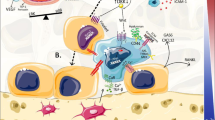
Abstract
Purpose of Review
Dormant disseminated tumor cells are thought to play a pivotal role in driving tumor growth in bone and are likely responsible for disease recurrence following chemotherapy; however, the mechanisms regulating these processes remain unclear. Herein, we discuss recent advances controlling the mechanisms of tumor cell dormancy in bone and discuss the clinical implications of these findings.
Recent Findings
Recent studies have defined gene expression signatures for dormant tumor cells in bone, identifying novel pathways that we can potentially exploit to target these cells. Using intravital imaging and cell fate tracking, bone cells within the bone microenvironment have been shown to play a critical role in regulating tumor cell dormancy and growth, highlighting local bone cell activity as a novel avenue to control tumor cell growth and a role for bone cell niches in supporting dormancy and treatment resistance.
Summary
Due to advances in pre-clinical imaging and sequencing tools, we have a greater understanding of the phenomenon of tumor cell dormancy in bone, ultimately opening avenues for novel targeted treatments.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s–9s. https://doi.org/10.1158/1078-0432.ccr-06-0931.
Bussard KM, Gay CV, Mastro AM. The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Rev. 2008;27(1):41–55. https://doi.org/10.1007/s10555-007-9109-4.
Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350(16):1655–64. https://doi.org/10.1056/NEJMra030831.
Klein CA, Holzel D. Systemic cancer progression and tumor dormancy: mathematical models meet single cell genomics. Cell Cycle. 2006;5(16):1788–98. https://doi.org/10.4161/cc.5.16.3097.
•• Lawson MA, McDonald MM, Kovacic N, Hua Khoo W, Terry RL, Down J, et al. Osteoclasts control reactivation of dormant myeloma cells by remodelling the endosteal niche. Nat Commun. 2015;6:8983. https://doi.org/10.1038/ncomms9983. This study demonstrates the importance of the endosteal niche in controlling myleoma cell dormancy in bone. The authors show that dormancy is a reversible state which can be switched on through the engagement with bone lining cells/osteoblasts and switched off by osteoclasts remodelling the endosteal niche.
•• Yumoto K, Eber MR, Wang J, Cackowski FC, Decker AM, Lee E, et al. Axl is required for TGF-β2-induced dormancy of prostate cancer cells in the bone marrow. Sci Rep. 2016;6:36520. https://doi.org/10.1038/srep36520. This study demonstrates the first evidence that AXL is a crucial regulator of prostate cancer dormancy in bone induced by TGF-β signalling.
Li J, Jiang E, Wang X, Shangguan AJ, Zhang L, Yu Z. Dormant cells: the original cause of tumor recurrence and metastasis. Cell Biochem Biophys. 2015;72(2):317–20. https://doi.org/10.1007/s12013-014-0477-4.
Linde N, Fluegen G, Aguirre-Ghiso JA. The relationship between dormant cancer cells and their microenvironment. Adv Cancer Res. 2016;132:45–71. https://doi.org/10.1016/bs.acr.2016.07.002.
Magbanua MJM, Das R, Polavarapu P, Park JW. Approaches to isolation and molecular characterization of disseminated tumor cells. Oncotarget. 2015;6(31):30715–29.
• Croucher PI, McDonald MM, Martin TJ. Bone metastasis: the importance of the neighbourhood. Nat Rev Cancer. 2016;16(6):373–86. https://doi.org/10.1038/nrc.2016.44. This review discusses the role of different bone cells in supporting dormancy and reactivation and highlights the therapeutic opportunities they may provide.
Massagué J, Obenauf AC. Metastatic colonization. Nature. 2016;529(7586):298–306. https://doi.org/10.1038/nature17038.
Van Der Toom EE, Verdone JE, Pienta KJ. Disseminated tumor cells and dormancy in prostate cancer metastasis. Curr Opin Biotechnol. 2016;40:9–15. https://doi.org/10.1016/j.copbio.2016.02.002.
Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353(8):793–802. https://doi.org/10.1056/NEJMoa050434.
Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8(5):329–40. https://doi.org/10.1038/nrc2375.
Gimbrone MA Jr, Leapman SB, Cotran RS, Folkman J. Tumor dormancy in vivo by prevention of neovascularization. J Exp Med. 1972;136(2):261–76.
Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14(9):611–22. https://doi.org/10.1038/nrc3793.
•• Lam HM, Vessella RL, Morrissey C. The role of the microenvironment-dormant prostate disseminated tumor cells in the bone marrow. Drug Discov Today Technol. 2014;11:41–7. https://doi.org/10.1016/j.ddtec.2014.02.002. This paper describes the important role of cell instrinsic factors and signals from the microenvionment in controlling tumour cell dormancy in bone.
Lawson DA, Bhakta NR, Kessenbrock K, Prummel KD, Yu Y, Takai K, et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature. 2015;526(7571):131–5. https://doi.org/10.1038/nature15260.
Arai F, Suda T. Maintenance of quiescent hematopoietic stem cells in the osteoblastic niche. Ann N Y Acad Sci. 2007;1106:41–53. https://doi.org/10.1196/annals.1392.005.
Trumpp A, Essers M, Wilson A. Awakening dormant haematopoietic stem cells. Nat Rev Immunol. 2010;10(3):201–9. https://doi.org/10.1038/nri2726.
Quayle L, Ottewell PD, Holen I. Bone metastasis: molecular mechanisms implicated in tumour cell dormancy in breast and prostate cancer. Curr Cancer Drug Targets. 2015;15(6):469–80.
Zhang XHF, Giuliano M, Trivedi MV, Schiff R, Kent Osborne C. Metastasis dormancy in estrogen receptor-positive breast cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2013;19(23). doi:https://doi.org/10.1158/078-0432.CCR-13-838.
Welte T, Yu C, Zhang XHF. Retrieval of disseminated tumor cells colonizing the bone in murine breast cancer metastasis models. J Mammary Gland Biol Neoplasia. 2015;20(3–4):103–8. https://doi.org/10.1007/s10911-015-9347-y.
Adam AP, George A, Schewe D, Bragado P, Iglesias BV, Ranganathan AC, et al. Computational identification of a p38(SAPK) regulated transcription factor network required for tumor cell quiescence. Cancer Res. 2009;69(14):5664–72. https://doi.org/10.1158/0008-5472.CAN-08-3820.
Almog N, Ma L, Raychowdhury R, Schwager C, Erber R, Short S, et al. Transcriptional switch of dormant tumors to fast-growing angiogenic phenotype. Cancer Res. 2009;69(3):836–44. https://doi.org/10.1158/0008-5472.can-08-2590.
Kim RS, Avivar-Valderas A, Estrada Y, Bragado P, Sosa MS, Aguirre-Ghiso JA, et al. Dormancy signatures and metastasis in estrogen receptor positive and negative breast cancer. PLoS One. 2012;7(4):e35569. https://doi.org/10.1371/journal.pone.0035569.
Gomis RR, Gawrzak S. Tumor cell dormancy. Mol Oncol. 2017;11(1):62–78. https://doi.org/10.1016/j.molonc.2016.09.009.
Han HH, Lee SH, Kim BG, Lee JH, Kang S, Cho NH. Estrogen receptor status predicts late-onset skeletal recurrence in breast cancer patients. Medicine. 2016;95(8):e2909. https://doi.org/10.1097/md.0000000000002909.
Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7(5):e1000279. https://doi.org/10.1371/journal.pmed.1000279.
Chery L, Lam HM, Coleman I, Lakely B, Coleman R, Larson S, et al. Characterization of single disseminated prostate cancer cells reveals tumor cell heterogeneity and identifies dormancy associated pathways. Oncotarget. 2014;5(20):9939–51. https://doi.org/10.18632/oncotarget.2480.
