Abstract
Purpose of review
This review comprehensively examines host-vaginal microbiota interactions, the composition of the vaginal microbiota, and its dynamic changes throughout a woman's lifespan. Furthermore, the intricate interplay between the host and beneficial bacterial communities, such as Lactobacillus species, and opportunistic pathogens, such as Gardnerella spp., associated with BV development, has been explored. Additionally, the current and advanced therapeutic strategies for managing complications related to vaginal microbiota along with the challenges faced in this field have been discussed.
Recent findings
Recent findings have shown that the microbial communities inhabiting the female vagina, known as the vaginal microbiota, play a critical role in maintaining women's health and supporting reproductive activities. Imbalanced vaginal microbiota can predispose individuals to a range of diseases, including bacterial vaginosis (BV), sexually transmitted infections (STI), miscarriage, and preterm birth. While the exact mechanisms by which a Lactobacillus-dominated vaginal microenvironment improves vaginal health remain elusive, gaining insight into the interactions between the host and vaginal microbiota, as well as with opportunistic pathogens, can help address unanswered questions.
Summary
A deeper understanding of the reciprocal interactions between the host and vaginal microbiota has the potential to pave the way for the development of novel diagnostic and therapeutic interventions and the improvement of women's health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human microbiota comprises a diverse community of microorganisms, including bacteria, viruses, fungi, and protozoa residing both inside and on the human body in various organs, such as the gut, mouth, skin, lungs, and vagina [1, 2]. While the vaginal microbiota may exhibit a lower microbial diversity in comparison to other organs, it serves as the first line of defense within the vaginal ecosystem, undertaking multifaceted roles that significantly impact the reproductive and sexual activities of women [3]. Throughout a woman's lifespan, the vaginal microbiota undergoes dynamic transformations, adapting to physiological and hormonal fluctuations.
The vaginal microenvironment is composed of various bacterial species, which can vary among individuals [4•]. However, a healthy vaginal microbiota is a low-diversity environment and is typically dominated by Lactobacillus species including L. crispatus, L. jensenii, L. gasseri and L. iners (107- 108 CFUs per gram of vaginal fluid) [4•]. Other bacterial species in the vaginal microbiota, such as various anaerobic, Prevotella spp., Peptostreptococcus spp, Atopobium vaginae, and others, exist albeit in low abundance [5, 6]. This microbial composition has a crucial role in maintaining vaginal health and ensures a balanced pH and actively supports reproductive activities in females [7].
However, any significant reduction in or depletion of Lactobacillus species can create an optimal environment for the growth of microbial pathogens, potentially leading to severe consequences in women, including bacterial vaginosis (BV), sexually transmitted infections (STIs), HIV, infertility, preterm birth, and other reproductive health conditions [8••].
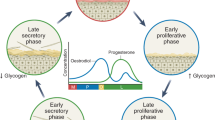
The vaginal microbiota is influenced by several intrinsic and extrinsic factors. Intrinsic factors include host physiology, such as the innate and adaptive immune systems, while extrinsic factors range from age, menstrual cycle, pregnancy, sexual activity, contraception, hormonal fluctuations, antibiotic usage, smoking, ethnicity, lifestyle, and personal hygiene [9••]. The complex interactions between the vaginal microbiota and the host have led to co-evolutionary processes in which the vaginal microbiota maintains the vaginal health and the host supplies vital resources like glycogen for microbial function [4•].
Traditionally, the vaginal microbiota was analyzed using culture-dependent methods that involved examining vaginal secretions and vaginal smear samples through techniques like gram-staining and microscopy visualization. Recent advancements in omics approaches (molecular-based methods) have significantly enhanced our understanding of the composition and dynamics of the vaginal microenvironment, including fastidious and difficult-to-grow bacteria that were previously underestimated using culture-based methods [10••].
The present review investigates the vaginal microbiota’s composition and its dynamic changes over a woman's lifetime. The pivotal role of Lactobacillus in preserving vaginal health and the mechanisms that enable Gardnerella spp. to disrupt this equilibrium and trigger conditions such as BV will also be discussed. Current treatment methods and new strategies for modulating the vaginal microbiota and challenges encountered in the field will also be discussed. This comprehensive understanding holds the potential to pave the way for new strategies in disease diagnosis and personalized treatments aimed at promoting women's health and improving the quality of their lives.
Vaginal microbiota composition
The vaginal microbiota is a diverse community of microorganisms that includes bacteria, fungi, viruses, and protozoa. However, our understanding of the relative abundance of non-bacterial communities is still in the process of being fully explained. In one of the early studies investigating the vaginal microbiota, a cohort of 396 reproductive-aged North American women representing four ethnic backgrounds was examined. The study categorized microbial communities within the vagina into five distinct Community State Types (CSTs). Subsequently, this classification system expanded to include thirteen categories: CST-I is primarily dominated by Lactobacillus crispatus. CST-II is characterized by a prevalence of L. gasseri. CST-III is marked by L. iners as the dominant species. CST-V is primarily represented by L. jensenii. While CST-IV with a higher diversity and evenness comprises a diverse community of facultative and anaerobic bacteria including Gardnerella, Atopobium, Prevotella, Dialister, Megasphaera, Peptoniphilus, Sneathia, Eggerthella, Aerococcus, Finegoldia, Mobiluncus, and lower abundance of Lactobacillus [10••]. In 2021, a subsequent study further divided CST IV into two sub-states; CST IV-A was found to be predominantly composed of anaerobic genera, including Anaerococcus, Prevotella, Streptococcus, and L. iners. On the other hand, CST IV-B exhibited higher proportions of genera such as Gardnerella, Atopobium, Megasphaera, Leptotrichia, and Sneathia spp., and other bacterial vaginosis–associated microorganisms [11] (Table 1). This classification has provided a valuable framework for understanding the variations in vaginal microbiota composition among individuals.
Historically, much of the research on the vaginal microbiota has focused on bacteria. Non-bacterial communities in the vagina have received less attention, leading to a lack of well-specialized techniques and reference databases for their identification. Among non-bacterial communities which comprise a small portion of the vaginal ecosystem, Candida albicans is a well-studied fungus responsible for vaginal infections. Other species such as C. albicans, C. glabrata, C. krusei, C. parapsilosis, C. dubliensis, C. tropicalis, and C. alimentaria have also been detected in vaginal microbiota [12]. Some studies have also explored the impact of bacteriophages (such as Myoviridae, Siphoviridae, Podoviridae) and viruses (e.g., human papillomaviruses, HPV, and Herpesvirales,) on vaginal health [13•, 14•]. Archaea, like Methanobrevibacter smithii have also been associated with BV [15, 16].
Future studies should focus on expanding research in non-bacterial communities of the vaginal microbiota, developing standardized techniques such as optimized extraction methods and sequencing platforms, and establishing comprehensive reference databases to improve their identification and understanding.
Vaginal microbiota in different backgrounds and over the lifespan
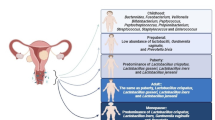
The composition of vaginal microbiota undergoes changes over a woman's lifespan in response to various internal and external stimuli, including hormonal fluctuations, sexual activity, contraceptive methods, pregnancy, menopause, as well as external factors like medications, diet, smoking, stress, as well as race and ethnicity [17•] (Fig. 1). Unlike other body organs where microbiota changes occur over months or years, the vaginal microbiota can shift rapidly between healthy and diseased states within days [18••].
