). FlaB also suppressed IL‑4 and IL-17 production in an IL-10-dependent manner by invariant (i)NKT cells isolated ex vivo both from asthmatic patients and healthy controls [106]. Moreover, FlaB-induced, DC-derived IL-10 contributed to the induction of (non-IL-10-producing) FOXP3+ regulatory iNKT cells from ex vivo-isolated PBMCs of asthmatic patients (Fig. 3; [106]).
Pre-clinical immunologic effects of flagellin in allergen-specific immunotherapy. Both flagellin mixed with allergens and fusion proteins combining flagellin with the respective allergen into a single molecule were shown to induce the differentiation of both mouse and human tolerogenic dendritic cells (DCs). These DCs are characterized by IL-10 and transforming growth factor beta (TGF‑β) secretion as well as a phosphoinositide 3‑kinase (PI3K)-, mammalian target of rapamycin (mTOR) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF𝜅B)-dependent metabolic reprogramming. These tolerogenic DCs may induce the differentiation of T helper 1 (Th1), regulatory (Treg), and FOXP3+ regulatory invariant (i)NKT cells. Flagellin was also described to induce a B-cell lymphoma 6 (Bcl6)-dependent differentiation of Th2 cells that promoted allergic reactions. The induction of Th1 responses by flagellin and flagellin:allergen fusion proteins promotes the production of thymus-dependent allergen-specific IgG and IgA antibodies. Furthermore, thymus-independent production of allergen-specific IgG antibodies can be triggered directly by the flagellin:allergen fusion protein from naïve B cells. For more information, see the text. TLR Toll-like receptor
However, flagellin was also reported to have Th2-promoting effects. Flagellin, in concert with IL‑4, promoted IgE production from ex vivo-isolated mouse B cells via down-regulation of B‑cell lymphoma 6 (Bcl6; Fig. 3; [107]), suggesting that flagellin has Th2-promoting properties. Additionally, flagellin increased OVA-specific IgE levels, cell infiltration in the intestine, temperature drop, and the frequency of diarrhea in an OVA food allergy mouse model [107].
In line with these results, Salmonella typhimurium flagellin C (FliC) molecules were found in the house dust of allergic patients [108]. FliC predominantly induced Th1 and Th17 responses with co-exposure of FliC and OVA exacerbating Th1-like allergic airway inflammation in a mouse model of allergic asthma [108].
Moreover, flagellin:allergen fusion proteins were reported to have strong immune-modulating properties. Tan and colleagues described a recombinant fusion protein combining FlaB to the C‑terminus of the HDM allergen Der p 2 (rDerp2:FlaB) [109]. In an HDM-induced mouse asthma model, rDerp2:FlaB suppressed airway hyperactivity, serum IgE levels, and Th2 cytokine secretion into bronchoalveolar lavage fluid more efficiently than the mixture of flagellin and allergen [109].
In a pre-clinical study (partially funded by Biomay AG, Vienna, Austria), fusion proteins combining a truncated version of Salmonella typhimurium FliC with either the N‑ or C‑terminus of the major birch pollen allergen Bet v 1 (rFliC:Betv1 or rBetv1:FliC, respectively) were shown to activate monocyte-derived DCs isolated ex vivo from birch pollen allergic patients [110]. In mice, these FliC:Betv1 fusion proteins promoted Bet v 1‑specific IgG while reducing IgE production [110].
In our own previous pre-clinical studies, we reported Listeria monocytogenes flagellin A (FlaA) conjugated to OVA [111, 112], the major mugwort allergen Art v 1 [113], or the major birch pollen allergen Bet v 1 [114, 115]. All generated FlaA fusion proteins strongly activated myeloid dendritic cells (mDC) and suppressed allergen-specific Th2 responses.
These tolerogenic properties of mDCs activated by the fusion proteins were shown to depend on a mTOR-mediated rewiring of mDC metabolism toward increased rates of glycolysis (Fig. 3; [114,115,116]). Furthermore, mTOR activation was shown to be critical for flagellin fusion protein-induced IL-10 secretion that in turn suppressed Th2 responses in mice [114]. These results were recently supported in mice by Mishra et al. using a flagellin fusion protein incorporating either the cockroach allergen Per a 10 or Per a 10-derived peptides [117]. The fusion proteins induced (1) up-regulation of co-stimulatory molecules CD80, CD83, and CD86 on BALB/c mouse bone marrow-derived DCs and (2) enhanced IL‑6, IL-1β, and IL-12p70 as well as phosphoinositide 3‑kinase (PI3K)/mTOR/nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)-dependent IL-10 secretion [117]. The activated mouse DCs modulated Per a 10-induced Th2 responses in co-cultures with allergen-specific T cells isolated from Per a 10-immunized mice toward Th1/Treg responses [117].
For the FlaA:Betv1 fusion protein, a series of papers furthermore demonstrated its broad immune-activating capacity. Here, the fusion protein induced a pronounced activation of mouse and human macrophages [118, 119], mouse epithelial cells [120], and the ex vivo thymus-independent induction of mouse IL-10-producing functional regulatory B cells that also secreted Bet v 1‑specific IgG1 and IgG3 antibodies (Fig. 3; [121]).
In summary, the available results on flagellin and flagellin-containing fusion proteins in the context of allergies suggest flagellin to be a robust adjuvant with the capacity to induce tolerogenic responses toward either co-applied or fused allergens. While flagellin has repeatedly demonstrated its efficacy in mouse models and the capacity to activate many immune cell types (DCs, macrophages, epithelial cells, B cells), the mechanisms underlying flagellin’s adjuvant effects are complex (including, e.g., the modulation of immune metabolism in APCs and a thymus-independent B‑cell activation) and require further investigation.
