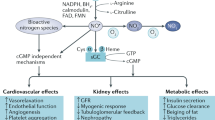
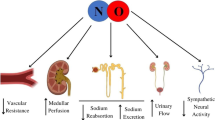
Abstract
Nitric oxide (NO) is a gas with biological and regulatory properties, produced from arginine by the way of nitric oxide synthases (NOS), and with a very short half-life (few seconds). A “coupled” NOS activity leads to NO generation, whereas its uncoupling produces the reactive oxygen species peroxynitrite (ONOO−). Uncoupling is usually due to inflammation, oxidative stress, decreased cofactor availability, or excessive NO production. Competitive inhibitors of NO production are post-translationally methylated arginine residues in proteins, which are constantly released into the circulation. NO availability is altered in many clinical conditions associated with vascular dysfunction, such as diabetes mellitus. The kidney plays an important role in body NO homeostasis. This article provides an overview of current literature, on NO production/availability, with a focus on diabetic nephropathy. In diabetes, NO availability is usually decreased (with exception of the early, hyper filtration phase of nephropathy in Type 1 diabetes), and it could constitute a factor of the generalized vasculopathy present in diabetic nephropathy. NO generation in Type 2 diabetes with nephropathy is inversely associated with the dimethyl-arginine concentrations, which are therefore important modulators of NO synthesis independently from the classic stimulatory pathways (such as the insulin effect). A disturbed NO metabolism is present in diabetes associated with nephropathy. Although modulation of NO production is not yet a common therapeutical strategy, a number of yet experimental compounds need to be tested as potential interventions to treat the vascular dysfunction and nephropathy in diabetes, as well as in other diseased states. Finally, in diabetic nephropathy NO deficiency may be associated to that of hydrogen sulfide, another interesting gaseous mediator which is increasingly investigated.



Similar content being viewed by others
References
Moncada S, Higgs A (1993) The L-arginine-nitric oxide pathway. N Engl J Med 329(27):2002–2012
Furchgott RF, Zawadzki JV (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288(5789):373–376
Förstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33(7):829–837
Boudko DY (2007) Bioanalytical profile of the L-arginine/nitric oxide pathway and its evaluation by capillary electrophoresis. J Chromatogr B Analyt Technol Biomed Life Sci 851(1–2):186–210
Dejam A, Hunter CJ, Pelletier MM et al (2005) Erythrocytes are the major intravascular storage sites of nitrite in human blood. Blood 106(2):734–739
Recchia FA, Vogel TR, Hintze TH (2000) NO metabolites accumulate in erythrocytes in proportion to carbon dioxide and bicarbonate concentration. Am J Physiol Heart Circ Physiol 279(2):H852–H856
Nathan C, Xie QW (1994) Regulation of biosynthesis of nitric oxide. J Biol Chem 269(19):13725–13728
Chen K, Popel AS (2007) Vascular and perivascular nitric oxide release and transport: biochemical pathways of neuronal nitric oxide synthase (NOS1) and endothelial nitric oxide synthase (NOS3). Free Radic Biol Med 42(6):811–822
Haque MM, Panda K, Tejero J et al (2007) A connecting hinge represses the activity of endothelial nitric oxide synthase. Proc Natl Acad Sci USA 104(22):9254–9259
Mann GE, Yudilevich DL, Sobrevia L (2003) Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol Rev 83(1):183–252
