Abstract
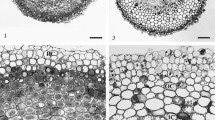
The distribution of essential nutrients such as potassium (K), phosphorous (P), calcium (Ca), sulfur (S) and chlorine (Cl) within root tissues is crucial aspect of plant growth but nothing is known regarding this subject in seedlings of dicotyledons such as peanut (Arachis hypogaea). We used scanning electron microscopy/energy-dispersive X-ray spectroscopy (SEM/EDS) and inductively coupled plasma optical emission spectrometry (ICP-OES) to analyze element distributions in developing root tissues. Distribution patterns and concentrations of endogenous K, P, S and exogenous Ca from seeds grown in water varied along the length of peanut radicle and were tissue specific. Semi-quantitative SEM/EDS data indicated 24 mM K in the cortex and 46 mM K in the stele at a distance of 5 mm from the root tip. The P concentration in the same region was 15 mM in cortex and 22 mM in stele. The concentration of K and P decreased in the more mature parts of the root. SEM–EDS element concentrations values were lower for K and higher for P when compared with the ICP-OES data of root segments. Peanut roots grown in 10 mM Ca(NO3)2 solution for hours showed tissue-specific Ca distribution at 25 mm from the root tip with the highest levels detected in portions of the cortex. The parent root cortex centrifugal to the tip of developing lateral root primordia had lower levels of calcium than could be detected by SEM/EDS.









Similar content being viewed by others
References
Acharya A (2021a) Global agriculture management system. Crop Sci 61(5):2861–2862. https://doi.org/10.1002/csc2.20499
Acharya A (2021b) Tissue specific nutrient localization in the Arachis hypogaea seedling root (Doctoral dissertation, University of Louisiana at Lafayette)
Bavaresco LG, Osco LP, Araujo ASF, Mendes LW, Bonifacio A, Araujo FF (2020) Bacillus subtilis can modulate the growth and root architecture in soybean through volatile organic compounds. Theor Exp Plant Physiol 32(2):99–108
Baxter I, Ouzzani M, Orcun S, Kennedy B, Jandhyala SS, Salt DE (2007) Purdue ionomics information management system. An integrated functional genomics platform. Plant Physiol 143(2):600–611
Beauford W, Barber J, Barringer AR (1977) Uptake and distribution of mercury within higher plants. Physiol Plant 39(4):261–265
Boogerd FC, van Rossum D (1997) Nodulation of groundnut by Bradyrhizobium: a simple infection process by crack entry. FEMS Microbiol Rev 21(1):5–27
Brunner I, Luster J, Günthardt-Goerg MS, Frey B (2008) Heavy metal accumulation and phytostabilisation potential of tree fine roots in a contaminated soil. Environ Pollut 152(3):559–568
Bucking H, Heyser W (2000) Subcellular compartmentation of elements in non-mycorrhizal and mycorrhizal roots of Pinus sylvestris: an X- ray microanalytical study I. The distribution of phosphate. New Phytol 145(2):311–320
Bulgarelli RG, De Oliveira VH, de Andrade SAL (2020) Arbuscular mycorrhizal symbiosis alters the expression of PHT1 phosphate transporters in roots and nodules of P-starved soybean plants. Theor Exp Plant Physiol 32(3):243–253
Casero PJ, Casimiro I, Lloret PG (1995) Lateral root initiation by asymmetrical transverse divisions of pericycle cells in four plant species: Raphanus sativus, Helianthus annuus, Zea mays, and Daucus carota. Protoplasma 188(1):49–58
Chassagne-Berces S, Poirier C, Devaux MF, Fonseca F, Lahaye M, Pigorini G et al (2009) Changes in texture, cellular structure and cell wall composition in apple tissue as a result of freezing. Food Res Int 42(7):788–797
