Abstract
Purpose
Patients submitted to curative surgery for non-functioning pancreatic neuroendocrine neoplasms (NF-PanNENs) exhibit a variable risk of disease relapse. Aims of this meta-analysis were to estimate the rate of disease recurrence and to investigate the risk factors for disease relapse in patients submitted to curative surgery for NF-PanNENs.
Methods
Medline/Pubmed and Web of Science databases were searched for relevant studies. A meta-regression analysis was performed to investigate the source of recurrence rate heterogeneity. Pooled hazard ratios (HRs) and 95% confidence intervals (95% CI) were used to assess the effect of each possible prognostic factor on disease-free survival.
Results
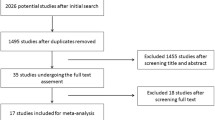
Fifteen studies, involving 2754 patients submitted to curative surgery for NF-PanNENs, were included. The pooled rate of disease recurrence was 21% (95% CI 15–26%). Study quality (Odds ratio, OR 0.94, P = 0.016) and G3-PanNENs rate (OR 2.18, P = 0.040) independently predicted the recurrence rate variability. Nodal metastases (HR 1.63, P < 0.001), tumor grade G2-G3 (G1 versus G2: HR 1.72, P < 0.001, G1 versus G3 HR 2.57, P < 0.001), microvascular (HR 1.25, P = 0.046) and perineural (HR 1.29, P = 0.019) invasion were identified as significant prognostic factors. T stage (T1-T2 versus T3-T4, P = 0.253) and status of resection margins (R0 versus R1, P = 0.173) did not show any significant relationship with NF-PanNENs recurrence.
Conclusion
Disease relapse occurs in approximately one out of five patients submitted to curative surgery for NF-PanNENs. Nodal involvement, tumor grade, microvascular and perineural invasion are relevant prognostic factors, that should be taken into account for follow-up and for possible trials investigating adjuvant or neoadjuvant treatments.



Similar content being viewed by others
Availability of data and material
All the data generated or analysed during this study are included in the manuscript or in the supplementary material.
Code availability
Not applicable.
References
Kuo EJ, Salem RR (2013) Population-level analysis of pancreatic neuroendocrine tumors 2 cm or less in size. Ann Surg Oncol 20:2815–2821. https://doi.org/10.1245/s10434-013-3005-7
Dasari A, Shen C, Halperin D et al (2017) Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 3:1335–1342. https://doi.org/10.1001/jamaoncol.2017.0589
Sandvik OM, Søreide K, Gudlaugsson E et al (2016) Epidemiology and classification of gastroenteropancreatic neuroendocrine neoplasms using current coding criteria. Br J Surg 103:226–232. https://doi.org/10.1002/bjs.10034
Falconi M, Eriksson B, Kaltsas G et al (2016) ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology 103:153–171. https://doi.org/10.1159/000443171
Howe JR, Merchant NB, Conrad C et al (2020) The north American neuroendocrine tumor society consensus paper on the surgical management of pancreatic neuroendocrine tumors. Pancreas 49:1–33. https://doi.org/10.1097/MPA.0000000000001454
Partelli S, Bartsch DK, Capdevila J et al (2017) ENETS consensus guidelines for standard of care in neuroendocrine tumours: surgery for small intestinal and pancreatic neuroendocrine tumours. Neuroendocrinology 105:255–265. https://doi.org/10.1159/000464292
Cloyd JM, Wiseman JT, Pawlik TM (2020) Surgical management of pancreatic neuroendocrine liver metastases. J Gastrointest Oncol 11:590–600. https://doi.org/10.21037/jgo.2019.11.02
Andreasi V, Muffatti F, Guarneri G et al (2020) Surgical principles in the management of pancreatic neuroendocrine neoplasms. Curr Treat Options Oncol 21:48. https://doi.org/10.1007/s11864-020-00736-w
Souche R, Hobeika C, Hain E, Gaujoux S (2020) Surgical management of neuroendocrine tumours of the pancreas. J Clin Med 9:2993. https://doi.org/10.3390/jcm9092993