Guzvic M, Braun B, Ganzer R, Burger M, Nerlich M, Winkler S, et al. Combined genome and transcriptome analysis of single disseminated cancer cells from bone marrow of prostate cancer patients reveals unexpected transcriptomes. Cancer Res. 2014;74(24):7383–94. https://doi.org/10.1158/0008-5472.can-14-0934.
Gawrzak S, Rinaldi L, Gregorio S, Arenas EJ, Salvador F, Urosevic J, et al. MSK1 regulates luminal cell differentiation and metastatic dormancy in ER(+) breast cancer. Nat Cell Biol. 2018;20(2):211–21. https://doi.org/10.1038/s41556-017-0021-z.
•• Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15(7):807–17. https://doi.org/10.1038/ncb2767. This paper illustrates that stable microvasculature (rich in thrombospondin-1) constitutes a dormant niche whereas sprouting neovasculature (enriched with TGF-β1 and periostin) accelerates micrometastatic outgrowth.
• Price TT, Burness ML, Sivan A, Warner MJ, Cheng R, Lee CH, et al. Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Sci Transl Med. 2016;8(340):340ra73. https://doi.org/10.1126/scitranslmed.aad4059. This paper provides insights into the mechanisms controlling the movement and anchoring of breast cancer cells in the bone marrow microenvironment. The authors show that breast cancer cells in the bone marrow reside predominantly in E-selectin and stromal cell-derived factor rich perisinusoidal vascular regions.
Wang H, Yu C, Gao X, Welte T, Muscarella Aaron M, Tian L, et al. The Osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell. 2015;27(2):193–210.
Humtsoe JO, Kramer RH. Differential epidermal growth factor receptor signaling regulates anchorage-independent growth by modulation of the PI3K/AKT pathway. Oncogene. 2010;29(8):1214–26. https://doi.org/10.1038/onc.2009.419.
Avivar-Valderas A, Bobrovnikova-Marjon E, Alan Diehl J, Bardeesy N, Debnath J, Aguirre-Ghiso JA. Regulation of autophagy during ECM detachment is linked to a selective inhibition of mTORC1 by PERK. Oncogene. 2013;32(41):4932–40. https://doi.org/10.1038/onc.2012.512.
Bambang IF, Lu D, Li H, Chiu LL, Lau QC, Koay E, et al. Cytokeratin 19 regulates endoplasmic reticulum stress and inhibits ERp29 expression via p38 MAPK/XBP-1 signaling in breast cancer cells. Exp Cell Res. 2009;315(11):1964–74. https://doi.org/10.1016/j.yexcr.2009.02.017.
Dey-Guha I, Wolfer A, Yeh AC, GA J, Darp R, Leon E, et al. Asymmetric cancer cell division regulated by AKT. Proc Natl Acad Sci U S A. 2011;108(31):12845–50. https://doi.org/10.1073/pnas.1109632108.
Jung Y, Wang J, Lee E, McGee S, Berry JE, Yumoto K, et al. Annexin 2–CXCL12 interactions regulate metastatic cell targeting and growth in the bone marrow. Mol Cancer Res. 2015;13(1):197–207. https://doi.org/10.1158/1541-7786.mcr-14-0118.
• Lu X, Mu E, Wei Y, Riethdorf S, Yang Q, Yuan M, et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell. 2011;20(6):701–14. https://doi.org/10.1016/j.ccr.2011.11.002. This study sheds light on the molecular understanding of tumor dormancy by showing that VCAM-1 is an essential protein which reactivates indolent micrometastasis in the bone microenvironment.
Nakamura T, Shinriki S, Jono H, Guo J, Ueda M, Hayashi M, et al. Intrinsic TGF-beta2-triggered SDF-1-CXCR4 signaling axis is crucial for drug resistance and a slow-cycling state in bone marrow-disseminated tumor cells. Oncotarget. 2015;6(2):1008–19. https://doi.org/10.18632/oncotarget.2826.
Adwan H, Bauerle T, Najajreh Y, Elazer V, Golomb G, Berger MR. Decreased levels of osteopontin and bone sialoprotein II are correlated with reduced proliferation, colony formation, and migration of GFP-MDA-MB-231 cells. Int J Oncol. 2004;24(5):1235–44.