Cheong YH, Pandey GK, Grant JJ, Batistic O, Li L, Kim BG et al (2007) Two calcineurin B-like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in Arabidopsis. Plant J 52(2):223–239
Cholewa E, Peterson CA (2004) Evidence for symplastic involvement in the radial movement of calcium in onion roots. Plant Physiol 134(4):1793–1802
Clarkson DT, Hanson JB (1980) The mineral nutrition of higher plants. Annu Rev Plant Physiol 31(1):239–298
Clarkson DT, Hopper MJ, Jones LHP (1986) The effect of root temperature on the uptake of nitrogen and the relative size of the root system in Lolium perenne I. Solutions containing both NH4+ and NO3−. Plant Cell Environ 9(7):535–545
Conn S, Gilliham M (2010) Comparative physiology of elemental distributions in plants. Ann Bot 105(7):1081–1102
da Silva BHP, Rossatto DR (2019) Are underground organs able to store water and nutrients? A study case in non-arboreal species from the Brazilian Cerrado. Theor Exp Plant Physiol 31(3):413–421
De Smet I, Vanneste S, Inzé D, Beeckman T (2006) Lateral root initiation or the birth of a new meristem. Plant Mol Biol 60(6):871–887
Delhaize E, Craig S, Beaton CD, Bennet RJ, Jagadish VC, Randall PJ (1993) Aluminum tolerance in wheat (Triticum aestivum L.) (I. Uptake and distribution of aluminum in root apices). Plant Physiol 103(3):685–693
Dembinsky D, Woll K, Saleem M, Liu Y, Fu Y, Borsuk LA et al (2007) Transcriptomic and proteomic analyses of pericycle cells of the maize primary root. Plant Physiol 145(3):575–588
Demidchik V, Bowen HC, Maathuis FJ, Shabala SN, Tester MA, White PJ, Davies JM (2002) Arabidopsis thaliana root non-selective cation channels mediate calcium uptake and are involved in growth. Plant J 32(5):799–808
Ehrhardt-Brocardo NCM, Coelho CMM, Souza CA, Mathias V (2019) Callose accumulation in roots of soybean seedlings under water deficit. Theor Exp Plant Physiol 31(4):475–481
Ensikat HJ, Weigend M (2013) Cryo-scanning electron microscopy of plant samples without metal coating, utilizing bulk conductivity. Microsc Anal 27(6):7–10
Esau K (1977) Anatomy of seed plants, 2nd edn. Wiley, New York
Escamez S, André D, Sztojka B, Bollhöner B, Hall H, Berthet B et al (2020) Cell death in cells overlying lateral root primordia facilitates organ growth in Arabidopsis. Curr Biol 30(3):455–464
Fu X, Harberd NP (2003) Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 421(6924):740–743
Garnik EY, Tarasenko VI, Gorbunova AI, Shmakov VN, Konstantinov YM (2019) Genome uncoupled (gun) phenotype is associated with root growth repression in Arabidopsis seedlings grown on lincomycin. Theor Exp Plant Physiol 31(4):445–454
Gierth M, Mäser P, Schroeder JI (2005) The potassium transporter AtHAK5 functions in K+ deprivation-induced high-affinity K+ uptake and AKT1 K+ channel contribution to K+ uptake kinetics in Arabidopsis roots. Plant Physiol 137(3):1105–1114
Grignon N, Touraine B, Durand M (1989) 6 (5) Carboxyfluorescein as a tracer of phloem sap translocation. Am J Bot 76(6):871–877
Handley R, Overstreet R (1961) Uptake of calcium & chlorine in roots of Zea mays. Plant Physiol 36(6):766
Heim A, Brunner I, Frey B, Frossard E, Luster J (2001) Root exudation, organic acids, and element distribution in roots of Norway spruce seedlings treated with aluminum in hydroponics. J Plant Nutr Soil Sci 164(5):519–526
Jeschke WD, Stelter W (1976) Mesurement of longitudinal ion profiles in single roots of Hordeum and Atriplex by use of flameless atomic absorption spectroscopy. Planta 128(2):107–112