Pulvirenti A, Pea A, Chang DK, Jamieson NB (2020) Clinical and molecular risk factors for recurrence following radical surgery of well-differentiated pancreatic neuroendocrine tumors. Front Med 7:385. https://doi.org/10.3389/fmed.2020.00385
Singh S, Chan DL, Moody L et al (2018) Recurrence in resected gastroenteropancreatic neuroendocrine tumors. JAMA Oncol 4:583–585. https://doi.org/10.1001/jamaoncol.2018.0024
Singh S, Moody L, Chan DL et al (2018) Follow-up recommendations for completely resected gastroenteropancreatic neuroendocrine tumors. JAMA Oncol 4:1597–1604. https://doi.org/10.1001/jamaoncol.2018.2428
Partelli S, Gaujoux S, Boninsegna L et al (2013) Pattern and clinical predictors of lymph node involvement in nonfunctioning pancreatic neuroendocrine tumors (NF-PanNETs). JAMA Surg 148:932–939. https://doi.org/10.1001/jamasurg.2013.3376
Birnbaum DJ, Gaujoux S, Cherif R et al (2014) Sporadic nonfunctioning pancreatic neuroendocrine tumors: prognostic significance of incidental diagnosis. Surgery (United States) 155:13–21. https://doi.org/10.1016/j.surg.2013.08.007
Genc CG, Falconi M, Partelli S et al (2018) Recurrence of pancreatic neuroendocrine tumors and survival predicted by Ki67. Ann Surg Oncol 25:2467–2474. https://doi.org/10.1245/s10434-018-6518-2
Nanno Y, Toyama H, Otani K et al (2016) Microscopic venous invasion in patients with pancreatic neuroendocrine tumor as a potential predictor of postoperative recurrence. Pancreatology 16:882–887. https://doi.org/10.1016/j.pan.2016.06.008
Zhou H, Wang Y, Guo C et al (2021) Microscopic invasion of nerve is associated with aggressive behaviors in pancreatic neuroendocrine tumors. Front Oncol 11:630316. https://doi.org/10.3389/fonc.2021.630316
Genç CG, Jilesen AP, Partelli S et al (2018) A new scoring system to predict recurrent disease in grade 1 and 2 nonfunctional pancreatic neuroendocrine tumors. Ann Surg 267:1148–1154. https://doi.org/10.1097/SLA.0000000000002123
Pulvirenti A, Javed AA, Landoni L et al (2019) Multi-institutional development and external validation of a nomogram to predict recurrence after curative resection of pancreatic neuroendocrine tumors. Ann Surg. https://doi.org/10.1097/SLA.0000000000003579
Zaidi MY, Lopez-Aguiar AG, Switchenko JM et al (2019) A novel validated recurrence risk score to guide a pragmatic surveillance strategy after resection of pancreatic neuroendocrine tumors: an international study of 1006 patients. Ann Surg 270:422–433. https://doi.org/10.1097/SLA.0000000000003461
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62:1006–1012. https://doi.org/10.1016/j.jclinepi.2009.06.005
Riley RD, Moons KGM, Snell KIE et al (2019) A guide to systematic review and meta-analysis of prognostic factor studies. BMJ 364:k4597. https://doi.org/10.1136/bmj.k4597
Goossen K, Tenckhoff S, Probst P et al (2018) Optimal literature search for systematic reviews in surgery. Langenbeck’s Arch Surg 403:119–129. https://doi.org/10.1007/s00423-017-1646-x
Partelli S, Javed AA, Andreasi V et al (2018) The number of positive nodes accurately predicts recurrence after pancreaticoduodenectomy for nonfunctioning neuroendocrine neoplasms. Eur J Surg Oncol 44:778–783. https://doi.org/10.1016/j.ejso.2018.03.005
Landoni L, Marchegiani G, Pollini T et al (2019) The evolution of surgical strategies for pancreatic neuroendocrine tumors (Pan-NENs): time-trend and outcome analysis from 587 consecutive resections at a high-volume institution. Ann Surg 269:725–732. https://doi.org/10.1097/SLA.0000000000002594
Zhou B, Zhan C, Wu J et al (2017) Prognostic significance of preoperative gamma-glutamyltransferase to lymphocyte ratio index in nonfunctional pancreatic neuroendocrine tumors after curative resection. Sci Rep 7:13372. https://doi.org/10.1038/s41598-017-13847-6