At birth, neonates experience increased estrogen levels, influenced by their mother's circulating hormones. However, these levels undergo a decline in the first weeks of life, and they typically remain low until the onset of puberty. Estrogen fluctuation has a significant role in the composition of vaginal microbiota. During periods of low estrogens, such as before puberty, the vaginal epithelium may be thinner, and glycogen production, a nutrient source for beneficial bacteria like lactobacilli, may be reduced. As estrogen levels rise with the onset of puberty, the vaginal environment becomes more favorable for lactobacilli growth, contributing to the establishment of a balanced and protective vaginal microbiota [19].
While there are not enough details available about gut microbiota composition before puberty or during menopause, studies have shown that women's vaginal microbiota undergo significant changes throughout their lifespan correlated with hormonal fluctuations and sexual activity. For instance, vaginal microbiota is believed to have an impact on urinary tract infections in girls before they reach puberty (3–7% of premenarchal girls) and sexual dysfunction and atrophic vaginitis in post-menopausal women [20, 21]. In these two age groups, lower estrogen levels may lead to a thinner vaginal epithelium with reduced glycogen which is a less favorable environment for lactobacilli [22, 23••].
A study on vaginal samples from 10 girls aged 3–9 years, comparing those with and without vulvovaginitis, revealed the dominance of Prevotella, Porphyromonas, Ezakiella, and Peptoniphilus species in healthy girls, while the case group was characterized by the dominance of Streptococcus, Prevotella, Haemophilus, and Granulicatella [24•]. In contrast, in a longitudinal study involving 31 healthy premenarcheal girls (aged 10 to 12 years), followed for 3 years, contrary to expectations, lactic acid bacteria, mainly Lactobacillus spp., dominated the microbiota well before the onset of menstruation in early to middle puberty. G. vaginalis, associated with bacterial vaginosis in adults, was detected in about one-third of subjects. Importantly, this study showed the resemblance of girls' vaginal microbiota to adults before the onset of menstruation and also emphasized the importance of longitudinal changes in vaginal microbiota before and after puberty [25].
Although the vaginal microbiota is typically a stable ecosystem in most women, longitudinal studies have shown a variation and shifts between certain Community State Types in women [26]. It has been reported that CST-I dominated by L. crispatus seems to be the most stable community associated with vaginal health, while CST-IV dominated by bacterial vaginosis–associated microorganisms appear to transit to other CSTs and lead to the development of BV and vaginal infection [27].
Anevaluation of vaginal bacterial communities in asymptomatic North American women, including four ethnic groups—white, black, Hispanic, and Asian—has shown that the vaginal microbiota in Asian and white women is dominated by 80.2% and 89.7% of lactobacilli, primarily belonging to groups CST I, II, III, and V, respectively. In contrast, in black and Hispanic women, lactobacilli are less dominant, with these ethnic groups mainly categorized in CST III and CST IV (59.6% and 61.9%, respectively) [10••].Although a healthy vaginal microbiota belonging to Black and Hispanic women may lack a high abundance of Lactobacillus species like other ethnic groups, other non-pathogenic lactic acid-producing bacteria such as Leuconostoc, Atopobium, Megasphaera, Leptotrichia, Pediococcus, and Weissella play crucial roles in maintaining microbial balance and vaginal health in these populations [10••, 28].
During pregnancy, the composition of the vaginal microbiota can undergo changes. For instance, in a study on the vaginal microbiome in a British cohort of pregnant women (n = 42) revealed significant changes postpartum, characterized by reduced Lactobacillus dominance and increased alpha diversity [29]. Unlike Northern American populations, some women exhibited a pregnancy microbiome dominated by L. jensenii, particularly in those of Asian and Caucasian ethnicity, with L. gasseri absent in samples from Black women. This research highlights biogeographical and ethnic influences on the pregnancy and postpartum vaginal microbiome, emphasizing the need for further exploration of its relationship with host health and pregnancy outcomes [29].
In another study using 16S rRNA gene pyrosequencing, bacterial communities from mothers and their newborns (four born vaginally and six via Cesarean section) were characterized across multiple body habitats [30]. Results revealed that neonates exhibited undifferentiated bacterial communities across various body habitats, regardless of delivery mode. In this study vaginally delivered infants displayed microbiota resembling their mothers' vaginal microbiota, dominated by Lactobacillus, Prevotella, and Sneathia spp., while C-section infants harbored communities similar to skin surface bacteria, dominated by Staphylococcus, Corynebacterium, and Propionibacterium spp. These findings provide a crucial baseline for understanding the early development of the human microbiome in different body regions following different delivery modes and its potential implications for infant health [30].
Menopause in women, which typically occurs around the age of 50, is known as the end of a woman's reproductive years and can significantly influence hormonal activity (resulting in a sharp decline in estrogen levels) and vaginal microbiota composition [31•]. A study comparing vaginal microbiota in premenopausal, perimenopausal, and postmenopausal women and its association with vulvovaginal atrophy (VVA) showed that bacterial communities were categorized into six types in these groups, including those dominated by various Lactobacillus species. Significant associations were found between menopausal stage and bacterial community types, as well as between VVA and specific community types. Women with VVA were more likely to have a distinct bacterial community state (CST IV-A) with low Lactobacillus abundance. The findings suggest a potential link between the vaginal microbiota composition and VVA, emphasizing the need for future studies to further explore this relationship and its implications for the treatment and prevention of atrophic vaginitis in menopause [32].
Vaginal dryness is the most common complication associated with decreased levels of estrogen and menopause. In a study aiming to explore the vaginal microbiota in post-menopausal women with and without vaginal dryness and symptoms of atrophy (n = 32), as well as differences in epithelial gene expression associated with atrophy, the bacterial abundance remained relatively stable over 10 weeks, with an inverse correlation between Lactobacillus ratio and dryness [33]. Also, increased bacterial diversity was observed in women with moderate to severe vaginal dryness in this study. Contrary to traditional belief, healthy participants showed the prevalence of L. iners and L. crispatus. Also, vaginal dryness and atrophy in this study were linked to down-regulation of genes involved in epithelial structure maintenance and barrier function and up-regulation of genes associated with inflammation [33].
Role of lactobacillus in vaginal health
Since the identification of Döderlein's bacillus (known as Lactobacilli genus; a Gram-positive, facultative, and anaerobic bacterium) by Albert Döderlein in 1892, significant changes have occurred in the field of microbiology and women's health. One notable change has been the recognition of the critical role of Lactobacilli in maintaining a healthy vaginal environment [18••].
Vaginal Lactobacillus spp. are essential for preserving a balanced and healthy vaginal environment [4•]. Lactobacillus accomplish this by a number of mechanisms, one of which is the production of lactic acid (L-lactic acid and D-lactic acid) through the metabolism of glycogen, which lowers the pH of the vagina (< 4.5) and prevents bacterial pathogens colonization [34].
D-lactic acid produced by L. crispatus, L. gasseri, and L. jensenii, but not L. iners, not only can lower the pH but also has several anti-inflammatory properties. In order to prevent infections, Lactobacillus spp. produce bacteriocins and antimicrobial compounds (such as hydrogen peroxide (H2O2)), compete with pathogens for resources and adhesion sites, and stimulate host immune responses against infections [35]. Antimicrobial peptides selectively target and inhibit the growth of harmful pathogens through various mechanisms of action, including pore formation in the cell membranes of pathogens and enzymatic activity that interferes with vital cellular processes such as cell wall synthesis, protein synthesis, and DNA replication [36, 37]. These actions together protect against vaginal infections and support the health of the vaginal ecosystem (Fig. 2).