Summary and conclusion
Recent developments have shown that novel adjuvants such as mannan, virus-like particle (VLPs), and flagellin have the capacity to efficiently increase the immunogenicity of allergens that are either conjugated to or co-applied with these adjuvants. Here, the different adjuvants have individual immune-activating profiles resulting in the induction of distinct immune cell subsets. Mannan induces regulatory responses and modulation of antibody production in mice and humans. Mannan–allergoid conjugates have recently seen their first application in phase II and III clinical trials. Here, initial results are promising but need further investigation. Currently investigated in many pre-clinical approaches, VLPs combine a repetitive display of antigens and immune stimulation via packaged RNA sequences or display of, e.g., tetanus toxin antigens, and a size-dependent efficient drainage to lymph nodes with a reduced capacity to activate mast cells and to induce IgE production. These properties result in strong VLP-mediated immune activation most often leading to a potent induction of blocking IgG antibodies. While peanut-containing VLPs are currently undergoing clinical phase I evaluation (NCT05476497), many approaches are still in the pre-clinical stage. Flagellin (either co-administered with allergens or fused to them) was shown to predominantly induce the differentiation of regulatory/tolerogenic immune cell subsets such as tolerogenic dendritic cells or regulatory B cells in mice. Furthermore, recent results on mannan and flagellin have shown these novel adjuvants to have immune-metabolic effects that distinctly shape the overall immune responses induced toward these adjuvants and the co-applied allergens. Therefore, more studies are necessary to better understand the complex effects of these potent immune modulators in order to safely and efficiently apply them for the treatment of allergic diseases.
Abbreviations
- AIT:
-
Allergen-specific immunotherapy
- APC:
-
Antigen-presenting cell
- AS:
-
Adjuvant system
- Bcl6:
-
B‑cell lymphoma 6
- CD206/Mannose R:
-
Mannose receptor
- CP:
-
Calcium phosphate
- CSMS:
-
Combined symptom and medication score
- CuMVTT :
-
Cucumber mosaic virus-derived VLPs containing the tetanus toxin-derived universal T cell epitope tt830-843
- DAMP:
-
Damage-associated molecular pattern
- DBPC:
-
Double-blind placebo-controlled
- DC:
-
Dendritic cell
- DC-SIGN:
-
DC-specific intracellular adhesion molecule-3-grabbing non-integrin
- FlaA/B/C:
-
Flagellin A/B/C
- FliC:
-
Salmonella typhimurium flagellin C
- FOXP3:
-
Forkhead-Box-Protein P3
- GM-CSF:
-
Granulocyte macrophage-colony stimulating factor
- HDM:
-
House dust mite
- HEL:
-
Hen egg lysozyme
- IFN:
-
Interferon
- IgE/G/A:
-
Immunoglobulin E/G/A
- IL:
-
Interleukin
- ILC2:
-
Innate lymphocyte cell
- (i)NKT:
-
Invariant NKT cell
- MATA:
-
Modified allergen tyrosine adsorbed
- MCT:
-
Microcrystalline tyrosine
- mDC:
-
Myeloid DCs
- moDC:
-
Monocyte-derived DCs
- MoMLV:
-
Moloney murine leukemia virus
- MPLA:
-
Monophosphoryl lipid A
- mTOR:
-
Mammalian target of rapamycin
- mTU:
-
Mannan-conjugated therapeutic units
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NLRC4:
-
NLR family caspase activation and recruitment domain (CARD) containing 4
- NLRP3:
-
Nucleotide binding oligomerization domain (NOD)-like receptor (NLR) protein 3
- NPT:
-
Nasal provocation test
- OIT:
-
Oral immunotherapy
- OVA:
-
Ovalbumin
- PAMP:
-
Pathogen-associated molecular pattern
- PBMC:
-
Peripheral blood mononuclear cell
- PI3K:
-
Phosphoinositide 3‑kinase
- PRR:
-
Pattern recognition receptor
- RBL:
-
Rat basophil leukemia cell
- s.c.:
-
Subcutaneous
- s. l.:
-
Sublingual
- SCIT:
-
Subcutaneous immunotherapy
- SLIT:
-
Sublingual immunotherapy
- TGF‑β:
-
Transforming growth factor beta
- Th 1/2:
-
T helper 1/2
- TLR:
-
Toll-like receptor
- Treg :
-
Regulatory T cell
- VLP:
-
Virus-like particle
- VSV:
-
Vesicular stomatitis virus
References
Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J. 2004;24(5):758–64. https://doi.org/10.1183/09031936.04.00013904.
de Monchy JG, Demoly P, Akdis CA, Cardona V, Papadopoulos NG, Schmid-Grendelmeier P, et al. Allergology in Europe, the blueprint. Allergy. 2013;68(10):1211–8. https://doi.org/10.1111/all.12225.
Bousquet J, Anto JM, Bachert C, Baiardini I, Bosnic-Anticevich S, Canonica WG, et al. Allergic rhinitis. Nat Rev Dis Primers. 2020;6(1):95. https://doi.org/10.1038/s41572-020-00227-0.
Shin YH., Hwang J., Kwon R., Lee SW., Kim MS., GBD 2019 Allergic Disorders Collaborators, et al. Global, regional, and national burden of allergic disorders and their risk factors in 204 countries and territories, from 1990 to 2019: A systematic analysis for the Global Burden of Disease Study 2019. Allergy 2023;78(8):2232–54. https://doi.org/10.1111/all.15807.
Abbas M, Moussa M, Akel H. Type I Hypersensitivity. React Statpearls Treasure Isl (fl): Statpearls Publ. 2024;.
Jutel M, Agache I, Zemelka-Wiacek M, Akdis M, Chivato T, Del Giacco S, et al. Nomenclature of allergic diseases and hypersensitivity reactions: Adapted to modern needs: An EAACI position paper. Allergy. 2023;78(11):2851–74. https://doi.org/10.1111/all.15889.