Zhou B, Deng J, Chen L, Zheng S (2017) Preoperative neutrophil-to-lymphocyte ratio and tumor-related factors to predict lymph node metastasis in nonfunctioning pancreatic neuroendocrine tumors. Sci Rep 7:17506. https://doi.org/10.1038/s41598-017-17885-y
Rindi G, Kloppel G, Alhman H et al (2006) TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch 449:395–401. https://doi.org/10.1007/s00428-006-0250-1
Brierley J, Gospodarowicz MK, Wittekind C (eds) (2017) AJCC cancer staging manual, 8th edn. Wiley, Oxford
Bosman FT, Carneiro F, Hruban RH, Theise ND (eds) (2010) WHO classification of tumours of the digestive system, 4th edn. IARC Press, Lyon
Lloyd RV, Osamura RY, Kloppel G, Rosai J (eds) (2017) WHO classification of tumours of endocrine organs, 4th edn. IARC Press, Lyon
Slim K, Nini E, Forestier D et al (2003) Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 73:712–716. https://doi.org/10.1046/j.1445-2197.2003.02748.x
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13. https://doi.org/10.1186/1471-2288-5-13
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14:135. https://doi.org/10.1186/1471-2288-14-135
Clarke M, Horton R (2001) Bringing it all together: lancet-cochrane collaborate on systematic reviews. Lancet (London, England) 357:1728. https://doi.org/10.1016/S0140-6736(00)04934-5
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 283:2008–2012. https://doi.org/10.1001/jama.283.15.2008
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22:719–748. https://doi.org/10.1093/jnci/22.4.719
Hoaglin DC (2016) Misunderstandings about Q and “Cochran’s Q test” in meta-analysis. Stat Med 35:485–495. https://doi.org/10.1002/sim.6632
Higgins JPT, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558. https://doi.org/10.1002/sim.1186
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634. https://doi.org/10.1136/bmj.315.7109.629
Thompson SG, Sharp SJ (1999) Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med 18:2693–2708. https://doi.org/10.1002/(sici)1097-0258(19991030)18:20%3c2693::aid-sim235%3e3.0.co;2-v
Jiang Y, Bin JJ, Zhan Q et al (2015) Impact and clinical predictors of lymph node metastases in nonfunctional pancreatic neuroendocrine tumors. Chin Med J (Engl) 128:3335–3344. https://doi.org/10.4103/0366-6999.171427
Sallinen V, Haglund C, Seppanen H (2015) Outcomes of resected nonfunctional pancreatic neuroendocrine tumors: do size and symptoms matter? Surgery 158:1556–1563. https://doi.org/10.1016/j.surg.2015.04.035
Choi SH, Kim HJ, Kim SY et al (2017) Computed tomography features predictive of lymph node involvement in patients with a nonfunctioning pancreatic neuroendocrine tumor. Pancreas 46:1056–1063. https://doi.org/10.1097/MPA.0000000000000888
Bu J, Youn S, Kwon W et al (2018) Prognostic factors of non-functioning pancreatic neuroendocrine tumor revisited: the value of WHO 2010 classification. Ann Hepato-Biliary-Pancreatic Surg 22:66–74. https://doi.org/10.14701/ahbps.2018.22.1.66
Capretti G, Nappo G, Smiroldo V et al (2019) The number of metastatic lymph nodes is a useful predictive factor for recurrence after surgery for nonmetastatic nonfunctional neuroendocrine neoplasm of the pancreas. Gastroenterol Res Pract 2019:6856329. https://doi.org/10.1155/2019/6856329
Dong D-H, Zhang X-F, Lopez-Aguiar AG et al (2019) Tumor burden score predicts tumor recurrence of non-functional pancreatic neuroendocrine tumors after curative resection. HPB (Oxford) 22:1149–1157. https://doi.org/10.1016/j.hpb.2019.11.009
Feretis M, Wang T, Ghorani E et al (2019) A rational approach to postoperative surveillance for resected non-functional pancreatic neuro-endocrine tumours. Pancreatology 19:1000–1007. https://doi.org/10.1016/j.pan.2019.08.005