Boyerinas B, Zafrir M, Yesilkanal AE, Price TT, Hyjek EM, Sipkins DA. Adhesion to osteopontin in the bone marrow niche regulates lymphoblastic leukemia cell dormancy. Blood. 2013;121(24):4821–31. https://doi.org/10.1182/blood-2012-12-475483.
Fremder E, Munster M, Aharon A, Miller V, Gingis-Velitski S, Voloshin T, et al. Tumor-derived microparticles induce bone marrow-derived cell mobilization and tumor homing: a process regulated by osteopontin. Int J Cancer. 2014;135(2):270–81. https://doi.org/10.1002/ijc.28678.
Wang J, Wang L, Xia B, Yang C, Lai H, Chen X. BSP gene silencing inhibits migration, invasion, and bone metastasis of MDA-MB-231BO human breast cancer cells. PLoS One. 2013;8(5):e62936. https://doi.org/10.1371/journal.pone.0062936.
•• Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121(4):1298–312. https://doi.org/10.1172/jci43414. This study demonstrates the first evidence that disseminated tumor cells can home to compete with HSCs in their niches, thereby supporting their dormant state.
Shiozawa Y, Pedersen EA, Patel LR, Ziegler AM, Havens AM, Jung Y, et al. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia. 2010;12(2):116–27.
• Taichman RS, Patel LR, Bedenis R, Wang J, Weidner S, Schumann T, Yumoto K, Berry JE, Shiozawa Y, Pienta KJ GAS6 receptor status is associated with dormancy and bone metastatic tumor formation. PLoS One 2013;8(4):e61873. https://doi.org/10.1371/journal.pone.0061873. This study demonstrates a possible association with the expression ratio of AXL and TYRO3 and the ability of prostate cancer cells to switch between dormant and proliferative states.
Decker AM, Jung Y, Cackowski FC, Yumoto K, Wang J, Taichman RS. Sympathetic signaling reactivates quiescent disseminated prostate cancer cells in the bone marrow. Mol Cancer Res. 2017;15(12):1644–55. https://doi.org/10.1158/1541-7786.mcr-17-0132.
Cackowski FC, Eber MR, Rhee J, Decker AM, Yumoto K, Berry JE, et al. MER tyrosine kinase regulates disseminated prostate cancer cellular dormancy. J Cell Biochem. 2017;118(4):891–902. https://doi.org/10.1002/jcb.25768.
• Johnson RW, Finger EC, Olcina MM, Vilalta M, Aguilera T, Miao Y, et al. Induction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrow. Nat Cell Biol. 2016;18(10):1078–89. https://doi.org/10.1038/ncb3408. This study implicates the LIFR:STAT3:SOCS3 signaling pathway in breast cancer dormancy through the maintenance of STAT3 signaling.
Guise TA, Yin JJ, Taylor SD, Kumagai Y, Dallas M, Boyce BF, et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest. 1996;98(7):1544–9. https://doi.org/10.1172/JCI118947.
Ooi LL, Zheng Y, Zhou H, Trivedi T, Conigrave AD, Seibel MJ, et al. Vitamin D deficiency promotes growth of MCF-7 human breast cancer in a rodent model of osteosclerotic bone metastasis. Bone. 2010;47(4):795–803. https://doi.org/10.1016/j.bone.2010.07.012.
Ooi LL, Zhou H, Kalak R, Zheng Y, Conigrave AD, Seibel MJ, et al. Vitamin D deficiency promotes human breast cancer growth in a murine model of bone metastasis. Cancer Res. 2010;70(5):1835–44. https://doi.org/10.1158/0008-5472.CAN-09-3194.
• Ottewell PD, Wang N, Meek J, Fowles CA, Croucher PI, Eaton CL, et al. Castration-induced bone loss triggers growth of disseminated prostate cancer cells in bone. Endocr Relat Cancer. 2014;21(5):769–81. https://doi.org/10.1530/ERC-14-0199. This study was pivotal in revealing the impact of altered bone turnover on tumor growth in bone demonstrating that accelerated bone resorption led to increased bone metastatic tumors and this was blocked with anti-resorptive therapy.