Pfaar O, Creticos PS, Kleine-Tebbe J, Canonica GW, Palomares O, Schülke S. One Hundred Ten Years of Allergen Immunotherapy: A Broad Look Into the Future. J Allergy Clin Immunol Pract. 2021;9(5):1791–803. https://doi.org/10.1016/j.jaip.2020.12.067.
Wheeler AW, Woroniecki SR. Allergy vaccines—new approaches to an old concept. Expert Opin Biol Ther. 2004;4(9):1473–81. https://doi.org/10.1517/14712598.4.9.1473.
Satitsuksanoa P, Angelina A, Palomares O, Akdis M. Mechanisms in AIT: Insights. Allergol Sel. 2021;2022(6):259–66. https://doi.org/10.5414/ALX02300E.
Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102:558–62.
Pfaar O., Ankermann T., Augustin M., Bubel P., Böing S., Brehler R., et al. Guideline on allergen immunotherapy in IgE-mediated allergic diseases: S2K Guideline of the German Society of Allergology and Clinical Immunology (DGAKI), Society of Pediatric Allergology and Environmental Medicine (GPA), Medical Association of German Allergologists (AeDA), Austrian Society of Allergology and Immunology (ÖGAI), Swiss Society for Allergology and Immunology (SSAI), German Dermatological Society (DDG), German Society of Oto-Rhino-Laryngology, Head and Neck Surgery (DGHNO-KHC), German Society of Pediatrics and Adolescent Medicine (DGKJ), Society of Pediatric Pulmonology (GPP), German Respiratory Society (DGP), German Professional Association of Otolaryngologists (BVHNO), German Association of Paediatric and Adolescent Care Specialists (BVKJ), Federal Association of Pneumologists, Sleep and Respiratory Physicians (BdP), Professional Association of German Dermatologists (BVDD). Allergol Select 2022;6:167–232. https://doi.org/10.5414/ALX02331E.
Pfaar O, Becker S, Calabria C, Hartenstein D, Jung J, Zimmer J, et al. Comparison of allergen immunotherapy practice patterns in inhalant allergies in the United States of America and Europe: Similarities and differences 2023. World Allergy Organ J. 2023;16(5):100766. https://doi.org/10.1016/j.waojou.2023.100766.
Fujita H, Soyka MB, Akdis M. Akdis C a. Mech Allergen-specific Immunother Clin Transl Allergy. 2012;2(1):2. https://doi.org/10.1186/2045-7022-2-2.
Liu J, Li W, Zhu R. The Role of Innate Immune Cells in Allergen Immunotherapy. Curr Treat Options Allergy. 2023;10(2):148–65. https://doi.org/10.1007/s40521-023-00337-6.
Golebski K, Layhadi JA, Sahiner U, Steveling-Klein EH, Lenormand MM, Li RCY, et al. Induction of IL-10-producing type 2 innate lymphoid cells by allergen immunotherapy is associated with clinical response. Immunity. 2021;54(2):291–307.e7. https://doi.org/10.1016/j.immuni.2020.12.013.
Mitthamsiri W, Pradubpongsa P, Sangasapaviliya A, Boonpiyathad T. Decreased CRTH2 Expression and Response to Allergen Re-stimulation on Innate Lymphoid Cells in Patients With Allergen-Specific Immunotherapy. Allergy Asthma Immunol Res. 2018;10(6):662–74. https://doi.org/10.4168/aair.2018.10.6.662.
Eljaszewicz A, Ruchti F, Radzikowska U, Globinska A, Boonpiyathad T, Gschwend A, et al. Trained immunity and tolerance in innate lymphoid cells, monocytes, and dendritic cells during allergen-specific immunotherapy. J Allergy Clin Immunol. 2020; https://doi.org/10.1016/j.jaci.2020.08.042.
Committee For Medicinal Products For Human Use (CHMP): Guideline On Adjuvants In Vaccines For Human Usecommittee For Medicinal Products For Human Use. EMEA/CHMP/VEG/134716/2004. London, 20 January 2005n . d.
Hem SL, White JL. Structure and properties of aluminum-containing adjuvants. Pharm Biotechnol. 1995;6:249–76. https://doi.org/10.1007/978-1-4615-1823-5_9.
Cox JC., Coulter AR. Adjuvants—a classification and review of their modes of action. Vaccine 1997;15(3):248–56. https://doi.org/10.1016/s0264-410x(96)00183-1.
Awate S, Babiuk LA, Mutwiri G. Mechanisms of action of adjuvants. Front Immunol. 2013;4:114. https://doi.org/10.3389/fimmu.2013.00114.
Leuthard DS, Duda A, Freiberger SN, Weiss S, Dommann I, Fenini G, et al. Microcrystalline Tyrosine and Aluminum as Adjuvants in Allergen-Specific Immunotherapy Protect from IgE-Mediated Reactivity in Mouse Models and Act Independently of Inflammasome and TLR Signaling. J Immunol. 2018;200(9):3151–9. https://doi.org/10.4049/jimmunol.1800035.
Lin Y‑J, Zimmermann J, Schülke S. Novel adjuvants in allergen-specific immunotherapy: where do we stand? Front Immunol. 2024;15:1348305. https://doi.org/10.3389/fimmu.2024.1348305.
Glenny AT, Pope CG, Waddington H, Wallace U. Immunological notes. XVII–XXIV. J Pathol Bacteriol. 1926;29(1):31–40. https://doi.org/10.1002/path.1700290106.
Council of Europe; Strasbourg, France, 2024. EDQM. European Pharmacopoeia. 11.4th edition. n. d.
HogenEsch H. Mechanisms of stimulation of the immune response by aluminum adjuvants. Vaccine 2002;20 Suppl 3:S34–39. https://doi.org/10.1016/s0264-410x(02)00169-x.