Montezano AC, Touyz RM (2012) Reactive oxygen species and endothelial function—role of NOS uncoupling and Nox family NADPH oxidases. Basic Clin Pharmacol Toxicol 110(1):87–94
Leiper J, Nandi M (2011) The therapeutic potential of targeting endogenous inhibitors of nitric oxide synthesis. Nat Rev Drug Discov 10(4):277–291
Davids M, Swieringa E, Palm F et al (2012) Simultaneous determination of asymmetric and symmetric dimethylarginine, L-monomethylarginine, L-arginine, and L-homoarginine in biological samples using stable isotope dilution liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 900:38–47
López-Jaramillo P, Arenas WD, García RG et al (2008) The role of the L-arginine-nitric oxide pathway in preeclampsia. Ther Adv Cardiovasc Dis 2(4):261–275
Leighton F, Urquiaga I (2007) Endothelial nitric oxide synthase as a mediator of the positive health effects of Mediterranean diets and wine against metabolic syndrome. World Rev Nutr Diet 97:33–51
Shpektor A (2010) Cardiogenic shock: the role of inflammation. Acute Card Care 12(4):115–118
Kayali Z, Herring J, Baron P et al (2009) Increased plasma nitric oxide, L-arginine, and arginase-1 in cirrhotic patients with progressive renal dysfunction. J Gastroenterol Hepatol 24(6):1030–1037
Böger RH, Diemert A, Schwedhelm E et al (2010) The role of nitric oxide synthase inhibition by asymmetric dimethylarginine in the pathophysiology of preeclampsia. Gynecol Obstet Invest 69(1):1–13
Münzel T, Daiber A, Gori T (2011) Nitrate therapy: new aspects concerning molecular action and tolerance. Circulation 123(19):2132–2144 Review
Mount PF, Power DA (2006) Nitric oxide in the kidney: functions and regulation of synthesis. Acta Physiol (Oxf) 187(4):433–446
Rippin JD, Patel A, Belyaev ND et al (2003) Nitric oxide synthase gene polymorphisms and diabetic nephropathy. Diabetologia 46(3):426–428
Welch WJ, Wilcox CS (1997) Macula densa arginine delivery and uptake in the rat regulates glomerular capillary pressure. Effects of salt intake. J Clin Invest 100(9):2235–2242
Hall CN, Garthwaite J (2009) What is the real physiological NO concentration in vivo? Nitric Oxide 21(2):92–103
Brosnan ME, Brosnan JT (2004) Renal arginine metabolism. J Nutrition 134:2791S–2797S
Kakoki M, Kim HS, Edgell CJS et al (2006) Amino acids as modulators of endothelium-derived nitric oxide. Am J Physiol Renal Physiol 291(2):F297–F304
Wu F, Cholewa B, Mattson DL (2000) Characterization of L-arginine transporters in rat renal inner medullary collecting duct. Am J Physiol Regul Integr Comp Physiol 278(6):R1506–R1512
Zoccali C (2007) The endothelium as a target in renal diseases. J Nephrol 20(Suppl 12):S39–S44
Lahera V, Salom MG, Miranda-Guardiola F et al (1991) Effects of NG-nitro-L-arginine methyl ester on renal function and blood pressure. Am J Physiol 261(6 Pt 2):F1033–F1037
Aulak KS, Liu J, Wu J et al (1996) Molecular sites of regulation of expression of the rat cationic amino acid transporter gene. J Biol Chem 271:29799–29806
Kakoki M, Kim H-S, Arendshorst W et al (2004) L-Arginine uptake affects nitric oxide production and blood flow in the renal medulla. Am J Physiol Regul Integr Comp Physiol 287(6):R1478–R1485
Dallinger S, Sieder A, Strametz J et al (2003) Vasodilator effects of L-arginine are stereospecific and augmented by insulin in humans. Am J Physiol Endocrinol Metab 284(6):E1106–E1106