Jones R, Ougham H, Thomas H, Waaland S (2012) Molecular life of plants. Wiley-Blackwell, Chichester
Kumari A, Das P, Parida AK, Agarwal PK (2015) Proteomics, metabolomics, and ionomics perspectives of salinity tolerance in halophytes. Front Plant Sci 6:537
Lahner B, Gong J, Mahmoudian M, Smith EL, Abid KB, Rogers EE et al (2003) Genomic scale profiling of nutrient and trace elements in Arabidopsis thaliana. Nat Biotechnol 21(10):1215–1221
Langer K, Ache P, Geiger D, Stinzing A, Arend M, Wind C et al (2002) Poplar potassium transporters capable of controlling K+ homeostasis and K+-dependent xylogenesis. Plant J 32(6):997–1009
Läuchli A, Spurr AR, Epstein E (1971) Lateral transport of ions into the xylem of corn roots: II. Evaluation of a stelar pump. Plant Physiol 48(2):118–124
Lee S, Oh MM (2021) Electric stimulation promotes growth, mineral uptake, and antioxidant accumulation in kale (Brassica oleracea var. acephala). Bioelectrochemistry 138:107727
Lee RB, Ratcliffe RG (1993) Subcellular distribution of inorganic phosphate, and levels of nucleoside triphosphate, in mature maize roots at low external phosphate concentrations: measurements with 31P-NMR. J Exp Bot 44(3):587–598
Li L, Luo Y, Li R, Zhou Q, Peijnenburg WJ, Yin N et al (2020) Effective uptake of submicrometre plastics by crop plants via a crack-entry mode. Nat Sustain 3(11):929–937
Lombi E, Zhao FJ, Fuhrmann M, Ma LQ, McGrath SP (2002) Arsenic distribution and speciation in the fronds of the hyperaccumulator Pteris vittata. New Phytol 156(2):195–203
Lyubenova L, Pongrac P, Vogel-Mikuš K, Mezek GK, Vavpetič P, Grlj N et al (2013) The fate of arsenic, cadmium and lead in Typha latifolia: a case study on the applicability of micro-PIXE in plant ionomics. J Hazard Mater 248:371–378
Malangisha GK, Yang Y, Moustafa-Farag M, Fu Q, Shao W, Wang J et al (2020) Subcellular distribution of aluminum associated with differential cell ultra-structure, mineral uptake, and antioxidant enzymes in root of two different Al+3-resistance watermelon cultivars. Plant Physiol Biochem 155:613–625
Malhotra H, Sharma S, Pandey R (2018) Phosphorus nutrition: plant growth in response to deficiency and excess. In: Plant nutrients and abiotic stress tolerance, pp 171–190. Springer, Singapore
Mäser P, Gierth M, Schroeder JI (2002) Molecular mechanisms of potassium and sodium uptake in plants. In: Progress in Plant Nutrition: Plenary Lectures of the XIV International Plant Nutrition Colloquium, pp 43–54. Springer, Dordrecht
Miller AJ, Cookson SJ, Smith SJ, Wells DM (2001) The use of microelectrodes to investigate compartmentation and the transport of metabolized inorganic ions in plants. J Exp Bot 52(356):541–549
Moore DP, Overstreet R, Jacobson L (1961) Uptake of magnesium & its interaction with calcium in excised barley roots. Plant Physiol 36(3):290
Moradi AB, Swoboda S, Robinson B, Prohaska T, Kaestner A, Oswald SE et al (2010) Mapping of nickel in root cross-sections of the hyperaccumulator plant Berkheya coddii using laser ablation ICP-MS. Environ Exp Bot 69(1):24–31
Murphy JA (2002) Designing a microscopy/analytical instrumentation facility: step by step procedure. Microsc Today 10(6):36–41
Newman IA (2001) Ion transport in roots: measurement of fluxes using ion-selective microelectrodes to characterize transporter function. Plant Cell Environ 24(1):1–14
Norton GJ, Aitkenhead MJ, Khowaja FS, Whalley WR, Price AH (2008) A bioinformatic and transcriptomic approach to identifying positional candidate genes without fine mapping: an example using rice root-growth QTLs. Genomics 92(5):344–352