Vaginal epithelial cells can also generate host antimicrobial peptides, including lactoferrin and lysozyme which are an essential part of the innate immune system's defense against infections [38]. The reciprocal interaction between vaginal epithelial cells and H2O2-producing lactobacilli can result in increased host antimicrobial activity and enhanced lactobacilli growth when compared to non-H2O2-producing lactobacilli [39]. However, since not all Lactobacilli can produce H2O2, there is debate over the function of H2O2 in preserving the homeostasis of the vaginal ecology [40]. Furthermore, lactic acid seems to be more important than H2O2 in preserving this balance. Moreover, previous research on H2O2's role lacks universal support, so more studies are needed to understand its function in the vaginal ecosystem and how it affects women's health.
Role of Gardnerella in bacterial vaginosis
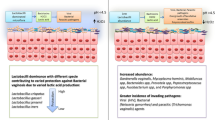
While a reduced microbial diversity in the gut can indicate various health issues, the context of the vaginal microbiota is distinct. In the vagina, a lower microbial diversity often signifies optimal health. This is due to specific conditions, such as an acidic pH, that support the growth of a particular group of microbial communities [41, 42]. As a result, imbalanced vaginal microbiota exhibits high diversity, and an overgrowth of various anaerobic microbes, including G. vaginalis which has been linked to Bacterial vaginosis (BV) development (Fig. 3).
BV is the primary reason for vaginal discharge in women during their reproductive years. The prevalence of BV differs geographically. However, based on a recent systematic review and analysis, the worldwide occurrence of BV among women of reproductive age ranges from 23 to 29% [43]. The overgrowth of BV-associated microbes such as facultative or obligate anaerobic microbes and a sharp decline in the total number of Lactobacillus has been linked to several gynecologic and obstetric disorders including BV [44].
The exact mechanisms of BV development are still not fully understood, but G. vaginalis is believed to play a key role in its pathogenesis. G. vaginalis and the overgrowth of anaerobic bacteria can induce inflammatory responses in the vaginal mucosa and lead to several symptoms such as increased vaginal discharge, itching, and discomfort [45••]. Using several virulence factors, G. vaginalis can adhere to epithelial cells and compete with Lactobacilli for nutrients and resources successfully.
G. vaginalis can also produce enzymes and metabolites, including sialidase (NanH2 and NanH3) and hydrolyze, which can break down sialic acid residues, a crucial component of the protective mucin layer, resulting in decreased viscosity and thickness of the mucus. These alterations are associated with the reduced ability of the mucus to effectively trap and prevent pathogens, as well as the characteristic "fishy" odor in BV (37). G. vaginalis can also produce a pore-forming toxin known as vaginolysin (a cholesterol-dependent cytolysin), which specifically affects human cells [46].
Vaginolysin plays a significant role in the pathogenesis of BV and is produced by some strains of G. vaginalis. The cytolytic activity of vaginolysin can damage vaginal epithelial cells, contributing to the pathogenic effects of BV [47]. Additionally, microorganisms associated with BV such as Peptostreptococcus, Megasphaera, Parvimonas, Dialister, Prevotella, and Mobiluncus are capable of producing biogenic amines, including tyramine, putrescine, and cadaverine. These compounds are associated with vaginal odor and is a crucial factor for the growth of BV-associated bacteria in the vagina [48].
Of utmost significance, biofilm formation by G. vaginalis is a critical strategy that facilitates adhesion to vaginal epithelial cells and other bacteria [49••]. The biofilm, consisting of a matrix of extracellular polymeric substances and complex microbial communities, creates a protective environment, enabling G. vaginalis and other anaerobic bacteria to evade the immune system and presents numerous challenges for diagnosis and treatment approaches [49••] (Fig. 3). G. vaginalis, as the initial colonizer of the vaginal biofilm, can facilitate the colonization of other BV-associated microorganisms, including Atopobium vaginae, leading to the formation of polymicrobial biofilms [50]. According to a comparative transcriptomic analysis, G. vaginalis in biofilms exhibited higher expression of genes associated with antimicrobial resistance, along with distinct responses to stress and reduced metabolic activity. These findings suggest a potential contribution to the chronic nature of BV when compared to free and planktonic G. vaginalis. Surprisingly, vaginolysin expression was reduced in biofilms, highlighting G. vaginalis's ability to adapt its phenotypic response to external and internal stimuli [51].
To combat the BV infection and inflammation, several cytokines and chemokines are induced. T cells, antigen-presenting cells (APCs) present in vaginal mucosal tissue, macrophages, and neutrophils respond to the infection by releasing various cytokines and chemokines. This includes pro-inflammatory cytokines like IL-1β, IL-6, and TNF-α, as well as chemokines such as IL-8, which recruit neutrophils to the infection site. Additionally, IFN-γ is involved in immune cell activation. Examination of vaginal samples in a longitudinal study on 40 women diagnosed with BV (Nugent score 7–10) showed a high concentration of several immune factors including IL-1a, IL-1b, IL-8, IL-6, IL-12p70, and tumor necrosis factor-alpha (TNF α) compared to healthy controls [52]. Moreover, evaluation of 90 vaginal samples from Caucasian asymptomatic women (aged 18–40 years) showed that the presence of L. iners in CST III and BV-associated microorganisms in CST IV was associated with higher baseline levels of pro-inflammatory factors, such as macrophage migration inhibitory factor (MIF), interleukin-1α, interleukin-18, and TNF-α which are responsible for the activation of inflammatory responses in the vagina [53].
Diagnosis and treatment approaches
The two primary techniques used in BV diagnosis are the Amsel method and the Nugent method [54]. The Amsel approach combines clinical and laboratory findings. In the Amsel diagnosis method, BV is confirmed if at least three of the following criteria are met: a specific type of vaginal discharge, an elevated vaginal pH (> 4.5), the presence of clue cells in microscopic examination, and a positive amine test indicating a fishy odor upon the addition of potassium hydroxide to vaginal secretions [55].
The Nugent method considered the gold standard for BV diagnosis, involves a Gram stain and microscopic examination of vaginal bacteria. It scores the loss of lactobacilli and the presence of increasing numbers of certain Gram-variable and Gram-negative coccobacilli (e.g., Gardnerella and Bacteroides). A score of 0–3 indicates normal flora, 4–6 indicates intermediate flora and 7–10 indicates BV [56].
Furthermore, rapid and on-site diagnosis methods known as Point-of-Care (POC) methods offer immediate results at the location of patient care [57]. POC tests include;1) OSOM BV Blue test which detects increased vaginal fluid sialidase produced by BV-associated bacteria such as Gardnerella, Prevotella, Bacteroides, and Mobiluncus [58], 2) VGTest ion motility spectrometry (IMS) which determines the levels of the malodorous biogenic amines (e.g. trimethylamine) associated with BV [57], 3) FemExam test card measuring amines and pH level of vaginal fluid [59], 4) vaginal wet mount also known as a vaginal smear test or wet prep which is the microscopic examination of vaginal fluid to evaluate vagina fluid for presence of clue cells, bacterial vaginosis, yeast infections, and trichomoniasis [60].
Furthermore, molecular diagnostic approaches, such as PCR (e.g. NuSwab), qPCR (e.g. SureSwab), and next-generation sequencing-based methods offer genetic insights into the vaginal flora that may not be attainable through routine laboratory tests. For example, NuSwab is employed to detect the three most predictive marker organisms (Atopobium vaginae, BVAB-2, and Megasphaera-1) [61] and SureSwab detects BV-associated microorganisms (Megasphaera spp, A. vaginae, and G. vaginalis) and H2O2-producing Lactobacillus species (L. crispatus, L. acidophilus, and L. jensenii) (52). Also, BD Max vaginal panel can detect L. jensenii and L. crispatus and four BV-associated microorganisms (G. vaginalis, BVAB2, Megasphaera type 1, and A. vaginae) [62].