Jensen-Jarolim E. Aluminium in Allergies and Allergen immunotherapy. World Allergy Organ J. 2015;8(1):7. https://doi.org/10.1186/s40413-015-0060-5.
Kooijman S, Brummelman J, van Els CACM, Marino F, Heck AJR, van Riet E, et al. Vaccine antigens modulate the innate response of monocytes to Al(OH)3. PLoS ONE. 2018;13(5):e197885. https://doi.org/10.1371/journal.pone.0197885.
Fadugba OO, Wang L, Chen Q, Halasa NB. Immune responses to pertussis antigens in infants and toddlers after immunization with multicomponent acellular pertussis vaccine. Clin Vaccine Immunol. 2014;21(12):1613–9. https://doi.org/10.1128/CVI.00438-14.
Benito-Villalvilla C, Pérez-Diego M, Angelina A, Kisand K, Rebane A, Subiza JL, et al. Allergoid-mannan conjugates reprogram monocytes into tolerogenic dendritic cells via epigenetic and metabolic rewiring. J Allergy Clin Immunol. 2022;149(1):212–222.e9. https://doi.org/10.1016/j.jaci.2021.06.012.
McKee AS, Munks MW, MacLeod MKL, Fleenor CJ, Van Rooijen N, Kappler JW, et al. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol. 2009;183(7):4403–14. https://doi.org/10.4049/jimmunol.0900164.
Kool M, Soullié T, van Nimwegen M, Willart MAM, Muskens F, Jung S, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205(4):869–82. https://doi.org/10.1084/jem.20071087.
Mannhalter JW, Neychev HO, Zlabinger GJ, Ahmad R, Eibl MM. Modulation of the human immune response by the non-toxic and non-pyrogenic adjuvant aluminium hydroxide: effect on antigen uptake and antigen presentation. Clin Exp Immunol. 1985;61(1):143–51.
Iyer S., HogenEsch H., Hem SL. Relationship between the degree of antigen adsorption to aluminum hydroxide adjuvant in interstitial fluid and antibody production. Vaccine 2003;21(11–12):1219–23. https://doi.org/10.1016/s0264-410x(02)00556-x.
Rimaniol A‑C., Gras G., Verdier F., Capel F., Grigoriev VB., Porcheray F., et al. Aluminum hydroxide adjuvant induces macrophage differentiation towards a specialized antigen-presenting cell type. Vaccine 2004;22(23–24):3127–35. https://doi.org/10.1016/j.vaccine.2004.01.061.
Lindblad EB. Aluminium compounds for use in vaccines. Immunol Cell Biol. 2004;82(5):497–505. https://doi.org/10.1111/j.0818-9641.2004.01286.x.
Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453(7198):1122–6. https://doi.org/10.1038/nature06939.
Kool M, Pétrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, et al. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181(6):3755–9. https://doi.org/10.4049/jimmunol.181.6.3755.
Franchi L, Núñez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol. 2008;38(8):2085–9. https://doi.org/10.1002/eji.200838549.
Kuroda E, Ishii KJ, Uematsu S, Ohata K, Coban C, Akira S, et al. Silica crystals and aluminum salts regulate the production of prostaglandin in macrophages via NALP3 inflammasome-independent mechanisms. Immunity. 2011;34(4):514–26. https://doi.org/10.1016/j.immuni.2011.03.019.
Kool M, Willart MAM, van Nimwegen M, Bergen I, Pouliot P, Virchow JC, et al. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity. 2011;34(4):527–40. https://doi.org/10.1016/j.immuni.2011.03.015.
McDougall SA, Heath MD, Kramer MF, Skinner MA. Analysis of aluminium in rat following administration of allergen immunotherapy using either aluminium or microcrystalline-tyrosine-based adjuvants. Bioanalysis. 2016;8(6):547–56. https://doi.org/10.4155/bio.16.10.
Jensen-Jarolim E., Bachmann MF., Bonini S., Jacobsen L., Jutel M., Klimek L., et al. State-of-the-art in marketed adjuvants and formulations in Allergen Immunotherapy: A position paper of the European Academy of Allergy and Clinical Immunology (EAACI). Allergy 2020;75(4):746–60. https://doi.org/10.1111/all.14134.
Weisser K. Toxicokinetics of aluminium—novel insights in an old adjuvant. Allergo J Int. 2024; https://doi.org/10.1007/s40629-024-00288-7.
Weisser K, Stübler S, Matheis W, Huisinga W. Towards toxicokinetic modelling of aluminium exposure from adjuvants in medicinal products. Regul Toxicol Pharmacol. 2017;88:310–21. https://doi.org/10.1016/j.yrtph.2017.02.018.
Hethey C, Hartung N, Wangorsch G, Weisser K, Huisinga W. Physiology-based toxicokinetic modelling of aluminium in rat and man. Arch Toxicol. 2021;95(9):2977–3000. https://doi.org/10.1007/s00204-021-03107-y.
Hiller J, Göen T, Drexler H, Berking C, Wagner N. Elevated aluminum excretion in patients by long-term subcutaneous immunotherapy—A cross-sectional case-control study. Int J Hyg Environ Health. 2024;258:114337. https://doi.org/10.1016/j.ijheh.2024.114337.
Mahler V, Kleine-Tebbe J, Vieths S. Immunotherapy of allergies: current status. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2020;63(11):1341–56. https://doi.org/10.1007/s00103-020-03224-6.
Baldrick P, Richardson D, Wheeler AW. Review of L‑tyrosine confirming its safe human use as an adjuvant. J Appl Toxicol. 2002;22(5):333–44. https://doi.org/10.1002/jat.869.
Bell AJ, Heath MD, Hewings SJ, Skinner MA. The adsorption of allergoids and 3‑O-desacyl-4’-monophosphoryl lipid A (MPL®) to microcrystalline tyrosine (MCT) in formulations for use in allergy immunotherapy. J Inorg Biochem. 2015;152:147–53. https://doi.org/10.1016/j.jinorgbio.2015.08.007.