Izumo W, Higuchi R, Furukawa T et al (2019) Evaluation of the site and frequency of lymph node metastasis with non-functioning pancreatic neuroendocrine tumor. Eur Surg Res 60:219–228. https://doi.org/10.1159/000504410
Landoni L, Marchegiani G, Pollini T et al (2019) The evolution of surgical strategies for pancreatic neuroendocrine tumors (Pan-NENs). Ann Surg 269:725–732. https://doi.org/10.1097/sla.0000000000002594
Gong Y, Fan Z, Zhang P et al (2021) High pre-operative fasting blood glucose levels predict a poor prognosis in patients with pancreatic neuroendocrine tumour. Endocrine 71:494–501. https://doi.org/10.1007/s12020-020-02469-0
Tan Q-Q, Wang X, Yang L et al (2020) Analysis of recurrence after resection of well-differentiated non-functioning pancreatic neuroendocrine tumors. Medicine (Baltimore) 99:e20324. https://doi.org/10.1097/MD.0000000000020324
Li Y-L, Fan G, Yu F et al (2020) Meta-analysis of prognostic factors for recurrence of resected well-differentiated pancreatic neuroendocrine tumors. Neuroendocrinology. https://doi.org/10.1159/000514047
Crippa S, Zerbi A, Boninsegna L et al (2012) Surgical management of insulinomas: short- and long-term outcomes after enucleations and pancreatic resections. Arch Surg 147:261–266. https://doi.org/10.1001/archsurg.2011.1843
Gao Y, Gao H, Wang G et al (2018) A meta-analysis of prognostic factor of pancreatic neuroendocrine neoplasms. Sci Rep 8:7271. https://doi.org/10.1038/s41598-018-24072-0
Tanaka M, Heckler M, Mihaljevic AL et al (2021) Systematic review and metaanalysis of lymph node metastases of resected pancreatic neuroendocrine tumors. Ann Surg Oncol 28:1614–1624. https://doi.org/10.1245/s10434-020-08850-7
Zhang X-F, Wu Z, Cloyd J et al (2019) Margin status and long-term prognosis of primary pancreatic neuroendocrine tumor after curative resection: results from the US neuroendocrine tumor study group. Surgery 165:548–556. https://doi.org/10.1016/j.surg.2018.08.015
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FLTA (eds) (2010) AJCC cancer staging manual, 7th edn. Springer, Lyon
Acknowledgements
The authors are grateful to Gioja Bianca Costanza Fund for supporting the PhD Scholarship of Dr. Valentina Andreasi and the Research Fellowship of Dr. Francesca Muffatti. The Authors are also grateful to the Fondazione Umberto Veronesi for supporting the Reserach Fellowship of Dr. Giovanni Guarneri. Authors thank the ERN EURACAN initiative.
Funding
None.
Author information
Authors and Affiliations
Contributions
Study concept and design: VA, CR, SP, RC, MF. Data collection: VA, GG, SP. Analysis of the data: CR, VA, GG, CI. Interpretation of the data: VA, CR, SP, GG, CI, FM, SC, RC, MF. Drafting the manuscript: VA, CR, SP, GG. Critical revision of the manuscript: all the authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This meta-analysis is based on published studies performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki.
Research involving human participants and/or animals
As this meta-analysis is performed based on published studies, no ethical approval was required.
Consent to participate
Not applicable.
Consent for publication
As this meta-analysis is based on published studies, no patient consent for publication was required
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Andreasi, V., Ricci, C., Partelli, S. et al. Predictors of disease recurrence after curative surgery for nonfunctioning pancreatic neuroendocrine neoplasms (NF-PanNENs): a systematic review and meta-analysis. J Endocrinol Invest 45, 705–718 (2022). https://doi.org/10.1007/s40618-021-01705-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-021-01705-2