Although several BV diagnostic methods are currently available, the choice of methods is dependent on the availability of techniques and equipment, the time required for analysis, and the level of expertise of the healthcare professionals conducting the tests. Factors such as the diagnostic goals, patient characteristics, and the healthcare setting also influence the selection of an appropriate diagnostic approach.
Antibiotic therapy and biofilm-disrupting agents
The primary choices for BV treatment are metronidazole and clindamycin, effective antibiotics against anaerobic infections. In cases where these antibiotics are not suitable, tinidazole and secnidazole, which are also nitroimidazole antibiotics, are considered alternative options for BV treatment [63]. However, due to the limited effectiveness of antibiotic therapy in addressing BV biofilm and the high recurrence rate (< 50%) observed in patients with BV biofilm within one year of antibiotic therapy [64, 65], the use of commonly prescribed antibiotics may not be an efficient strategy.
According to an interventional study with 18 BV patients treated with metronidazole, a persistent biofilm, mainly containing G. vaginalis and A. vaginae re-emerged on the vaginal mucosa after termination of treatment. This suggests a lasting reservoir of core BV bacteria in biofilm form post-metronidazole treatment [66]. The evaluation of virulence potential and biofilm formation ability in 30 bacterial species associated with BV in Alves et al.'s study revealed that the majority of BV-associated bacteria tended to grow as biofilms. Among them, G. vaginalis exhibited higher adhesion and cytotoxicity, as well as greater biofilm formation ability [67].
These findings have led to the development of new therapeutic approaches aimed at targeting biofilm structures and selectively addressing vaginal pathogens. These approaches include the use of probiotics, prebiotics, DNases, antiseptics, acidifying agents, retrocyclins, vaginal microbiota transplantation (VMT), and natural antimicrobial agents.
As the extracellular DNA of G. vaginalis plays a significant role in maintaining the structure and integrity of the BV biofilm, enzymatic disruption of this component can be a potential nonantibiotic adjunct to decrease the risk of BV recurrence. Studies have shown greater than tenfold inhibition of G. vaginalis colonization by DNase in a murine model [68]. Similarly, the assessment of lysozyme's impact (30 μg/ml) on the biofilm formation capacities of 16 strains of microorganisms, including G. vaginalis, using the in vitro Test Tube method, revealed that G. vaginalis was highly sensitive to lysozyme, even at low concentrations (2.5 μg/ml). This sensitivity led to the inhibition of biofilm formation, showcasing the potential application of lysozyme in the treatment of vaginal infections [69].
Other substances, such as Lauramide Arginine Ethyl Ester [70], Amphoteric Tenside (Sodium Cocoamphoacetate) [71], Endolysin [72••], Thymol [73], Boric Acid [74], Octenidine [75], and Cationic Amphiphiles [76], have exhibited various properties that can be beneficial in preventing and treating BV. These substances, in particular, are known for their ability to disrupt biofilm formation and target the bacteria associated with this condition.
Some studies have also suggested that combining different treatment strategies such as antibiotics and biofilm-disrupting agents may enhance the efficacy of treatment by making it easier for antibiotics to penetrate the biofilm structure and reach the target pathogens bacteria [77•]. Furthermore, as estrogen plays a crucial role in maintaining the health of vaginal tissues, including promoting the growth of lactobacilli, and its levels decrease in postmenopause, estrogen therapy can help restore vaginal tissues and alleviate symptoms of atrophic vaginitis commonly reported in postmenopausal women (25% to 50%). Additionally, it can promote the growth of beneficial bacteria [78]. For instance, in a study on postmenopausal women with atrophic vaginitis, low doses of estrogen therapy showed rapid changes in vaginal microbiota composition, characterized by significant increases in the abundance of Lactobacillus spp. and a decrease in Gardnerella. These changes correlated with a four-fold increase in serum estradiol levels, decreased vaginal pH, and alleviation of symptoms [79]. Also, in a 12-week study on 144 postmenopausal women receiving low-dose vaginal estradiol tablets, the estradiol group showed a significant shift towards beneficial bacteria, with the dominance of Lactobacillus and Bifidobacterium communities [80•]. Additionally, changes in vaginal metabolites, including an increase in lactate, were observed in the estradiol group in this study. Estradiol group also exhibited a lower pH compared to the placebo. These findings suggested a positive impact of low-dose vaginal estradiol on the vaginal microenvironment, indicating potential benefits for postmenopausal women with genitourinary symptoms [80•].
Probiotics and prebiotics
As defined by the World Health Organization, probiotics are live microorganisms that when administered in adequate amounts confer a beneficial health effect on the host [81]. The probiotic properties of lactobacilli are associated with different components of lactobacilli, including lactobacillus surface-active molecules (SAMs) such as polysaccharides, lipoteichoic acid, surface layer associated proteins, fibronectin binding proteins, and mucin-binding proteins [82].
Recent studies have demonstrated the effectiveness of oral or vaginal lactobacilli probiotics in treating and preventing recurrent BV. According to a meta-analysis study involving fourteen randomized controlled trials comparing the efficacy of probiotics with antibiotic therapy (probiotics + antibiotics group) versus antibiotics alone or plus placebo (antibiotics + placebo group) for BV, combination therapy (probiotic + antibiotic), particularly oral administration of L. rhamnose (compared to vaginal application of L. rhamnose), played a significant role in adjuvant treatment of BV [83••]. Another study on 89 BV patients (aged 18–50 years) receiving oral or vaginal probiotic capsules (containing two or three L. crispatus strains), or placebo capsules for 3 months, showed that oral and vaginal L. crispatus capsules reduced symptoms in BV patients (such as improvement of Nugent score, discharge amount and smell, reduced itching and irritation). It also significantly increased the lactobacilli counts and decreased BV-associated bacteria in the vaginal samples [84•].
Also, findings from a systematic review and meta-analysis of 30 studies showed that probiotic consumption in BV patients, who were followed up after treatment, could reduce the recurrence of vaginitis rate (OR = 0.27, 95% CI: 0.18–0.41, P < 0.001) and improve the cure rate (OR = 2.28, 95% CI: 1.20–4.32, P = 0.011) [85••]. In contrast according to a study on the effects of oral probiotic supplements (oral capsules containing L. rhamnosus GR-1 and L. reuteri RC-14 each at 2.5 × 109 colony-forming units (CFUs)) in 238 Women aged 16 years or older from early pregnancy (9–14 weeks’ gestation) until delivery (case: 123, control: 115), there was no significant difference in BV rates between the two groups (15% in the probiotic group vs. 9% in the placebo group). Additionally, there were no differences in the colonization of specific bacteria or in the alpha diversity and composition of bacterial communities between the probiotic and placebo groups [86•].
In another similar study on low-risk pregnant women, orally administered L. rhamnosus GR-1 and L. reuteri RC-14 twice daily for 12 weeks showed no adverse effects. Vaginal microbiota remained variable, and there were no significant alterations. The study concluded that these probiotic strains did not adversely impact pregnancy outcomes or microbiota in low-risk pregnant women [87•].
