Abstract
Background
Hypercalcemia is highly prevalent in kidney transplant recipients with hyperparathyroidism. However, its long-term impact on graft function is uncertain.
Methods
We conducted a prospective cohort study investigating adverse graft outcomes associated with persistent hypercalcemia (free calcium > 5.2 mg/dL in ≥ 80% of measures) and inappropriately elevated intact parathyroid hormone (> 30 pg/mL) in kidney transplant recipients. Asymptomatic mild hypercalcemia was monitored unless complications developed.
Results
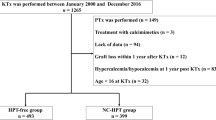
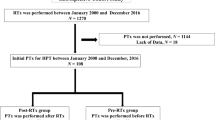
We included 385 kidney transplant recipients. During a 4-year (range 1–9) median follow-up time, 62% of kidney transplant recipients presented persistent hypercalcemia. Compared to kidney transplant recipients without hypercalcemia, there were no significant differences in graft dysfunction (10% vs. 12%, p = 0.61), symptomatic urolithiasis (5% vs. 3%, p = 0.43), biopsy-proven calcium deposits (6% vs. 5%, p = 1.0), fractures (6% vs. 4%, p = 0.64), and a composite outcome of urolithiasis, calcium deposits, fractures, and parathyroidectomy indication (16% vs. 13%, p = 0.55). In a subset of 76 kidney transplant recipients, subjects with persistent hypercalcemia had higher urinary calcium (median 84 [43–170] vs. 38 [24–64] mg/day, p = 0.03) and intact fibroblast growth factor 23 (median 36 [24–54] vs. 27 [19–40] pg/mL, p = 0.04), and lower 25-hydroxyvitamin D levels (11.3 ± 1.2 vs. 16.3 ± 1.4 ng/mL, p < 0.001). In multivariate analysis, pretransplant intact parathyroid hormone < 300 pg/mL was associated with a reduced risk of post-transplant hypercalcemia (OR 0.51, 95% CI 0.32–0.80).
Conclusions
Long-term persistent mild hypercalcemia (tertiary hyperparathyroidism) was frequent in kidney transplant recipients in our series. This condition presented with lower phosphate and 25-hydroxyvitamin D, and higher urinary calcium and intact fibroblast growth factor 23 levels compared to kidney transplant recipients without hypercalcemia, resembling a mild form of primary hyperparathyroidism. Despite these metabolic derangements, the risk of adverse graft outcomes was low.
Graphical abstract





Similar content being viewed by others
Data availability statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Abbreviations
- KTR:
-
Kidney transplant recipient.
- IQR:
-
Interquartile range.
- PTH:
-
Parathyroid hormone.
- iPTH:
-
Intact parathyroid hormone.
- DSA:
-
Donor-specific HLA-antibodies.
- eGFR:
-
Estimated glomerular filtration rate.
- ESRD:
-
End stage renal disease
- iFGF23:
-
Intact fibroblast growth factor 23.
- OPG:
-
Osteoprotegerin.
- OC:
-
Osteocalcin.
- OPN:
-
Osteopontin.
- 25(OH)D:
-
25-Hydroxyvitamin D.
- IL-6:
-
Interleukin-6.
- BMD:
-
Bone mineral density.
- DEXA:
-
Dual-energy X-ray absorptiometry.
- CV:
-
Coefficient of variation.
- SD:
-
Standard deviation.
- ROC:
-
Receiver operating characteristic.
- AUC:
-
Area under the curve.
- URL:
-
Upper reference limit.
- CI:
-
Confidence interval.
- ALP:
-
Alkaline phosphatase.
- CKD:
-
Chronic kidney disease.
- ADPKD:
-
Autosomal dominant polycystic kidney disease.
- SLE:
-
Systemic lupus erythematosus.