Heath MD, Mohsen MO, de Kam P‑J, Carreno Velazquez TL, Hewings SJ, Kramer MF, et al. Shaping Modern Vaccines: Adjuvant Systems Using MicroCrystalline Tyrosine (MCT®). Front Immunol. 2020;11:594911. https://doi.org/10.3389/fimmu.2020.594911.
Miller AC, Hart AP, Tees ECD. pteronyssinus-tyrosine adsorbate: biological and clinical properties. Acta Allergol. 1976;31(1):35–43. https://doi.org/10.1111/j.1398-9995.1976.tb01477.x.
He Q, Mitchell AR, Johnson SL, Wagner-Bartak C, Morcol T, Bell SJ. Calcium phosphate nanoparticle adjuvant. Clin Diagn Lab Immunol. 2000;7(6):899–903. https://doi.org/10.1128/CDLI.7.6.899-903.2000.
Jones S, Asokanathan C, Kmiec D, Irvine J, Fleck R, Xing D, et al. Protein coated microcrystals formulated with model antigens and modified with calcium phosphate exhibit enhanced phagocytosis and immunogenicity. Vaccine. 2014;32(33):4234–42. https://doi.org/10.1016/j.vaccine.2013.09.061.
Leynadier F, Banoun L, Dollois B, Terrier P, Epstein M, Guinnepain MT, et al. Immunotherapy with a calcium phosphate-adsorbed five-grass-pollen extract in seasonal rhinoconjunctivitis: a double-blind, placebo-controlled study. Clin Exp Allergy. 2001;31(7):988–96. https://doi.org/10.1046/j.1365-2222.2001.01145.x.
Pazár B, Ea H‑K, Narayan S, Kolly L, Bagnoud N, Chobaz V, et al. Basic calcium phosphate crystals induce monocyte/macrophage IL-1β secretion through the NLRP3 inflammasome in vitro. J Immunol. 2011;186(4):2495–502. https://doi.org/10.4049/jimmunol.1001284.
Hayashi M, Aoshi T, Kogai Y, Nomi D, Haseda Y, Kuroda E, et al. Optimization of physiological properties of hydroxyapatite as a vaccine adjuvant. Vaccine. 2016;34(3):306–12. https://doi.org/10.1016/j.vaccine.2015.11.059.
Ulrich JT, Myers KR. Monophosphoryl lipid A as an adjuvant. Past experiences and new directions. Pharm Biotechnol. 1995;6:495–524.
Schülke S, Flaczyk A, Vogel L, Gaudenzio N, Angers I, Löschner B, et al. MPLA shows attenuated pro-inflammatory properties and diminished capacity to activate mast cells in comparison with LPS. Allergy. n/a-n/a. 2015. https://doi.org/10.1111/all.12675.
Boland G, Beran J, Lievens M, Sasadeusz J, Dentico P, Nothdurft H, et al. Safety and immunogenicity profile of an experimental hepatitis B vaccine adjuvanted with AS04. Vaccine. 2004;23(3):316–20. https://doi.org/10.1016/j.vaccine.2004.06.006.
RTS,S Clinical Trials Partnership Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 2015;386(9988):31–45. https://doi.org/10.1016/S0140-6736(15)60721-8.
Mahler V, Bonertz A, Ruoff C, Hartenstein D, Mentzer D, Kaul S, et al. What we learned from TAO—10 years of German therapy allergen ordinance. Allergo J Int. 2019;28(8):330–7. https://doi.org/10.1007/s40629-019-0101-7.
Puggioni F, Durham SR, Francis JN. Monophosphoryl lipid A (MPLR)* promotes allergen-induced immune deviation in favour of Th1 responses. Allergy. 2005;60(5):678–84. https://doi.org/10.1111/j.1398-9995.2005.00762.x.
Drachenberg KJ, Wheeler AW, Stuebner P, Horak F. A well-tolerated grass pollen-specific allergy vaccine containing a novel adjuvant, monophosphoryl lipid A, reduces allergic symptoms after only four preseasonal injections. Allergy. 2001;56(6):498–505. https://doi.org/10.1034/j.1398-9995.2001.056006498.x.
Worm M, Higenbottam T, Pfaar O, Mösges R, Aberer W, Gunawardena K, et al. Randomized controlled trials define shape of dose response for Pollinex Quattro Birch allergoid immunotherapy. Allergy. 2018;73(9):1812–22. https://doi.org/10.1111/all.13478.
de Kam PJ, Zielen S, Bernstein JA, Berger U, Berger M, Cuevas M, et al. Short-course subcutaneous treatment with PQ Grass strongly improves symptom and medication scores in grass allergy. Allergy. 2023;78(10):2756–66. https://doi.org/10.1111/all.15788.
Wheeler a W., Marshall JS., Ulrich JT. A Th1-inducing adjuvant, MPL, enhances antibody profiles in experimental animals suggesting it has the potential to improve the efficacy of allergy vaccines. Int Arch Allergy Immunol 2001;126(2):135–9.
Patel P., Holdich T., Fischer von Weikersthal-Drachenberg KJ., Huber B. Efficacy of a short course of specific immunotherapy in patients with allergic rhinoconjunctivitis to ragweed pollen. J Allergy Clin Immunol 2014;133(1):121–129.e1–2. https://doi.org/10.1016/j.jaci.2013.05.032.
Drachenberg KJ, Heinzkill M, Urban E, Woroniecki SR. Efficacy and tolerability of short-term specific immunotherapy with pollen allergoids adjuvanted by monophosphoryl lipid A (MPL) for children and adolescents. Allergol Immunopathol (Madr). 2003;31(5):270–7.