Although recent findings have shown the effectiveness of probiotics in improving BV symptoms, more standardized large-sample randomized controlled trials are required to fully understand the synergistic effect of probiotic combined therapy and verify the efficacy and safety of probiotics in the treatment of BV in patients with different backgrounds and health conditions. Moreover, the lack of consistent regulation in the supplement industry raises concerns about the accuracy of labeled content, as some products may not contain the stated strains or amounts [88].
In addition, prebiotics are non-digestible fibers that promote the activity and growth of beneficial microorganisms such as Bifidobacteria and lactobacilli. This selective promotion helps maintain a balanced and healthy microbiota, regulate immune responses, and protect against infections and inflammation [66]. In a study by Hakimi et al., 100 patients were treated with a 5 mg prebiotic vaginal gel applicator (Trifolium vag) and three 250 mg metronidazole tablets per day for 7 days in the case group, while the control group received a 5 mg placebo vaginal gel applicator and three 250 mg metronidazole tablets per day for 7 days. The healing rate, assessed using Amsel and Nugent criteria, was significantly higher in the intervention group compared to the control group. On the 10th day, the healing rate was 76% in the intervention group and 30% in the control group (odds ratio 4.3; 95% confidence interval 2.7–9.4). Additionally, on the 90th day, the healing rate remained higher in the intervention group (84%) compared to the control group (62%) (odds ratio 3.7; 95% CI 1.3–8.9), indicating the beneficial effect of adjuvant treatment in BV therapy [89]. In another study assessing the role of intravaginal prebiotic gel (containing APP-14) in the improvement of BV symptoms in 42 subjects, after 8 days of treatment, all participants receiving the prebiotic achieved a normal Nugent score. In contrast, 33% of those treated with a placebo had an intermediate or positive Nugent score. By day 16, all subjects treated with the prebiotic maintained a normal Nugent score, while 24% of the placebo group still had an elevated Nugent score [90]. Also, in a study with freshly collected vaginal samples, various Lactobacillus strains and BV-associated microorganisms were isolated and tested for their response to prebiotics like lactitol, lactulose, raffinose, and oligofructose. The results indicated that lactulose significantly stimulated vaginal lactobacilli while inhibiting the growth of BV-associated microorganisms [91]. The evaluation of the role of prebiotics, specifically oligosaccharide series, has also demonstrated that prebiotics can effectively stimulate the growth of isolated lactobacilli strains from the normal vagina. In contrast, pathogenic microorganisms were unable to metabolize these oligosaccharides, suggesting the potential application of this formula in preventing vaginal infections [92].
Until recently, Photobiomodulation (PBM) primarily has been used in managing various women's health conditions, including menstrual pain, endometriosis, pelvic floor disorders, and more [93]. However, recent clinical and preclinical studies, particularly in the context of gut-microbiota-related diseases and gut-brain axis complications, have shown its potential as a non-invasive and drug-free approach to improve vaginal microbiota structure [94•]. PBM utilizes specific visible or near-infrared light wavelengths, impacting human physiology both locally and systemically. It can interact with cells, modulate cellular functions, promote tissue regeneration, stimulate immune responses, and aid in pH balance, tissue repair, and the growth of beneficial microbial communities while preventing pathogenic microbes. Nonetheless, it's crucial to recognize that PBM's application in reprogramming vaginal microbiota dysbiosis is an emerging field, necessitating further research to establish its safety and effectiveness in clinical settings [17•].
Vaginal microbiota transplantation (VMT)
Vaginal microbiota transplantation (VMT) involves transferring optimal vaginal microbiota from a healthy donor into the recipient's vagina to restore a more favorable microbial balance. The first exploratory study testing the use of VMT in five patients showed that VMT was associated with full long-term remission in four cases, lasting 5–21 months after the procedure, with no adverse effects. These patients exhibited improvements in symptoms, Amsel criteria, microscopic vaginal fluid appearance, and the reconstitution of a Lactobacillus-dominated vaginal microbiome. One patient experienced incomplete remission, and three patients required repeated VMT to achieve a sustained clinical response. This study suggests the potential efficacy of VMT in treating recurrent BV, but further research in randomized, placebo-controlled clinical trials is needed to confirm its therapeutic benefits [95]. In addition, the safety of VMT methods on a larger scale remains unclear, as each donation requires a comprehensive assessment for potential vaginal pathogens and viruses (such as HPV or HSV), resulting in a low amount of beneficial bacterial load [96].
In summary, there are multiple treatment options for imbalanced vaginal microbiota and BV management that can be used alone or in combination. The choice of treatment mainly depends on the severity of the condition, the individual's response to previous treatments, and the specific characteristics of vaginal microbiota composition.
Challenges and future recommendation
-
1.
The composition of the vaginal microbiota can significantly vary between individuals due to several confounding factors, such as age, hormonal fluctuations, sexual activity, and ethnicity [97•]. High heterogeneity and inter-individual variation can make it challenging to identify a normal or healthy vaginal microbiota composition. To minimize the impact of other contributing factors, it is important that future studies consider the influence of these factors when interpreting their findings. Moreover, designing longitudinal studies can help identify changes over time and in response to various factors, providing a more comprehensive understanding of vaginal microbiota dynamics.
-
2.
The dynamic nature of the vaginal ecosystem has also necessitated the development of better tools and approaches to analyze clinical findings. Systems biology research that uses mechanistic models, including computational or mathematical models and pre-existing knowledge about vaginal microbiota and their interactions, as well as their roles in the vaginal ecosystem, enable predictions regarding how vaginal dysbiosis might impact health or disease. Understanding the host-microbiota interaction using mechanistic models can aid in the development of more precise and effective treatment strategies [18••].
-
3.
Since our primary insights into the vaginal microbiota stem from culture-based and amplicon-based studies, our comprehension of host-microbiota mechanistic insights remains limited. To achieve a more comprehensive understanding, integrating multiple omics approaches, including metatranscriptomic, metagenomic, metabolomic, and immunology findings can provide a more holistic view of functional dynamics of vaginal microbiota. These approaches would broaden our knowledge of the microbial community composition and provide valuable information about metabolic aspects, host-microbe interactions, and the influence of the microbiota on immune responses [98•].
-
4.
Another challenge in the study of vaginal microbiota is model systems. Cultured models may not fully capture the dynamic of the microbial community in the human vagina. Moreover, translating findings from animal models to humans poses challenges due to variations in microbiota composition and immune system. To address these challenges, researchers are exploring advanced model systems, such as vaginal organoid models or co-culture systems, which can functionally recapitulate in vivo vaginal epithelium, enhancing our understanding of the vaginal microbiota and its role in health and disease. Recent advances in preclinical models, such as organ chip technology, have also introduced the "vagina-on-a-chip" model, which accurately replicates the microenvironment of the human vagina for diverse research applications [99]. This technology incorporates microfluidic channels, vaginal cells, and mucus, enabling controlled experiments to assess the real-time effects of microbiota dysbiosis, pathogens, and drugs with the potential for personalized therapeutic applications [99]. Vagina-on-a-chip models offer a promising solution to circumvent the limitations of traditional in vitro and in vivo methods in the study of vaginal health and related research, resulting in accelerated research progress, increased reproducibility, reduced costs, and addressing the ethical concerns associated with animal testing [100••].
-
5.
In addition, due to high heterogeneity across different studies, generalizing the findings to other settings may not be achievable. To enhance consistency across studies and facilitate meaningful comparisons, future studies should apply standardized protocols from sample collection to data analysis [101••].