Ruiz M, Singh P, Thomson SC et al (2008) L-arginine-induced glomerular hyperfiltration response: the roles of insulin and ANG-II. Am J Physiol Regul Integr Comp Physiol 294(5):R1744–R1751
Rajapakse NW, Mattson DL (2009) Role of L-arginine in nitric oxide production in health and hypertension. Clin Exp Pharm Physiol 36:249–255
Gruden G, Viberti G (2005) Pathogenesis of diabetic nephropathy. In: Kahn CR (ed) Joslin’s diabetes mellitus, 14th edn. PA. Lippincott Williams and Wilkins, Philadelphia, pp 853–866
Verzola D, Gandolfo MT, Ferrario F et al (2007) Apoptosis in the kidneys of patients with type II diabetic nephropathy. Kidney Int 72(10):1262–1272
Verzola D, Cappuccino L, D’Amato E, et al. (2014) Enhanced glomerular Toll-like receptor 4 expression and signaling in patients with type 2 diabetic nephropathy and microalbuminuria. Kidney Int [Epub ahead of print]
Williams ME, Stanton RC (2005) Management of Diabetic Kidney Disease. In: Kahn CR (ed) Joslin’s Diabetes Mellitus, 14th edn. Lippincot Williams & Wilkins, Philadelphia
Zelmanovitz T, Gerchman F, Balthazar APS et al (2009) Diabetic nephropathy. Diabetol Metab Syndr 1(1):10
Rossing P (2005) The changing epidemiology of diabetic microangiopathy in type 1 diabetes. Diabetologia 48:1439–1444
Valmadrid CT, Klein R, Moss SE, Klein BE (2000) The risk of cardiovascular disease mortality associated with microalbuminuria and gross proteinuria in persons with older-onset diabetes mellitus. Arch Intern Med 160:1093–1100
Amanda Adler I, Richard Stevens J, on behalf of the UKPDS Group et al (2003) Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 63:225–232
American Diabetes Association (2004) Nephropathy in diabetes. Position statement. Diabetes Care 27:s79–s83
Reutens AT (2013) Epidemiology of diabetic kidney disease. Med Clin North Am 97(1):1–18
Eschwège E (2003) The dysmetabolic syndrome, insulin resistance and increased cardiovascular (CV) morbidity and mortality in type 2 diabetes: aetiological factors in the development of CV complications. Diabetes Metab 29(4 Pt 2):6S19–6S27 (Review)
Nakagawa T, Segal M, Croker B, Johnson RJ (2007) A breakthrough in diabetic nephropathy: the role of endothelial dysfunction. Nephrol Dial Transplant 22(10):2775–2777
Tufro A, Veron D (2012) VEGF and podocytes in diabetic nephropathy. Semin Nephrol 32(4):385–393
Valiatti FB, Crispim D, Benfica C et al (2011) The role of vascular endothelial growth factor in angiogenesis and diabetic retinopathy. Arq Bras Endocrinol Metab 55(2):106–113
Hargrove GM, Dufresne J, Whiteside C et al (2000) Diabetes mellitus increases endothelin-1 gene transcription in rat kidney. Kidney Int 58(4):1534–1545
Muniyappa R, Quon MJ (2007) Insulin action and insulin resistance in vascular endothelium. Curr Opin Clin Nutr Metab Care 10(4):523–530
Tessari P, Inchiostro S, Biolo G et al (1991) Effects of acute systemic hyperinsulinemia on forearm muscle proteolysis in healthy man. J Clin Invest 88(1):27–33
Tessari P, Biolo G, Inchiostro S et al (1990) Effects of insulin on whole body and forearm leucine and KIC metabolism in type 1 diabetes. Am J Physiol 259(1 Pt 1):E96–E103
Zanetti M, Barazzoni R, Kiwanuka E, Tessari P (1999) Effects of branched-chain-enriched amino acids and insulin on forearm leucine kinetics. Clin Sci (Lond) 97(4):437–448
Reikerås O, Gunnes P (1986) Effects of high doses of insulin on systemic haemodynamics and regional blood flows in dogs. Clin Physiol 6(2):129–138