Mothes N, Heinzkill M, Drachenberg KJ, Sperr WR, Krauth MT, Majlesi Y, et al. Allergen-specific immunotherapy with a monophosphoryl lipid A‑adjuvanted vaccine: reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy-induced blocking antibodies. Clin Exp Allergy. 2003;33(9):1198–208.
Sheng K‑C, Pouniotis DS, Wright MD, Tang CK, Lazoura E, Pietersz GA, et al. Mannan derivatives induce phenotypic and functional maturation of mouse dendritic cells. Immunology. 2006;118(3):372–83. https://doi.org/10.1111/j.1365-2567.2006.02384.x.
Sánchez-Herrero S, Benito-Villalvilla C, Palomares O. Purified Free Mannan Promotes Tolerogenic Responses in Peanut-Stimulated Human Dendritic Cells. Int Arch Allergy Immunol. 2024; https://doi.org/10.1159/000537989.
Sirvent S, Soria I, Cirauqui C, Cases B, Manzano AI, Diez-Rivero CM, et al. Novel vaccines targeting dendritic cells by coupling allergoids to nonoxidized mannan enhance allergen uptake and induce functional regulatory T cells through programmed death ligand 1. J Allergy Clin Immunol. 2016; https://doi.org/10.1016/j.jaci.2016.02.029.
Benito-Villalvilla C, Pérez-Diego M, Subiza JL, Palomares O. Allergoid-mannan conjugates imprint tolerogenic features in human macrophages. Allergy. 2022;77(1):320–3. https://doi.org/10.1111/all.15118.
Nieto A, Mazón Á, Nieto M, Ibáñez E, Jang D‑T, Calaforra S, et al. First-in-human phase 2 trial with mite allergoids coupled to mannan in subcutaneous and sublingual immunotherapy. Allergy. 2022;77(10):3096–107. https://doi.org/10.1111/all.15374.
Mösges R, Zeyen C, Raskopf E, Acikel C, Sahin H, Allekotte S, et al. A randomized, double-blind, placebo-controlled trial with mannan-conjugated birch pollen allergoids. Allergy. 2023; https://doi.org/10.1111/all.15910.
Li S, Toriumi H, Takahashi D, Kamasaki T, Fujioka Y, Nagatoishi S, et al. Safe and efficient oral allergy immunotherapy using one-pot-prepared mannan-coated allergen nanoparticles. Biomaterials. 2023;303:122381. https://doi.org/10.1016/j.biomaterials.2023.122381.
Li S, Murakami D, Nagatoishi S, Liu Y, Tsumoto K, Katayama Y, et al. One-pot preparation of mannan-coated antigen nanoparticles using human serum albumin as a matrix for tolerance induction. J Colloid Interface Sci. 2023;649:955–65. https://doi.org/10.1016/j.jcis.2023.06.170.
Johnson JE., Chiu W. Structures of virus and virus-like particles. Curr Opin Struct Biol 2000;10(2):229–35. https://doi.org/10.1016/s0959-440x(00)00073-7.
Schmitz N, Dietmeier K, Bauer M, Maudrich M, Utzinger S, Muntwiler S, et al. Displaying Fel d1 on virus-like particles prevents reactogenicity despite greatly enhanced immunogenicity: a novel therapy for cat allergy. J Exp Med. 2009;206(9):1941–55. https://doi.org/10.1084/jem.20090199.
Sani MZ, Bargahi A, Momenzadeh N, Dehghani P, Moghadam MV, Maleki SJ, et al. Genetically engineered fusion of allergen and viral-like particle induces a more effective allergen-specific immune response than a combination of them. Appl Microbiol Biotechnol. 2021;105(1):77–91. https://doi.org/10.1007/s00253-020-11012-0.
Jitthamstaporn S, Sander AF, Jacquet A. Virus-like particles displaying recombinant Der p 1 zymogen to optimize IgG blocking antibody response. Allergy. 2022;77(2):664–7. https://doi.org/10.1111/all.15129.
Chanasit S, Johnston E, Thanasarnthungcharoen C, Kamath SD, Bohle B, Lopata AL, et al. Hypoallergenic chimeric virus-like particles for the development of shrimp tropomyosin allergen Pen m 1‑specific blocking antibodies. Allergy. 2023; https://doi.org/10.1111/all.15892.
Soongrung T, Mongkorntanyatip K, Peepim T, Jitthamstaporn S, Pitakpolrat P, Kaewamatawong T, et al. Virus-like particles displaying major house dust mite allergen Der p 2 for prophylactic allergen immunotherapy. Allergy. 2020;75(5):1232–6. https://doi.org/10.1111/all.14096.
Senti G, Johansen P, Haug S, Bull C, Gottschaller C, Müller P, et al. Use of A‑type CpG oligodeoxynucleotides as an adjuvant in allergen-specific immunotherapy in humans: a phase I/IIa clinical trial. Clin Exp Allergy. 2009;39(4):562–70. https://doi.org/10.1111/j.1365-2222.2008.03191.x.
Klimek L, Willers J, Hammann-Haenni A, Pfaar O, Stocker H, Mueller P, et al. Assessment of clinical efficacy of CYT003-QbG10 in patients with allergic rhinoconjunctivitis: a phase IIb study. Clin Exp Allergy. 2011;41(9):1305–12. https://doi.org/10.1111/j.1365-2222.2011.03783.x.
Beeh K‑M, Kanniess F, Wagner F, Schilder C, Naudts I, Hammann-Haenni A, et al. The novel TLR‑9 agonist QbG10 shows clinical efficacy in persistent allergic asthma. J Allergy Clin Immunol. 2013;131(3):866–74. https://doi.org/10.1016/j.jaci.2012.12.1561.
Casale TB., Cole J., Beck E., Vogelmeier CF., Willers J., Lassen C., et al. CYT003, a TLR9 agonist, in persistent allergic asthma—a randomized placebo-controlled Phase 2b study. Allergy 2015;70(9):1160–8. https://doi.org/10.1111/all.12663.