Zheng Y, Zhou H, Fong-Yee C, Modzelewski JR, Seibel MJ, Dunstan CR. Bone resorption increases tumour growth in a mouse model of osteosclerotic breast cancer metastasis. Clin Exp Metastasis. 2008;25(5):559–67. https://doi.org/10.1007/s10585-008-9172-4.
Zheng Y, Zhou H, Ooi LL, Snir AD, Dunstan CR, Seibel MJ. Vitamin D deficiency promotes prostate cancer growth in bone. Prostate. 2011;71(9):1012–21. https://doi.org/10.1002/pros.21316.
Corey E, Brown LG, Quinn JE, Poot M, Roudier MP, Higano CS, et al. Zoledronic acid exhibits inhibitory effects on osteoblastic and osteolytic metastases of prostate cancer. Clin Cancer Res. 2003;9:295–306.
Kiefer JA, Vessella RL, Quinn JE, Odman AM, Zhang J, Keller ET, et al. The effect of osteoprotegerin administration on the intra-tibial growth of the osteoblastic LuCaP 23.1 prostate cancer xenograft. Clin Exp Metastasis. 2004;21(5):381–7.
Ottewell PD, Wang N, Brown HK, Fowles CA, Croucher PI, Eaton CL, et al. OPG-Fc inhibits ovariectomy-induced growth of disseminated breast cancer cells in bone. Int J Cancer. 2015;137(4):968–77. https://doi.org/10.1002/ijc.29439.
• Ottewell PD, Wang N, Brown HK, Reeves KJ, Fowles CA, Croucher PI, et al. Zoledronic acid has differential antitumor activity in the pre- and postmenopausal bone microenvironment in vivo. Clin Cancer Res. 2014;20(11):2922–32. https://doi.org/10.1158/1078-0432.CCR-13-1246. This study provides the first pre-clincal data, in support of clinical data, suggesting that anti-resorptive therapies can suppress tumor growth in bone only in the post-menopausal setting.
• Holen I, Walker M, Nutter F, Fowles A, Evans CA, Eaton CL, et al. Oestrogen receptor positive breast cancer metastasis to bone: inhibition by targeting the bone microenvironment in vivo. Clin Exp Metastasis. 2016;33(3):211–24. https://doi.org/10.1007/s10585-015-9770-x. This study provides the first evidence for inhibition of ER − breast cancer metastasis to bone through the suppression of bone turnover. ER + breast cancer cells, on the other hand, grew independently of bone turnover.
• Mundy GR. Mechanisms of bone metastasis. Cancer. 1997;80(8 Suppl):1546–56. This was a seminal review describing the “vicious cycle” which exists between tumor cells and the cells of the bone microenvironment.
Ottewell PD. The role of osteoblasts in bone metastasis. J Bone Oncol. 2016;5(3):124–7. https://doi.org/10.1016/j.jbo.2016.03.007.
• Wang N, Docherty FE, Brown HK, Reeves KJ, Fowles AC, Ottewell PD, et al. Prostate cancer cells preferentially home to osteoblast-rich areas in the early stages of bone metastasis—evidence from in vivo models. J Bone Min Res the Off J Am Soc Bone Miner Res. 2014;29:2688–96. https://doi.org/10.1002/jbmr.2300. This study highlights a role for osteoblasts supporting engraftment of tumor cells metastasizing to bone.
Wang N, Reeves KJ, Brown HK, Fowles AC, Docherty FE, Ottewell PD, et al. The frequency of osteolytic bone metastasis is determined by conditions of the soil, not the number of seeds; evidence from in vivo models of breast and prostate cancer. J Exp Clin Cancer Res. 2015;34:124. https://doi.org/10.1186/s13046-015-0240-8.