Brownlee M (1992) Glycation products and the pathogenesis of diabetic complications. Diabetes Care 15(12):1835–1843 (Review)
Selemidis S, Sobey CG, Wingler K et al (2008) NADPH oxidases in the vasculature: molecular features, roles in disease and pharmacological inhibition. Pharmacol Ther 120(3):254–291 (Review)
Tian XY, Wang WT, Xu A et al (2012) Uncoupling protein-2 protects endothelial function in diet-induced obese mice. Circ Res 110(9):1211–1216
Tomita H, Sanford RB, Smithies O, Kakoki M (2012) The kallikrein-kinin system in diabetic nephropathy. Kidney Int 81(8):733–744
Conway BR, Maxwell AP (2009) Genetics of diabetic nephropathy: are there clues to the understanding of common kidney diseases? Nephron Clin Pract 112(4):c213–c221
Dellamea BS, Pinto LC, Leitão CB et al (2014) Endothelial nitric oxide synthase gene polymorphisms and risk of diabetic nephropathy: a systematic review and meta-analysis. BMC Med Genet 15:9
Kagawa Y, Cha SH, Hasegawa K et al (1999) Regulation of energy metabolism in human cells in aging and diabetes: FoF(1), mtDNA, UCP, and ROS. Biochem Biophys Res Commun 266(3):662–676
Lim SC, Goh SK, Lai YR et al (2006) Relationship between common functional polymorphisms of the p22phox gene (−930 A > G and +242 C > T) and nephropathy as a result of type 2 diabetes in a Chinese population. Diabet Med 23(9):1037–1041
Vozarova B, Fernández-Real JM et al (2003) The interleukin-6 (−174) G/C promoter polymorphism is associated with type-2 diabetes mellitus in Native Americans and Caucasians. Hum Genet 112(4):409–413
Kang P, Tian C, Jia C (2012) Association of RAGE gene polymorphisms with type 2 diabetes mellitus, diabetic retinopathy and diabetic nephropathy. Gene 500(1):1–9
Abhary S, Burdon KP, Kuot A et al (2010) Sequence variation in DDAH1 and DDAH2 genes is strongly and additively associated with serum ADMA concentrations in individuals with type 2 diabetes. PLoS One 5(3):e9462
Tanhäuserová V, Tomand J, Pácal L et al (2012) ADMA, SDMA and L-arginine/ADMA ratio but not DDAH genetic polymorphisms are reliable predictors of diabetic nephropathy progression as identified by competing risk analysis. Kidney Blood Press Res 36(1):200–208
Pieper GM (1999) Enhanced, unaltered and impaired nitric oxide-mediated endothelium-dependent relaxation in experimental diabetes mellitus: importance of disease duration. Diabetologia 42(2):204–213
Ishii N, Patel KP, Lane PH et al (2001) Nitric oxide synthesis and oxidative stress in the renal cortex of rats with diabetes mellitus. J Am Soc Nephrol 12(8):1630–1639
Komers R, Anderson S (2003) Paradoxes of nitric oxide in the diabetic kidney. Am J Physiol Renal Physiol 284(6):F1121–F1137
Prabhakar SS (2004) Role of nitric oxide in diabetic nephropathy. Semin Nephrol 24(4):333–344
Goligorsky, Chen J, Brodsky S (2001) Workshop: endothelial cell dysfunction leading to diabetic nephropathy: focus on nitric oxide. Hypertension 37(2 Part 2):744–748
Prabhakar S, Starnes J, Shi S et al (2007) Diabetic nephropathy is associated with oxidative stress and decreased renal nitric oxide production. J Am Soc Nephrol 18(11):2452–2945
Stehouver CD, Henry RM, Dekker JM et al (2004) Microalbuminuria is associated with impaired endothelium dependent, flow mediated vasodilation in elderly individuals without and with diabetes: further evidence for a link between microalbuminuria and endothelial dysfunction-the Hoorn Study. Kidney Int 92:S42–S44