Storni F, Zeltins A, Balke I, Heath MD, Kramer MF, Skinner MA, et al. Vaccine against peanut allergy based on engineered virus-like particles displaying single major peanut allergens. J Allergy Clin Immunol. 2020;145(4):1240–1253.e3. https://doi.org/10.1016/j.jaci.2019.12.007.
Sobczak JM, Krenger PS, Storni F, Mohsen MO, Balke I, Reseviča G, et al. The next generation virus-like particle platform for the treatment of peanut allergy. Allergy. 2023;78(7):1980–96. https://doi.org/10.1111/all.15704.
Krenger PS, Josi R, Sobczak J, Velazquez TLC, Balke I, Skinner MA, et al. Influence of antigen density and TLR ligands on preclinical efficacy of a VLP-based vaccine against peanut allergy. Allergy. 2024;79(1):184–99. https://doi.org/10.1111/all.15897.
Thoms F, Jennings GT, Maudrich M, Vogel M, Haas S, Zeltins A, et al. Immunization of cats to induce neutralizing antibodies against Fel d 1, the major feline allergen in human subjects. J Allergy Clin Immunol. 2019;144(1):193–203. https://doi.org/10.1016/j.jaci.2019.01.050.
Thoms F, Haas S, Erhart A, Nett CS, Rüfenacht S, Graf N, et al. Immunization of Cats against Fel d 1 Results in Reduced Allergic Symptoms of Owners. Viruses. 2020;12(3):288. https://doi.org/10.3390/v12030288.
Goksøyr L, Funch AB, Okholm AK, Theander TG, de Jongh WA, Bonefeld CM, et al. Preclinical Efficacy of a Capsid Virus-like Particle-Based Vaccine Targeting IL-1β for Treatment of Allergic Contact Dermatitis. Vaccines (basel). 2022;10(5):828. https://doi.org/10.3390/vaccines10050828.
Kim J, Oh J, Kang C‑S, Virus-like Particle CYS. (VLP) Mediated Antigen Delivery as a Sensitization Tool of Experimental Allergy Mouse Models. Immune Netw. 2020;20(4):e35. https://doi.org/10.4110/in.2020.20.e35.
Kratzer B, Köhler C, Hofer S, Smole U, Trapin D, Iturri J, et al. Prevention of allergy by virus-like nanoparticles (VNP) delivering shielded versions of major allergens in a humanized murine allergy model. Allergy. 2018; https://doi.org/10.1111/all.13573.
Pazos-Castro D, Margain C, Gonzalez-Klein Z, Amores-Borge M, Yuste-Calvo C, Garrido-Arandia M, et al. Suitability of potyviral recombinant virus-like particles bearing a complete food allergen for immunotherapy vaccines. Front Immunol. 2022;13:986823. https://doi.org/10.3389/fimmu.2022.986823.
Hatai H, Lepelley A, Zeng W, Hayden MS, Ghosh S. Toll-Like Receptor 11 (TLR11) Interacts with Flagellin and Profilin through Disparate Mechanisms. PLoS ONE. 2016;11(2):e148987. https://doi.org/10.1371/journal.pone.0148987.
Lee SE, Kim SY, Jeong BC, Kim YR, Bae SJ, Ahn OS, et al. A Bacterial Flagellin , Vibrio vulnificus FlaB , Has a Strong Mucosal Adjuvant Activity To Induce Protective. Immunity. 2006;74(1):694–702. https://doi.org/10.1128/IAI.74.1.694.
Honko AN, Sriranganathan N, Lees CJ, Mizel SB. Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis. Infect Immun. 2006;74(2):1113–20. https://doi.org/10.1128/IAI.74.2.1113-1120.2006.
Huleatt JW, Jacobs AR, Tang J, Desai P, Kopp EB, Huang Y, et al. Vaccination with recombinant fusion proteins incorporating Toll-like receptor ligands induces rapid cellular and humoral immunity. Vaccine. 2007;25(4):763–75. https://doi.org/10.1016/j.vaccine.2006.08.013.
Turley CB, Rupp RE, Johnson C, Taylor DN, Wolfson J, Tussey L, et al. Safety and immunogenicity of a recombinant M2e-flagellin influenza vaccine (STF2.4xM2e) in healthy adults. Vaccine. 2011;29(32):5145–52. https://doi.org/10.1016/j.vaccine.2011.05.041.
Tussey L, Strout C, Davis M, Johnson C, Lucksinger G, Umlauf S, et al. Phase 1 Safety and Immunogenicity Study of a Quadrivalent Seasonal Flu Vaccine Comprising Recombinant Hemagglutinin-Flagellin Fusion Proteins. Open Forum Infect Dis. 2016;3(1):ofw15. https://doi.org/10.1093/ofid/ofw015.
Kim EH, Kim JH, Samivel R, Bae J‑S, Chung Y‑J, Chung P‑S, et al. Intralymphatic treatment of flagellin-ovalbumin mixture reduced allergic inflammation in murine model of allergic rhinitis. Allergy. 2016;71(5):629–39. https://doi.org/10.1111/all.12839.
Bae SJ, Koh JT, Ryu H‑J, Choy HE, Lee SE, Rhee JH, et al. Inhibition of Airway Allergic Disease by Co-Administration of Flagellin with Allergen. J Clin Immunol. 2007;28(2):157–65. https://doi.org/10.1007/s10875-007-9138-3.
Shim J‑U, Lee SE, Hwang W, Lee C, Park J‑W, Sohn J‑H, et al. Flagellin suppresses experimental asthma by generating regulatory dendritic cells and T cells. J Allergy Clin Immunol. 2016;137(2):426–35. https://doi.org/10.1016/j.jaci.2015.07.010.