Pearce RS (2001) Plant freezing and damage. Ann Bot 87(4):417–424
Pesacreta TC, Acharya A, Hasenstein KH (2021) Endogenous nutrients are concentrated in specific tissues in the Zea mays seedling. Protoplasma 258(4):863–878
Pesacreta TC, Carley WW, Webb WW, Parthasarathy MV (1982) F-actin in conifer roots. Proc Natl Acad Sci USA 79(9):2898–2901
Pesacreta TC, Lucas WJ (1985) Presence of a partially-coated reticulum in angiosperms. Protoplasma 125(3):173–184
Pesacreta TC (2015) F-actin distribution in root primary tissues of several seed plant species. Am J Bot 102(9):1422–1433
Pesacreta TC, Hasenstein KH (2018) Tissue accumulation patterns and concentrations of potassium, phosphorus, and carboxyfluorescein translocated from pine seed to the root. Planta 248(2):393–407
Pesacreta TC, Purpera MA (2014) Light microscopy survey of extant gymnosperm root protophloem and comparison with basal angiosperms. Botany 92(5):388–401
Phothiset S, Charoenrein S (2014) Effects of freezing and thawing on texture, microstructure and cell wall composition changes in papaya tissues. J Sci Food Agric 94(2):189–196
Pita-Barbosa A, Ricachenevsky FK, Flis PM (2019) One “OMICS” to integrate them all: ionomics as a result of plant genetics, physiology and evolution. Theor Exp Plant Physiol 31(1):71–89
Pitman MG (1969) Adaptation of barley roots to low oxygen supply and its relation to potassium and sodium uptake. Plant Physiol 44(9):1233–1240
Pritchard J, Fricke W, Tomos D (1997) Turgor-regulation during extension growth and osmotic stress of maize roots. An example of single-cell mapping. In: Plant roots-from cells to systems, pp 11–21. Springer, Dordrecht
Punshon T, Guerinot ML, Lanzirotti A (2009) Using synchrotron X-ray fluorescence microprobes in the study of metal homeostasis in plants. Ann Bot 103(5):665–672
Quadir QF, Watanabe T, Chen Z, Osaki M, Shinano T (2011) Ionomic response of Lotus japonicus to different root-zone temperatures. Soil Sci Plant Nutr 57(2):221–232
Rahman M, Mostofa MG, Keya SS, Rahman A, Das AK, Islam R et al (2021) Acetic acid improves drought acclimation in soybean: an integrative response of photosynthesis, osmoregulation, mineral uptake and antioxidant defense. Physiol Plant 172(2):334–350
Ramos I, Esteban E, Lucena JJ, Gárate A (2002) Cadmium uptake and subcellular distribution in plants of Lactuca sp. Cd–Mn interaction. Plant Sci 162(5):761–767
Robinson BH, Lombi E, Zhao FJ, McGrath SP (2003) Uptake and distribution of nickel and other metals in the hyperaccumulator Berkheya coddii. New Phytol 158(2):279–285
Roudier F, Fedorova E, Lebris M, Lecomte P, Györgyey J, Vaubert D et al (2003) The Medicago species A2-type cyclin is auxin regulated and involved in meristem formation but dispensable for endoreduplication-associated developmental programs. Plant Physiol 131(3):1091–1103
Santos MP, Zandonadi DB, de Sa AFL, Costa EP, de Oliveira CJL, Perez LE, Façanha AR, Bressan-Smith R (2020) Abscisic acid-nitric oxide and auxin interaction modulates salt stress response in tomato roots. Theor Exp Plant Physiol 32(4):301–313
Schachtman DP, Reid RJ, Ayling SM (1998) Phosphorus uptake by plants: from soil to cell. Plant Physiol 116(2):447–453
Serralta-Interian AA, de Lourdes Miranda-Ham M, Echevarría-Machado I (2020) Stimulation of root growth and enhanced nitrogenous metabolite content in habanero pepper (Capsicum chinense Jacq.) treated with ad-amino acid mixture. Theor Exp Plant Physiol 32(1):31–47