Chu S, Bohlen HG (2004) High concentration of glucose inhibits glomerular endothelial eNOS through a PKC mechanism. Am J Physiol Renal Physiol 287(3):F384–F392
Tessari P, Cecchet D, Cosma A et al (2010) Nitric oxide synthesis is reduced in subjects with type 2 diabetes and nephropathy. Diabetes 59(9):2152–2159
Avogaro A, Toffolo G, Kiwanuka E et al (2003) L-arginine-nitric oxide kinetics in normal and type 2 diabetic subjects: a stable-labelled 15N arginine approach. Diabetes 52(3):795–802
Kashyap SR, Roman LJ, Lamont J et al (2005) Insulin resistance is associated with impaired nitric oxide synthase activity in skeletal muscle of type 2 diabetic subjects. J Clin Endocrinol Metab 90(2):1100–1105
Tessari P, Cecchet D, Artusi C et al (2013) Roles of insulin, age, and asymmetric dimethylarginine on nitric oxide synthesis in vivo. Diabetes 62(8):2699–2708
Badal SS, Danesh FR (2012) Strategies to reverse endothelial dysfunction in diabetic nephropathy. Kidney Int 82(11):1151–1154
Noori N, Tabibi H, Hosseinpanah F et al (2013) Effects of combined lipoic acid and pyridoxine on albuminuria, advanced glycation end-products, and blood pressure in diabetic nephropathy. Int J Vitam Nutr Res 83(2):77–85
Imanishi M, Okada N, Konishi Y et al (2013) Angiotensin II receptor blockade reduces salt sensitivity of blood pressure through restoration of renal nitric oxide synthesis in patients with diabetic nephropathy. J Renin Angiotensin Aldosterone Syst 14(1):67–73
Arima S, Kohagura K, Takeuchi K et al (2002) Biphasic vasodilator action of troglitazone on the renal microcirculation. J Am Soc Nephrol 13:342e9
Bolignano D, Zoccali C (2012) Glitazones in chronic kidney disease: potential and concerns. Nutr Metab Cardiovasc Dis 22(3):167–175
Pistrosch F, Passauer J, Herbrig K et al (2012) Effect of thiazolidinedione treatment on proteinuria and renal hemodynamic in type 2 diabetic patients with overt nephropathy. Horm Metab Res 44(12):914–918
Nakamura T, Ushiyama C, Shimada N et al (2000) Comparative effects of pioglitazone, glibenclamide, and voglibose on urinary endothelin-1 and albumin excretion. In diabetes patients. J Diabetes Complicat 14:250e4
Hanefeld M, Brunetti P, Schernthaner GH, QUARTET study group et al (2004) One-year glycemic control with a sulfonylurea plus pioglitazone versus a sulfonylurea plus metformin in patients with type 2 diabetes. Diabetes Care 27:141e7
Sironi AM, Vichi S, Gastaldelli A et al (1997) Effects of troglitazone on insulin action and cardiovascular risk factors in patients with non-insulin-dependent diabetes. Clin Pharmacol Ther 62:194–202
Agarwal R, Saha C, Battiwala M et al (2005) A pilot randomized controlled trial of renal protection with pioglitazone in diabetic nephropathy. Kidney Int 68:285–292
Cherney DZ, Perkins BA, Soleymanlou N et al (2014) Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129(5):587–597
Hills CE, Brunskill NJ, Squires PE (2010) C-peptide as a therapeutic tool in diabetic nephropathy. Am J Nephrol 31(5):389–397
Turgut F, Bolton WK (2010) Potential new therapeutic agents for diabetic kidney disease. Am J Kidney Dis 55(5):928–940
Coronel I, Arellano-Mendoza MG, del Valle-Mondragon L et al (2010) L-arginine and antioxidant diet supplementation partially restores nitric oxide-dependent regulation of phenylephrine renal vasoconstriction in diabetics rats. J Ren Nutr 20(3):158–168