Li L‑J, Ma N, Zeng L, Mo L‑H, Li X‑X, Xu L‑Z, et al. Flagellin modulates IgE expression in B cells to initiate food allergy in mice. Am J Transl Res. 2016;8(6):2748–57.
Whitehead GS, Hussain S, Fannin R, Trempus CS, Innes CL, Schurman SH, et al. TLR5 Activation Exacerbates Airway Inflammation in Asthma. Am J Physiol. 2020;198(2):289–98. https://doi.org/10.1007/s00408-020-00337-2.
Tan W, Zheng JH, Duong TMN, Koh YI, Lee SE, Rhee JH. A Fusion Protein of Derp2 Allergen and Flagellin Suppresses Experimental Allergic Asthma. Allergy Asthma Immunol Res. 2019;11(2):254–66. https://doi.org/10.4168/aair.2019.11.2.254.
Kitzmüller C, Kalser J, Mutschlechner S, Hauser M, Zlabinger GJ, Ferreira F, et al. Fusion proteins of flagellin and the major birch pollen allergen Bet v 1 show enhanced immunogenicity, reduced allergenicity, and intrinsic adjuvanticity. J Allergy Clin Immunol. 2018;141(1):293–299.e6. https://doi.org/10.1016/j.jaci.2017.02.044.
Schülke S., Burggraf M., Waibler Z., Wangorsch A., Wolfheimer S., Kalinke U., et al. A fusion protein of flagellin and ovalbumin suppresses the TH2 response and prevents murine intestinal allergy. J Allergy Clin Immunol 2011;128(6):1340–1348.e12. https://doi.org/10.1016/j.jaci.2011.07.036.
Schülke S, Wolfheimer S, Gadermaier G, Wangorsch A, Siebeneicher S, Briza P, et al. Prevention of intestinal allergy in mice by rflaA:Ova is associated with enforced antigen processing and TLR5-dependent IL-10 secretion by mDC. Plos One. 2014;9(2):e87822. https://doi.org/10.1371/journal.pone.0087822.
Schülke S, Kuttich K, Wolfheimer S, Duschek N, Wangorsch A, Reuter A, et al. Author Correction: Conjugation of wildtype and hypoallergenic mugwort allergen Art v 1 to flagellin induces IL-10-DC and suppresses allergen-specific TH2-responses in vivo. Sci Rep. 2018;8(1):2745. https://doi.org/10.1038/s41598-018-20635-3.
Schülke S., Fiedler A‑H., Junker A‑C., Flaczyk A., Wolfheimer S., Wangorsch A., et al. Critical role of mammalian target of rapamycin for IL-10 dendritic cell induction by a flagellin A conjugate in preventing allergic sensitization. J Allergy Clin Immunol 2018;141(5):1786–1798.e11. https://doi.org/10.1016/j.jaci.2017.07.002.
Moeller T, Wolfheimer S, Goretzki A, Scheurer S, Schülke S. NFκB- and MAP-Kinase Signaling Contribute to the Activation of Murine Myeloid Dendritic Cells by a Flagellin A:Allergen Fusion. Protein Cells. 2019; https://doi.org/10.3390/cells8040355.
Goretzki A, Lin Y‑J, Zimmermann J, Rainer H, Junker A‑C, Wolfheimer S, et al. Role of Glycolysis and Fatty Acid Synthesis in the Activation and T Cell-Modulating Potential of Dendritic Cells Stimulated with a TLR5-Ligand Allergen Fusion Protein. IJMS. 2022;23(20):12695. https://doi.org/10.3390/ijms232012695.
Mishra R., Sharma S., Arora N. TLR‑5 ligand conjugated with Per a 10 and T cell peptides potentiates Treg/Th1 response through PI3K/mTOR axis. Int Immunopharmacol 2022;113(Pt A):109389. https://doi.org/10.1016/j.intimp.2022.109389.
Lin Y‑J, Papp G, Miskey C, Fiedler A, Goretzki A, Wolfheimer S, et al. The Flagellin:Allergen Fusion Protein rFlaA:Betv1 Induces a MyD88- and MAPK-Dependent Activation of Glucose Metabolism in Macrophages. Cells. 2021;10(10):2614. https://doi.org/10.3390/cells10102614.
Lin Y‑J, Jamin A, Wolfheimer S, Fiedler A, Junker A‑C, Goretzki A, et al. A flagellin-conjugate protein induces dual NLRC4- and NLRP3-inflammasome activation which modulates inflammatory cytokine secretion from macrophages. Front Immunol. 2023;14.
Lin Y‑J., Flaczyk A., Wolfheimer S., Goretzki A., Jamin A., Wangorsch A., et al. The Fusion Protein rFlaA:Betv1 Modulates DC Responses by a p38-MAPK and COX2-Dependent Secretion of PGE2 from Epithelial Cells. Cells 2021;10(12):3415. https://doi.org/10.3390/cells10123415.
Goretzki A, Lin Y‑J, Meier C, Dorn B, Wolfheimer S, Jamin A, et al. Stimulation of naïve B cells with a fusion protein consisting of FlaA and Bet v 1 induces regulatory B cells ex vivo. Allergy. 2023;78(3):663–81. https://doi.org/10.1111/all.15542.
Acknowledgements
The authors would like to thank Prof. Dr. Vera Mahler (Paul-Ehrlich-Institut) for the critical revision of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
C. Pignard, H. Schiller, A. Seyffer and S. Schülke declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Authors’ Disclaimer
The views expressed in this review are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the respective national competent authority, the European Medicines Agency, or one of its committees, or the German Federal Ministry of Health.
The authors C. Pignard and H. Schiller contributed equally to the manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pignard, C., Schiller, H., Seyffer, A. et al. Mannan‑, VLP-, and flagellin-based adjuvants for allergen-specific immunotherapy: a review of the current literature. Allergo J Int (2024). https://doi.org/10.1007/s40629-024-00298-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40629-024-00298-5