Shen Q, Yu J, Fu L, Wu L, Dai F, Jiang L et al (2018) Ionomic, metabolomic and proteomic analyses reveal molecular mechanisms of root adaption to salt stress in Tibetan wild barley. Plant Physiol Biochem 123:319–330
Singh UM, Sareen P, Sengar RS, Kumar A (2013) Plant ionomics: a newer approach to study mineral transport and its regulation. Acta Physiol Plant 35(9):2641–2653
Storey R, Leigh RA (2004) Processes modulating calcium distribution in citrus leaves. An investigation using X-ray microanalysis with strontium as a tracer. Plant Physiol 136(3):3838–3848
Tajima R, Abe J, Lee ON, Morita S, Lux A (2008) Developmental changes in peanut root structure during root growth and root-structure modification by nodulation. Ann Bot 101(4):491–499
Thomas CL, Alcock TD, Graham NS, Hayden R, Matterson S, Wilson L et al (2016) Root morphology and seed and leaf ionomic traits in a Brassica napus L. diversity panel show wide phenotypic variation and are characteristic of crop habit. BMC Plant Biol 16(1):1–18
Tian SK, Lu LL, Yang XE, Labavitch JM, Huang YY, Brown P (2009) Stem and leaf sequestration of zinc at the cellular level in the hyperaccumulator Sedum alfredii. New Phytol 182(1):116–126
Toomer OT (2018) Nutritional chemistry of the peanut (Arachis hypogaea). Crit Rev Food Sci Nutr 58(17):3042–3053
Tromp J (1962) Interactions in the absorption of ammonium, potassium, and sodium ions by wheat roots. Acta Bot Neerl 11(2):147–192
Vaculík M, Konlechner C, Langer I, Adlassnig W, Puschenreiter M, Lux A, Hauser MT (2012) Root anatomy and element distribution vary between two Salix caprea isolates with different Cd accumulation capacities. Environ Pollut 163:117–126
Wakeel A, Farooq M, Qadir M, Schubert S (2011) Potassium substitution by sodium in plants. Crit Rev Plant Sci 30(4):401–413
Wang YH, Acharya A, Burrell AM, Klein RR, Klein PE, Hasenstein KH (2013) Mapping and candidate genes associated with saccharification yield in sorghum. Genome 56(11):659–665
Weigel HJ, Jäger HJ (1980) Subcellular distribution and chemical form of cadmium in bean plants. Plant Physiol 65(3):480–482
White PJ, Broadley MR (2003) Calcium in plants. Ann Bot 92(4):487–511
Woll K, Borsuk LA, Stransky H, Nettleton D, Schnable PS, Hochholdinger F (2005) Isolation, characterization, and pericycle-specific transcriptome analyses of the novel maize lateral and seminal root initiation mutant rum1. Plant Physiol 139(3):1255–1267
Yang SY, Huang TK, Kuo HF, Chiou TJ (2017) Role of vacuoles in phosphorus storage and remobilization. J Exp Bot 68(12):3045–3055
Yoshimoto N, Takahashi H, Smith FW, Yamaya T, Saito K (2002) Two distinct high-affinity sulfate transporters with different inducibilities mediate uptake of sulfate in Arabidopsis roots. Plant J 29(4):465–473
Zhong H, Läuchli A (1994) Spatial distribution of solutes, K, Na, Ca and their deposition rates in the growth zone of primary cotton roots: effects of NaCl and CaCl2. Planta 194(1):34–41
Zhu Z, Li D, Wang P, Li J, Lu X (2020) Transcriptome and ionome analysis of nitrogen, phosphorus and potassium interactions in sorghum seedlings. Theor Exp Plant Physiol 32(4):271–285
Acknowledgements
I acknowledge my dissertation committee member Dr. Karl H. Hasenstein for helping me with the ICP-OES analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Acharya, A., Pesacreta, T.C. Localization of seed-derived and externally supplied nutrients in peanut seedling root. Theor. Exp. Plant Physiol. 34, 37–51 (2022). https://doi.org/10.1007/s40626-021-00227-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40626-021-00227-9