Conclusion
In conclusion, this review underscores the critical role of a balanced vaginal microbiota in promoting women's health and enhancing their quality of life. While existing research highlights the significance of lactobacillus in maintaining vaginal health and implicates Gardenella spp. in the development of BV, the intricate, multifaceted, and polymicrobial nature of BV necessitates a comprehensive understanding beyond attributing the condition to a single bacterium or mechanism. Recognizing the limitations of current knowledge, the integration of metagenomics, meta-transcriptomics, and immunology findings can help facilitate a functional evaluation of the vaginal microbiota and its interactions with host cells and various microorganisms, including viruses, fungi, and other less-explored species. Also, promising yet sometimes inconsistent results in the methods for reestablishing the vaginal microbiota and treating BV highlight the need for well-designed studies following standardized methodologies. Moving forward, a collaborative effort among researchers, clinicians, and practitioners is needed to address the existing challenges and develop personalized therapeutic strategies in the management of vaginal complications.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
The integrative human microbiome project. Nature. 2019;569(7758):641–8.
Sadeghpour Heravi F. Gut Microbiota and Autoimmune Diseases: Mechanisms, Treatment, Challenges, and Future Recommendations. Current Clinical Microbiology Reports. 2024:1–16.
Holm JB, France MT, Gajer P, Ma B, Brotman RM, Shardell M, et al. Integrating compositional and functional content to describe vaginal microbiomes in health and disease. Microbiome. 2023;11(1):259.
• Chee WJY, Chew SY, Than LTL. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb Cell Fact. 2020;19(1):203. (Offering insights into the development of lactobacilli derivatives as a potential complementary or alternative medicine for maintaining vaginal health and preventing complications.)
Verhelst R, Verstraelen H, Claeys G, Verschraegen G, Van Simaey L, De Ganck C, et al. Comparison between Gram stain and culture for the characterization of vaginal microflora: definition of a distinct grade that resembles grade I microflora and revised categorization of grade I microflora. BMC Microbiol. 2005;5:1–11.
De Backer E, Verhelst R, Verstraelen H, Alqumber MA, Burton JP, Tagg JR, et al. Quantitative determination by real-time PCR of four vaginal Lactobacillus species, Gardnerella vaginalis and Atopobium vaginae indicates an inverse relationship between L. gasseri and L. iners. BMC microbiology. 2007;7:1–13.
Adapen C, Réot L, Menu E. Role of the human vaginal microbiota in the regulation of inflammation and sexually transmitted infection acquisition: Contribution of the non-human primate model to a better understanding? Front Reprod Health. 2022;4:992176.
•• Chen X, Lu Y, Chen T, Li R. The female vaginal microbiome in health and bacterial vaginosis. Front Cell Infect Microbiol. 2021;11:631972. (Underscores the global impact of bacterial vaginosis (BV) on women's reproductive health, emphasizing the urgent need for improved diagnostic and therapeutic strategies.)
•• Moosa Y, Kwon D, De Oliveira T, Wong EB. Determinants of vaginal microbiota composition. Front Cell Infect Microbiol. 2020;10:467. (Evaluation the influence of a woman's vaginal microbiota on sexual and reproductive health, addressing gaps in understanding the determinants of its composition and advocating for comprehensive research.)
•• Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proceedings of the National Academy of Sciences. 2011;108(supplement_1):4680–7. Evaluating the vaginal microbiomes of 396 asymptomatic women from diverse ethnic groups reveals distinct community clusters dominated by different Lactobacillus species.
Gajer P, Brotman RM, Bai G, Sakamoto J, Schütte UM, Zhong X, et al. Temporal dynamics of the human vaginal microbiota. Science translational medicine. 2012;4(132):132ra52-ra52.
Drell T, Lillsaar T, Tummeleht L, Simm J, Aaspõllu A, Väin E, et al. Characterization of the vaginal micro-and mycobiome in asymptomatic reproductive-age Estonian women. PLoS ONE. 2013;8(1):e54379.
• Happel A-U, Balle C, Maust BS, Konstantinus IN, Gill K, Bekker L-G, et al. Presence and persistence of putative lytic and temperate bacteriophages in vaginal metagenomes from South African adolescents. Viruses. 2021;13(12):2341. (Evaluating cervicovaginal microbiota in South African adolescents reveals diverse and persistent bacteriophages, suggesting potential interactions with vaginal bacteria.)
• Madere FS, Sohn M, Winbush AK, Barr B, Grier A, Palumbo C, et al. Transkingdom analysis of the female reproductive tract reveals bacteriophages form communities. Viruses. 2022;14(2):430. (Investigating the impact of the female reproductive tract (FRT) virome on vaginal health showed distinct bacteriophage signatures associated with bacterial vaginosis.)
Belay N, Mukhopadhyay B, Conway de Macario E, Galask R, Daniels L. Methanogenic bacteria in human vaginal samples. Journal of clinical microbiology. 1990;28(7):1666–8.
Grine G, Drouet H, Fenollar F, Bretelle F, Raoult D, Drancourt M. Detection of Methanobrevibacter smithii in vaginal samples collected from women diagnosed with bacterial vaginosis. Eur J Clin Microbiol Infect Dis. 2019;38:1643–9.
• Santos FP, Carvalhos CA, Figueiredo-Dias M. New Insights into Photobiomodulation of the Vaginal Microbiome—A Critical Review. Int J Mol Sci. 2023;24(17):13507. (Exploring the potential of Photobiomodulation (PBM), utilizing low-level light, to modulate the vaginal microbiome (VMB) offering insights into potential therapeutic applications.)
•• Lee CY, Dillard LR, Papin JA, Arnold KB. New perspectives into the vaginal microbiome with systems biology. Trends Microbiol. 2023;31(4):356–68. (Underscoring the complexity of optimal and non-optimal states, emphasizing the need for mechanistic models.)
Bidlingmaier F, Wagner-Barnack M, Butenandt O, Knorr D. Plasma estrogens in childhood and puberty under physiologic and pathologic conditions. Pediatr Res. 1973;7(11):901–7.
Mårild S, Jodal U. Incidence rate of first-time symptomatic urinary tract infection in children under 6 years of age. Acta Paediatr. 1998;87(5):549–52.
Brotman RM, Shardell M, Gajer P, Fadrosh D, Chang K, Silver M, et al. Association between the vaginal microbiota, menopause status and signs of vulvovaginal atrophy. Menopause-The Journal of the North American Menopause Society: Lippincott Williams & Wilkins 530 Walnut St, Philadelphia, PA 19106–3621 USA; 2013. p. 1318-.
Gliniewicz K, Schneider GM, Ridenhour BJ, Williams CJ, Song Y, Farage MA, et al. Comparison of the vaginal microbiomes of premenopausal and postmenopausal women. Front Microbiol. 2019;10:193.
•• Łaniewski P, Herbst-Kralovetz MM. Connecting microbiome and menopause for healthy ageing. Nat Microbiol. 2022;7(3):354–8. (Understanding the interplay between the microbiome and menopause holds promise for new interventions to alleviate menopausal symptoms and improve quality of life for women.)
• Xiaoming W, Jing L, Yuchen P, Huili L, Miao Z, Jing S. Characteristics of the vaginal microbiomes in prepubertal girls with and without vulvovaginitis. Eur J Clin Microbiol Infect Dis. 2021;40:1253–61. (Investigating vaginal microbiomes in prepubertal girls with and without vulvovaginitis, revealing a dominance of Prevotella, Porphyromonas, Ezakiella, and Peptoniphilus in control and Streptococcus, Prevotella, Haemophilus, and Granulicatella in cases.)