Perez EA, Weilbaecher K. Aromatase inhibitors and bone loss. Oncology (Williston Park, NY). 2006;20(9):1029–39. discussion 39–40, 42, 48
Hadji P, Aapro MS, Body JJ, Gnant M, Brandi ML, Reginster JY, et al. Management of aromatase inhibitor-associated bone loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: joint position statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J Bone Oncol. 2017;7:1–12. https://doi.org/10.1016/j.jbo.2017.03.001.
Handforth C, D'Oronzo S, Coleman R, Brown J. Cancer treatment and bone health. Calcif Tissue Int. 2018;102:251–64. https://doi.org/10.1007/s00223-017-0369-x.
Lipton A, Fizazi K, Stopeck AT, Henry DH, Smith MR, Shore N, et al. Effect of denosumab versus zoledronic acid in preventing skeletal-related events in patients with bone metastases by baseline characteristics. Eur J Cancer (Oxford, England : 1990). 2016;53:75–83. https://doi.org/10.1016/j.ejca.2015.09.011.
Nguyen PL, Alibhai SM, Basaria S, D’Amico AV, Kantoff PW, Keating NL, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67(5):825–36. https://doi.org/10.1016/j.eururo.2014.07.010.
Smith MR, Halabi S, Ryan CJ, Hussain A, Vogelzang N, Stadler W, et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance). J Clin Oncol Off J Am Soc Clin Oncol. 2014;32(11):1143–50. https://doi.org/10.1200/jco.2013.51.6500.
Smith MR, Saad F, Egerdie B, Sieber P, Tammela T, Leder BZ, et al. Denosumab and changes in bone turnover markers during androgen deprivation therapy for prostate cancer. J Bone Miner Res Off J Am Soc Bone Miner Res. 2011;26(12):2827–33. https://doi.org/10.1002/jbmr.492.
Coleman R, Gnant M, Morgan G, Clezardin P. Effects of bone-targeted agents on cancer progression and mortality. J Natl Cancer Inst. 2012;104(14):1059–67. https://doi.org/10.1093/jnci/djs263.
Coleman RE. Impact of bone-targeted treatments on skeletal morbidity and survival in breast cancer. Oncology (Williston Park, NY). 2016;30(8):695–702.
•• Early Breast Cancer Trialists’ Collaborative G. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet (London, England). 2015;386(10001):1353–61. https://doi.org/10.1016/S0140-6736(15)60908-4. This is a seminal study linking our understanding of bone cell regulation of tumor growth with clinical outcomes in patients with early breast cancer treated with anti-resorptives. Disease recurrence was decreased and disease-free survival increased with BP treatment.
• Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet (London, England). 2012;379(9810):39–46. https://doi.org/10.1016/s0140-6736(11)61226-9. This study provides data associating anti-resorptive therapy with increases in disease-free survival in men with advanced prostate cancer.
Nozawa M, Inagaki T, Nagao K, Nishioka T, Komura T, Esa A, et al. Phase II trial of zoledronic acid combined with androgen-deprivation therapy for treatment-naive prostate cancer with bone metastasis. Int J Clin Oncol. 2014;19(4):693–701. https://doi.org/10.1007/s10147-013-0604-z.
Okegawa T, Higaki M, Matsumoto T, Kase H, Murata A, Noda K, et al. Zoledronic acid improves clinical outcomes in patients with bone metastatic hormone-naive prostate cancer in a multicenter clinical trial. Anticancer Res. 2014;34(8):4415–20.
Dai J, Hensel J, Wang N, Kruithof-de Julio M, Shiozawa Y. Mouse models for studying prostate cancer bone metastasis. Bonekey Rep. 2016;5:777. https://doi.org/10.1038/bonekey.2016.4.
Wright LE, Ottewell PD, Rucci N, Peyruchaud O, Pagnotti GM, Chiechi A, et al. Murine models of breast cancer bone metastasis. Bonekey Rep. 2016;5:804. https://doi.org/10.1038/bonekey.2016.31.
Khorshed RA, Hawkins ED, Duarte D, Scott MK, Akinduro OA, Rashidi NM, et al. Automated identification and localization of hematopoietic stem cells in 3D intravital microscopy data. Stem Cell Rep. 2015;5(1):139–53. https://doi.org/10.1016/j.stemcr.2015.05.017.