- HLA:
-
Human leukocyte antigen
References
Brandenburg VM, Westenfeld R, Ketteler M (2004) The fate of bone after renal transplantation. J Nephrol 7:190–204
Reinhardt W, Bartelworth H, Jockenhövel F et al (1998) Sequential changes of biochemical bone parameters after kidney transplantation. Nephrol Dial Transplant 13:436–442. https://doi.org/10.1093/oxfordjournals.ndt.a027843
Alfieri C, Mattinzoli D, Messa P (2021) Tertiary, and postrenal transplantation hyperparathyroidism. Endocrinol Metab Clin N Am 50:649–662. https://doi.org/10.1016/j.ecl.2021.08.004
Evenepoel P, Claes K, Kuypers D, Maes B, Bammens B, Vanrenterghem Y (2004) Natural history of parathyroid function and calcium metabolism after kidney transplantation: a single-centre study. Nephrol Dial Transplant 19:1281–1287. https://doi.org/10.1093/ndt/gfh128
Wolf M, Weir MR, Kopyt N et al (2016) A prospective cohort study of mineral metabolism after kidney transplantation. Transplantation 100:184–193. https://doi.org/10.1097/TP.0000000000000823
Pihlstrøm H, Dahle DO, Mjøen G et al (2015) Increased risk of all-cause mortality and renal graft loss in stable renal transplant recipients with hyperparathyroidism. Transplantation 99:351–359. https://doi.org/10.1097/TP.0000000000000583
van der Plas WY, Gomes Neto AW, Berger SP et al (2021) Association of time-updated plasma calcium and phosphate with graft and patient outcomes after kidney transplantation. Am J Transplant 21:2437–2447. https://doi.org/10.1111/ajt.16457
Moe SM (2017) Calcium as a cardiovascular toxin in CKD-MBD. Bone 100:94–99. https://doi.org/10.1016/j.bone.2016.08.022
Çeltik A, Şen S, Yılmaz M et al (2016) The effect of hypercalcemia on allograft calcification after kidney transplantation. Int Urol Nephrol 48:1919–1925. https://doi.org/10.1007/s11255-016-1391-z
Ridefelt P, Helmersson-Karlqvist J (2017) Albumin adjustment of total calcium does not improve the estimation of calcium status. Scand J Clin Lab Investig 77:442–447. https://doi.org/10.1080/00365513.2017.1336568
Guerra R, Auyanet I, Fernández EJ et al (2011) Hypercalcemia secondary to persistent hyperparathyroidism in kidney transplant patients: analysis after a year with cinacalcet. J Nephrol 24:78–82. https://doi.org/10.5301/jn.2010.293
Ramírez-Sandoval JC (2020) Renal metabolic bone disease in the potential kidney transplant recipient. Rev Mex Traspl 9:68–74
Fonseca-Correa JI, Nava-Santana C, Tamez-Pedroza L et al (2021) Clinical factors associated with early and persistent hypocalcaemia after parathyroidectomy in patients on dialysis with severe hyperparathyroidism. Nephrology (Carlton) 26:408–419. https://doi.org/10.1111/nep.13854
Inker LA, Eneanya ND, Coresh J et al (2021) New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med 385:1737–1749. https://doi.org/10.1056/NEJMoa2102953
Bilezikian JP, Khan AA, Silverberg SJ et al (2022) International workshop on primary hyperparathyroidism. Evaluation and management of primary hyperparathyroidism: summary statement and guidelines from the fifth international workshop. J Bone Miner Res 37:2293–2314. https://doi.org/10.1002/jbmr.4677
Cusano NE, Bilezikian JP (2014) Parathyroid hormone in the evaluation of hypercalcemia. JAMA 312:680–681. https://doi.org/10.1001/jama.2014.9195
Ramirez-Sandoval JC, Diener-Cabieses P, Gutiérrez-Valle F et al (2022) Validation of an equation for free calcium estimation: accuracy improves after adjustment for phosphate and CO2. Int Urol Nephrol 54:2625–2635. https://doi.org/10.1007/s11255-022-03170-z
Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group (2011) KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl 7:1–59. https://doi.org/10.1016/j.kisu.2017.04.001
Miedziaszczyk M, Lacka K, Tomczak O, Bajon A, Primke M, Idasiak-Piechocka I (2022) Systematic review of the treatment of persistent hyperparathyroidism following kidney transplantation. Biomedicines 11(1):25. https://doi.org/10.3390/biomedicines11010025
Bonarek H, Merville P, Bonarek M et al (1999) Reduced parathyroid functional mass after successful kidney transplantation. Kidney Int 56:642–649. https://doi.org/10.1046/j.1523-1755.1999.00589.x
Westeel FP, Mazouz H, Ezaitouni F et al (2000) Cyclosporine bone remodeling effect prevents steroid osteopenia after kidney transplantation. Kidney Int 58:1788–1796. https://doi.org/10.1046/j.1523-1755.2000.00341.x
Rayego-Mateos S, Doladé N, García-Carrasco A, Diaz-Tocados JM, Ibarz M, Valdivielso JM (2022) The increase in FGF23 induced by calcium is partially dependent on vitamin D signaling. Nutrients 14:2576. https://doi.org/10.3390/nu14132576
David V, Dai B, Martin A, Huang J, Han X, Quarles LD (2013) Calcium regulates FGF-23 expression in bone. Endocrinology 154:4469–4482. https://doi.org/10.1210/en.2013-1627
Dusso AS, Rodriguez M (2012) Enhanced induction of Cyp24a1 by FGF23 but low serum 24,25-dihydroxyvitamin D in CKD: implications for therapy. Kidney Int 82:1046–1049. https://doi.org/10.1038/ki.2012.273
Stavroulopoulos A, Cassidy MJ, Porter CJ, Hosking DJ, Roe SD (2007) Vitamin D status in renal transplant recipients. Am J Transplant 7:2546–2552. https://doi.org/10.1111/j.1600-6143.2007.01978.x
Perrin P, Caillard S, Javier RM et al (2013) Persistent hyperparathyroidism is a major risk factor for fractures in the five years after kidney transplantation. Am J Transplant 13:2653–2663. https://doi.org/10.1111/ajt.12425