Hickey RJ, Zhou X, Settles ML, Erb J, Malone K, Hansmann MA, et al. Vaginal microbiota of adolescent girls prior to the onset of menarche resemble those of reproductive-age women. MBio. 2015;6(2):https://doi.org/10.1128/mbio. 00097–15.
Srinivasan S, Liu C, Mitchell CM, Fiedler TL, Thomas KK, Agnew KJ, et al. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS ONE. 2010;5(4):e10197.
DiGiulio DB, Callahan BJ, McMurdie PJ, Costello EK, Lyell DJ, Robaczewska A, et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci. 2015;112(35):11060–5.
Zhou X, Bent SJ, Schneider MG, Davis CC, Islam MR, Forney LJ. Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods. Microbiology. 2004;150(8):2565–73.
MacIntyre D, Chandiramani M, Lee Y, Kindinger L, Smith A, Angelopoulos N, et al. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci Rep. 2015;5:8988.
Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci. 2010;107(26):11971–5.
• Park MG, Cho S, Oh MM. Menopausal Changes in the Microbiome—A Review Focused on the Genitourinary Microbiome. Diagnostics. 2023;13(6):1193. (Providing insights into potential links to genitourinary syndrome and urinary tract diseases in women.)
Brotman RM, Shardell MD, Gajer P, Fadrosh D, Chang K, Silver MI, et al. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause. 2014;21(5):450–8.
Hummelen R, Macklaim JM, Bisanz JE, Hammond J-A, McMillan A, Vongsa R, et al. Vaginal microbiome and epithelial gene array in post-menopausal women with moderate to severe dryness. PLoS ONE. 2011;6(11):e26602.
Witkin SS, Linhares IM. Why do lactobacilli dominate the human vaginal microbiota? BJOG: An International Journal of Obstetrics & Gynaecology. 2017;124(4):606–11.
Smith SB, Ravel J. The vaginal microbiota, host defence and reproductive physiology. J Physiol. 2017;595(2):451–63.
Carmo MSd, Noronha FM, Arruda MO, Costa EPDS, Bomfim MR, Monteiro AS, et al. Lactobacillus fermentum ATCC 23271 displays in vitro inhibitory activities against Candida spp. Frontiers in Microbiology. 2016;7:1722.
Stoyancheva G, Marzotto M, Dellaglio F, Torriani S. Bacteriocin production and gene sequencing analysis from vaginal Lactobacillus strains. Arch Microbiol. 2014;196:645–53.
Madanchi H, Shoushtari M, Kashani H, Sardari S. Antimicrobial peptides of the vaginal innate immunity and their role in the fight against sexually transmitted diseases. New Microbes and New Infections. 2020;34:100627.
Sgibnev AV, Kremleva EA. Vaginal protection by H2O2-producing lactobacilli. Jundishapur journal of microbiology. 2015;8(10).
Tachedjian G, O’Hanlon DE, Ravel J. The implausible “in vivo” role of hydrogen peroxide as an antimicrobial factor produced by vaginal microbiota. Microbiome. 2018;6(1):1–5.
Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55(2):205–11.
Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353(18):1899–911.
Peebles K, Velloza J, Balkus JE, McClelland RS, Barnabas RV. High global burden and costs of bacterial vaginosis: a systematic review and meta-analysis. Sex Transm Dis. 2019;46(5):304–11.
Zozaya-Hinchliffe M, Lillis R, Martin DH, Ferris MJ. Quantitative PCR assessments of bacterial species in women with and without bacterial vaginosis. J Clin Microbiol. 2010;48(5):1812–9.
•• Morrill S, Gilbert NM, Lewis AL. Gardnerella vaginalis as a cause of bacterial vaginosis: appraisal of the evidence from in vivo models. Front Cell Infect Microbiol. 2020;10:168. (Assesses experimental models, focusing on Gardnerella vaginalis, in exploring the underlying causes and features of bacterial vaginosis (BV).)
Baruah FK, Sharma A, Das C, Hazarika NK, Hussain JH. Role of Gardnerella vaginalis as an etiological agent of bacterial vaginosis. Iranian J Microbiol. 2014;6(6):409.
Gelber SE, Aguilar JL, Lewis KL, Ratner AJ. Functional and phylogenetic characterization of Vaginolysin, the human-specific cytolysin from Gardnerella vaginalis. J Bacteriol. 2008;190(11):3896–903.
Nelson TM, Borgogna J-LC, Brotman RM, Ravel J, Walk ST, Yeoman CJ. Vaginal biogenic amines: biomarkers of bacterial vaginosis or precursors to vaginal dysbiosis? Frontiers in physiology. 2015;6:253.
•• Castro J, Machado D, Cerca N. Unveiling the role of Gardnerella vaginalis in polymicrobial bacterial vaginosis biofilms: the impact of other vaginal pathogens living as neighbors. ISME J. 2019;13(5):1306–17. (The findings underscore the significance of microbial interactions in bacterial vaginosis-associated biofilms.)
Castro J, Rosca AS, Cools P, Vaneechoutte M, Cerca N. Gardnerella vaginalis enhances Atopobium vaginae viability in an in vitro model. Front Cell Infect Microbiol. 2020;10:83.
Castro J, França A, Bradwell KR, Serrano MG, Jefferson KK, Cerca N. Comparative transcriptomic analysis of Gardnerella vaginalis biofilms vs. planktonic cultures using RNA-seq. NPJ biofilms and microbiomes. 2017;3(1):3.
Jespers V, Kyongo J, Joseph S, Hardy L, Cools P, Crucitti T, et al. A longitudinal analysis of the vaginal microbiota and vaginal immune mediators in women from sub-Saharan Africa. Sci Rep. 2017;7(1):11974.
De Seta F, Campisciano G, Zanotta N, Ricci G, Comar M. The vaginal community state types microbiome-immune network as key factor for bacterial vaginosis and aerobic vaginitis. Frontiers in Microbiology. 2019:2451.
Money D. The laboratory diagnosis of bacterial vaginosis. Canadian J Infectious Diseas Med Microbiol. 2005;16:77–9.
Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74(1):14–22.
Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29(2):297–301.
Blankenstein T, Lytton S, Leidl B, Atweh E, Friese K, Mylonas I. Point-of-care (POC) diagnosis of bacterial vaginosis (BV) using VGTest™ ion mobility spectrometry (IMS) in a routine ambulatory care gynecology clinic. Arch Gynecol Obstet. 2015;292:355–62.
Myziuk L, Romanowski B, Johnson SC. BVBlue test for diagnosis of bacterial vaginosis. J Clin Microbiol. 2003;41(5):1925–8.
West B, Morison L, Van Der Loeff MS, Gooding E, Awasana AA, Demba E, Mayaud P. Evaluation of a new rapid diagnostic kit (FemExam) for bacterial vaginosis in patients with vaginal discharge syndrome in The Gambia. Sexually transmitted diseases. 2003:483–9.
Frobenius W, Bogdan C. Diagnostic value of vaginal discharge, wet mount and vaginal pH–an update on the basics of gynecologic infectiology. Geburtshilfe Frauenheilkd. 2015;75(04):355–66.
Cartwright CP, Lembke BD, Ramachandran K, Body BA, Nye MB, Rivers CA, Schwebke JR. Development and validation of a semiquantitative, multitarget PCR assay for diagnosis of bacterial vaginosis. J Clin Microbiol. 2012;50(7):2321–9.
Gaydos CA, Beqaj S, Schwebke JR, Lebed J, Smith B, Davis TE, et al. Clinical validation of a test for the diagnosis of vaginitis. Obstet Gynecol. 2017;130(1):181.