Klahr S, Morrissey J (2004) L-arginine as a therapeutic tool in kidney disease. Semin Nephrol 24(4):389–394 (Review)
Verzola D, Bertolotto MB, Villaggio B et al (2002) Taurine prevents apoptosis induced by high ambient glucose in human tubule renal cells. J Investig Med 50(6):443–451
Pandya KG, Budhram R, Clark G, Lau-Cam CA (2013) Comparative evaluation of taurine and thiotaurine as protectants against diabetes-induced nephropathy in a rat model. Adv Exp Med Biol 775:371–394
Xiao L, Zhu X, Yang S et al (2014) Rap1 ameliorates renal tubular injury in diabetic nephropathy. Diabetes 63(4):1366–1380
Yuan F, Liu YH, Liu FY et al (2014) Intraperitoneal administration of the globular adiponectin gene ameliorates diabetic nephropathy in Wistar rats. Mol Med Rep 9(6):2293–2300
Matavelli LC, Siragy HM (2013) Reduction of aldosterone production improves renal oxidative stress and fibrosis in diabetic rats. J Cardiovasc Pharmacol 61(1):17–22
Ishizawa K, Izawa-Ishizawa Y, Yamano N et al (2014) Nitrosonifedipine ameliorates the progression of type 2 diabetic nephropathy by exerting antioxidative effects. PLoS One 9(1):e86335
Romero MJ, Yao L, Sridhar S et al (2013) l-Citrulline Protects from Kidney Damage in Type 1 Diabetic Mice. Front Immunol 4:480
Dessapt-Baradez C, Woolf AS, White KE et al (2014) Targeted glomerular angiopoietin-1 therapy for early diabetic kidney disease. J Am Soc Nephrol 25(1):33–42
Schildknecht S, Weber A, Gerding HR et al (2013) The NOX1/4 inhibitor GKT136901 as selective and direct scavenger of peroxynitrite. Curr Med Chem 21(3):365–376
You H, Gao T, Cooper TK et al (2013) Arginase inhibition mediates renal tissue protection in diabetic nephropathy by a nitric oxide synthase 3-dependent mechanism. Kidney Int 84(6):1189–1197
Gu Y, Gong Y, Zhang H et al (2013) Regulation of transforming growth factor beta 1 gene expression by dihydropteridine reductase in kidney 293T cells. Biochem Cell Biol 91(3):187–193
Kidokoro K, Satoh M, Channon KM et al (2013) Maintenance of endothelial guanosine triphosphate cyclohydrolase I ameliorates diabetic nephropathy. J Am Soc Nephrol 24(7):1139–1150
Cheng H, Wang H, Fan X et al (2012) Improvement of endothelial nitric oxide synthase activity retards the progression of diabetic nephropathy in db/db mice. Kidney Int 82(11):1176–1183
Asakura J, Hasegawa H, Takayanagi K et al (2012) Renoprotective effect of pioglitazone by the prevention of glomerular hyperfiltration through the possible restoration of altered macula densa signaling in rats with type 2 diabetic nephropathy. Nephron Exp Nephrol 122(3–4):83–94
Lin M, Yiu WH, Li RX et al (2013) The TLR4 antagonist CRX-526 protects against advanced diabetic nephropathy. Kidney Int 83(5):887–900
Ptilovanciv EO, Fernandes GS, Teixeira LC et al (2013) Heme oxygenase 1 improves glucoses metabolism and kidney histological alterations in diabetic rats. Diabetol Metab Syndr 5(1):3
Ojima A, Ishibashi Y, Matsui T et al (2013) Glucagon-like peptide-1 receptor agonist inhibits asymmetric dimethylarginine generation in the kidney of streptozotocin-induced diabetic rats by blocking advanced glycation end product-induced protein arginine methyltranferase-1 expression. Am J Pathol 182(1):132–141
Faria AM, Papadimitriou A, Silva KC et al (2012) Uncoupling endothelial nitric oxide synthase is ameliorated by green tea in experimental diabetes by re-establishing tetrahydrobiopterin levels. Diabetes 61(7):1838–1847