Saponaro F, Cetani F, Mazoni L et al (2020) Hypercalciuria: its value as a predictive risk factor for nephrolithiasis in asymptomatic primary hyperparathyroidism? J Endocrinol Investig 43:677–682. https://doi.org/10.1007/s40618-019-01162-y
Bilezikian JP (2000) Primary hyperparathyroidism. When to observe and when to operate. Endocrinol Metab Clin N Am 29:465–478. https://doi.org/10.1016/s0889-8529(05)70146-8
Sutton W, Chen X, Patel P et al (2022) Prevalence and risk factors for tertiary hyperparathyroidism in kidney transplant recipients. Surgery 171:69–76. https://doi.org/10.1016/j.surg.2021.03.067
National Kidney Foundation (2003) K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42:S1–201
Levine M, Ensom MH (2001) Post hoc power analysis: an idea whose time has passed? Pharmacotherapy 21:405–409. https://doi.org/10.1592/phco.21.5.405.34503
Zavatta G, Clarke BL (2021) Normocalcemic primary hyperparathyroidism: need for a standardized clinical approach. Endocrinol Metab (Seoul) 36(3):525–535. https://doi.org/10.3803/EnM.2021.1061
Ramirez-Sandoval JC, Casanova I, Villar A, Gomez FE, Cruz C, Correa-Rotter R (2016) Biomarkers associated with vascular calcification in peritoneal dialysis. Perit Dial Int 36:262–268. https://doi.org/10.3747/pdi.2014.00250
Acknowledgements
We thank the study participants and Dr. Hugo E. Chávez-Chávez for help in coding subjects data for the period from 2012 through 2014.
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
JCR-S: participated in all phases of the study: conception and design, patient recruitment and data acquisition, data analysis and interpretation, drafting of manuscript, revision and approval of final version. LM: participated in all phases of the study: conception and design, patient recruitment and data acquisition, data analysis and interpretation, drafting of manuscript, revision and approval of final version. GC-K: participated in data acquisition, data analysis and interpretation, drafting of manuscript, revision and approval of final version. ER-L: participated in patient recruitment and data acquisition and data analysis. NDP-C: participated in patient recruitment and data acquisition and data analysis. CC: participated in patient recruitment and data acquisition, data analysis, and contributed to new reagents or analytic tools. ENH-P: participated conception and design, patient recruitment and data acquisition, and data analysis and interpretation. NB-P: participated conception and design, patient recruitment and data acquisition, and data analysis and interpretation. VV-R: participated in the performance of the research. JMEL: participated in the performance of the research. RC-R: participated in conception and design, data analysis and interpretation, drafting of manuscript, revision and approval of final version. AAR-A: participated in conception and design, data analysis and interpretation, drafting of manuscript, revision and approval of final version. LEM-B: participated in conception and design, data analysis and interpretation, drafting of manuscript, revision and approval of final version.
Corresponding author
Ethics declarations
Conflict of interest
The results presented in this paper have not been published previously. The authors of this paper declare that the paper is original, is not plagiarized, is submitted for first publication in this journal, and has not been published or submitted for publication elsewhere, and that there is no affiliation with any organization with a direct or indirect financial interest in the subject matter discussed in the manuscript that may affect the reporting of the article submitted. No pharmaceutical or biotechnology company, foundation, or any other source participated in the design, monitoring, data collection, and analysis. Dr. Juan Carlos Ramírez-Sandoval is a member of the Review Board of Frontiers in medicine, has received honoraria from Bayer México, and has lectured for Abbvie, Amgen, Synthom, Bayer, and AstraZeneca. Dr. Ricardo Correa-Rotter is a member of the Review Board of Blood Purification, has received honoraria as consultant from AbbVie, AstraZeneca, GlaxoSmithKline, Bayer, and Boehringer Ingelheim, participates in research from: Boehringer Ingelheim, Baxter, AstraZeneca and has lectured for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim and Sanofi. Luis E. Morales-Buenrostro has received honoraria from AstraZeneca, Boehringer Ingelheim, Sanofi, Asofarma, and Roche. Lluvia Marino Gabriel Cojuc-Konigsberg, Estefania Reul-Linares, Nathalie Desire Pichardo-Cabrera, Cristino Cruz, Elisa Naomi Hernández-Paredes, Nathan Berman-Parks, Vanesa Vidal Ruiz, Jonathan Mauricio Estrada Linares, and Alfredo A. Reza-Albarrán have no competing interest to declare.
Ethical approval
This study was approved by the local Human Research and Ethics Boards (NMM-4220-22-23-1) and adhered to the principles outlined in the 1964 Helsinki Declaration and its subsequent amendments.
Human and animal rights
This study does not contain any studies with animals performed by any of the authors.
Informed consent
Participants who underwent biomarker assessment provided informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramirez-Sandoval, J.C., Marino, L., Cojuc-Konigsberg, G. et al. Long-term effects of hypercalcemia in kidney transplant recipients with persistent hyperparathyroidism. J Nephrol (2023). https://doi.org/10.1007/s40620-023-01815-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40620-023-01815-5