Demeulemeester J, Kumar P, Moller EK, Nord S, Wedge DC, Peterson A, et al. Tracing the origin of disseminated tumor cells in breast cancer using single-cell sequencing. Genome Biol. 2016;17(1):250. https://doi.org/10.1186/s13059-016-1109-7.
Delgado-Calle J, Anderson J, Cregor MD, Hiasa M, Chirgwin JM, Carlesso N, et al. Bidirectional notch signaling and osteocyte-derived factors in the bone marrow microenvironment promote tumor cell proliferation and bone destruction in multiple myeloma. Cancer Res. 2016;76(5):1089–100. https://doi.org/10.1158/0008-5472.CAN-15-1703.
Herroon MK, Rajagurubandara E, Diedrich JD, Heath EI, Podgorski I. Adipocyte-activated oxidative and ER stress pathways promote tumor survival in bone via upregulation of heme oxygenase 1 and survivin. Sci Rep. 2018;8(1):40. https://doi.org/10.1038/s41598-017-17800-5.
Templeton ZS, Lie WR, Wang W, Rosenberg-Hasson Y, Alluri RV, Tamaresis JS, et al. Breast Cancer cell colonization of the human bone marrow adipose tissue niche. Neoplasia. 2015;17(12):849–61. https://doi.org/10.1016/j.neo.2015.11.005.
Ghajar CM. Metastasis prevention by targeting the dormant niche. Nat Rev Cancer. 2015;15(4):238–47. https://doi.org/10.1038/nrc3910.
Sosa MS, Parikh F, Maia AG, Estrada Y, Bosch A, Bragado P, et al. NR2F1 controls tumour cell dormancy via SOX9- and RARbeta-driven quiescence programmes. Nat Commun. 2015;6:6170. https://doi.org/10.1038/ncomms7170.
El Touny LH, Vieira A, Mendoza A, Khanna C, Hoenerhoff MJ, Green JE. Combined SFK/MEK inhibition prevents metastatic outgrowth of dormant tumor cells. J Clin Invest. 2014;124(1):156–68. https://doi.org/10.1172/JCI70259.
Catena R, Bhattacharya N, El Rayes T, Wang S, Choi H, Gao D, et al. Bone marrow-derived Gr1+ cells can generate a metastasis-resistant microenvironment via induced secretion of thrombospondin-1. Cancer Discov. 2013;3(5):578–89. https://doi.org/10.1158/2159-8290.cd-12-0476.
Niraula S, Templeton AJ, Vera-Badillo F, Dodd A, Nugent Z, Joshua AM, et al. Duration of suppression of bone turnover following treatment with zoledronic acid in men with metastatic castration-resistant prostate cancer. Futur SciOA. 2018;4(1):FSO253. https://doi.org/10.4155/fsoa-2017-0094.
Wirth M, Tammela T, Cicalese V, Gomez Veiga F, Delaere K, Miller K, et al. Prevention of bone metastases in patients with high-risk nonmetastatic prostate cancer treated with zoledronic acid: efficacy and safety results of the Zometa European Study (ZEUS). Eur Urol. 2015;67(3):482–91. https://doi.org/10.1016/j.eururo.2014.02.014.
Yao H, Veine DM, Livant DL. Therapeutic inhibition of breast cancer bone metastasis progression and lung colonization: breaking the vicious cycle by targeting alpha5beta1 integrin. Breast Cancer Res Treat. 2016;157(3):489–501. https://doi.org/10.1007/s10549-016-3844-6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Nancy Haydar and Michelle M. McDonald declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Molecular Biology of Bone Metastasis
Rights and permissions
About this article
Cite this article
Haydar, N., McDonald, M.M. Tumor Cell Dormancy—a Hallmark of Metastatic Growth and Disease Recurrence in Bone. Curr Mol Bio Rep 4, 50–58 (2018). https://doi.org/10.1007/s40610-018-0088-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40610-018-0088-8