Park CH, Noh JS, Tanaka T, Yokozawa T (2012) 7-O-galloyl-D-sedoheptulose ameliorates renal damage triggered by reactive oxygen species-sensitive pathway of inflammation and apoptosis. J Pharm Pharmacol 64(12):1730–1740
Kuno Y, Iyoda M, Shibata T et al (2011) Sildenafil, a phosphodiesterase type 5 inhibitor, attenuates diabetic nephropathy in non-insulin-dependent Otsuka Long-Evans Tokushima Fatty rats. Br J Pharmacol 162(6):1389–1400
Zhou Y, Li JS, Zhang X et al (2010) Ursolic acid inhibits early lesions of diabetic nephropathy. Int J Mol Med 26(4):565–570
Schneider MP, Schneider A, Jumar A, et al. (2014) Effects of folic acid on renal endothelial function in patients with diabetic nephropathy: results from a randomized trial. Clin Sci (Lond) [Epub ahead of print]
Mose FH, Larsen T, Jensen JM, et al. (2014) Effects of atorvastatin on systemic and renal NO dependency in patients with non-diabetic stage II-III chronic kidney disease. Br J Clin Pharmacol. doi:10.1111/bcp.12390 [Epub ahead of print]
Mani S, Cao W, Wu L, et al. (2014) Hydrogen sulfide and the liver. Nitric Oxide. doi:10.1016/j.niox.2014.02.006 [Epub ahead of print] (Review)
Altaany Z, Moccia F, Munaron L, et al. (2014) Hydrogen sulfide and endothelial Dysfunction: relationship with nitric oxide. Curr Med Chem [Epub ahead of print]
Yuan P, Xue H, Zhou L et al (2011) Rescue of mesangial cells from high glucose-induced over-proliferation and extracellular matrix secretion by hydrogen sulfide. Nephrol Dial Transplant 26(7):2119–2126
Kundu S, Pushpakumar SB, Tyagi A et al (2013) Hydrogen sulfide deficiency and diabetic renal remodeling: role of matrix metalloproteinase-9. Am J Physiol Endocrinol Metab 304(12):E1365–E1378
Kundu S, Pushpakumar S, Khundmiri SJ, et al. (2014) Hydrogen sulfide mitigates hyperglycemic remodeling via liver kinase B1-adenosine monophosphate-activated protein kinase signaling. Biochim Biophys Acta. pii: S0167-4889(14)00304-8. doi:10.1016/j.bbamcr.2014.08.00
Xue H, Yuan P, Ni J et al (2013) H(2)S inhibits hyperglycemia-induced intrarenal renin-angiotensin system activation via attenuation of reactive oxygen species generation. PLoS One 8(9):e74366. doi:10.1371/journal.pone.0074366
Andrésdóttir G, Bakker SJ, Hansen HP et al (2013) Urinary sulphate excretion and progression of diabetic nephropathy in Type 1 diabetes. Diabet Med 30(5):563–566
Mizuno Y, Jacob RF, Mason RP (2010) Advances in pharmacologic modulation of nitric oxide in hypertension. Curr Cardiol Rep 12(6):472–480
Acknowledgment
Disclosure on financial support: the studies originally performed by the author have been supported by Grants from the Italian Minister of University and Research (“PRIN” Grants) and by Institutional Grants from the University of Padova.
Conflict of interest
Disclosure on conflict of interest: the Author doesn’t have any conflict of interest di declare.
Ethical standard
The clinical studies originally performed by the author have been approved by the local Institutional Review Board (IRB)/Ethics Committee, and have been performed according to the Declaration of Helsinki; a statement is made that informed consent was obtained by all participants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tessari, P. Nitric oxide in the normal kidney and in patients with diabetic nephropathy. J Nephrol 28, 257–268 (2015). https://doi.org/10.1007/s40620-014-0136-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-014-0136-2