Hoyme U. Bacterial vaginosis. Zentralbl Gynakol. 1989;111(24):1589–98.
Sobel J, Schmitt C, Meriwether C. Long-term follow-up of patients with bacterial vaginosis treated with oral metronidazole and topical clindamycin. J Infect Dis. 1993;167(3):783–4.
Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193(11):1478–86.
Swidsinski A, Mendling W, Loening-Baucke V, Swidsinski S, Dörffel Y, Scholze J, et al. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. American journal of obstetrics and gynecology. 2008;198(1):97. e1-. e6.
Alves P, Castro J, Sousa C, Cereija TB, Cerca N. Gardnerella vaginalis outcompetes 29 other bacterial species isolated from patients with bacterial vaginosis, using in an in vitro biofilm formation model. J Infect Dis. 2014;210(4):593–6.
Hymes SR, Randis TM, Sun TY, Ratner AJ. DNase inhibits Gardnerella vaginalis biofilms in vitro and in vivo. J Infect Dis. 2013;207(10):1491–7.
Hukić M, Seljmo D, Ramovic A, Ibrišimović MA, Dogan S, Hukic J, Bojic EF. The effect of lysozyme on reducing biofilms by Staphylococcus aureus, Pseudomonas aeruginosa, and Gardnerella vaginalis: an in vitro examination. Microb Drug Resist. 2018;24(4):353–8.
Turovskiy Y, Cheryian T, Algburi A, Wirawan RE, Takhistov P, Sinko PJ, Chikindas ML. Susceptibility of Gardnerella vaginalis biofilms to natural antimicrobials subtilosin, ε-poly-L-lysine, and lauramide arginine ethyl ester. Infectious Diseases in Obstetrics and Gynecology. 2012;2012.
Gottschick C, Deng Z-L, Vital M, Masur C, Abels C, Pieper DH, et al. Treatment of biofilms in bacterial vaginosis by an amphoteric tenside pessary-clinical study and microbiota analysis. Microbiome. 2017;5:1–15.
•• Landlinger C, Tisakova L, Oberbauer V, Schwebs T, Muhammad A, Latka A, et al. Engineered phage endolysin eliminates Gardnerella biofilm without damaging beneficial bacteria in bacterial vaginosis ex vivo. Pathogens. 2021;10(1):54. (The potential of endolysins from Gardnerella prophages, particularly the engineered endolysin PM-477, as a highly selective and effective alternative to antibiotics for treating bacterial vaginosis.)
Braga PC, Dal Sasso M, Culici M, Spallino A. Inhibitory activity of thymol on native and mature Gardnerella vaginalis biofilms: in vitro study. Arzneimittelforschung. 2010;60(11):675–81.
Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sexually transmitted diseases. 2009:732–4.
Swidsinski A, Loening-Baucke V, Swidsinski S, Verstraelen H. Polymicrobial Gardnerella biofilm resists repeated intravaginal antiseptic treatment in a subset of women with bacterial vaginosis: a preliminary report. Arch Gynecol Obstet. 2015;291:605–9.
Weeks RM, Moretti A, Song S, Uhrich KE, Karlyshev AV, Chikindas ML. Cationic amphiphiles against Gardnerella vaginalis resistant strains and bacterial vaginosis-associated pathogens. Pathogens and disease. 2019;77(8):ftz059.
• Tits J, Cammue BP, Thevissen K. Combination therapy to treat fungal biofilm-based infections. Int J Mol Sci. 2020;21(22):8873. (Combination therapy as a novel antibiofilm strategy, discussing in vitro methods for discovering new combinations and providing an overview of the main modes of action for promising antibiofilm treatments.)
Sturdee D, Panay Na. Recommendations for the management of postmenopausal vaginal atrophy. Climacteric. 2010;13(6):509–22.
Shen J, Song N, Williams C, Brown C, Yan Z, Xu C, Forney L. Effects of low dose estrogen therapy on the vaginal microbiomes of women with atrophic vaginitis. Sci Rep. 2016;6:24380.
• Srinivasan S, Hua X, Wu MC, Proll S, Valint D, Reed SD, et al. Impact of topical interventions on the vaginal microbiota and metabolome in postmenopausal women: a secondary analysis of a randomized clinical trial. JAMA Network Open. 2022;5(3):e225032-e. In postmenopausal women, vaginal estradiol tablets induced significant changes in the microbiota, metabolome, and pH, particularly in those with high-diversity bacterial communities, suggesting potential genitourinary health benefits.
FAO/WHO. Evaluation of health and nutritional properties of powder milk and live lactic acid bacteria. Joint FAO/WHO Expert Consultation Cordoba, Argentina; 2001. p. 1–34.
Lebeer S, Vanderleyden J, De Keersmaecker SC. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol. 2010;8(3):171–84.
•• Chen R, Li R, Qing W, Zhang Y, Zhou Z, Hou Y, et al. Probiotics are a good choice for the treatment of bacterial vaginosis: a meta-analysis of randomized controlled trial. Reprod Health. 2022;19(1):1–14. (Probiotic therapy, particularly as a supplementary remedy, is safe and may have both short-term and long-term beneficial effects for bacterial vaginosis (BV) treatment.)
• Mändar R, Sõerunurk G, Štšepetova J, Smidt I, Rööp T, Kõljalg S, et al. Impact of Lactobacillus crispatus-containing oral and vaginal probiotics on vaginal health: a randomised double-blind placebo controlled clinical trial. Beneficial Microbes. 2023;14(2):143–52. (The effectiveness of novel evidence-based probiotics containing Lactobacillus crispatus strains in reducing signs and symptoms of bacterial vaginosis (BV) and vulvovaginal candidiasis (VVC).)
•• Jeng HS, Yan TR, Chen JY. Treating vaginitis with probiotics in non-pregnant females: A systematic review and meta-analysis. Exp Ther Med. 2020;20(4):3749–65. (Probiotics have a significant short-term effect in the treatment of common vaginal infections, including bacterial vaginosis (BV) and vulvovaginal candidiasis (VVC), in non-pregnant females.)
• Husain S, Allotey J, Drymoussi Z, Wilks M, Fernandez‐Felix B, Whiley A, et al. Effects of oral probiotic supplements on vaginal microbiota during pregnancy: a randomised, double‐blind, placebo‐controlled trial with microbiome analysis. BJOG: an International Journal of Obstetrics & Gynaecology. 2020;127(2):275–84. Oral probiotics with Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14, administered from early pregnancy, did not alter rates of bacterial vaginosis or impact the vaginal microbiota in pregnant women.
• Yang S, Reid G, Challis JR, Gloor GB, Asztalos E, Money D, et al. Effect of oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on the vaginal microbiota, cytokines and chemokines in pregnant women. Nutrients. 2020;12(2):368. (Oral administration of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 to low-risk pregnant women did not significantly alter the vaginal microbiota or cytokine levels, suggesting no adverse effects during pregnancy.)
Reid G, Beuerman D, Heinemann C, Bruce AW. Probiotic Lactobacillus dose required to restore and maintain a normal vaginal flora. FEMS Immunol Med Microbiol. 2001;32(1):37–41.
Hakimi S, Farhan F, Farshbaf-Khalili A, Dehghan P, Javadzadeh Y, Abbasalizadeh S, Khalvati B. The effect of prebiotic vaginal gel with adjuvant oral metronidazole tablets on treatment and recurrence of bacterial vaginosis: a triple-blind randomized controlled study. Arch Gynecol Obstet. 2018;297:109–16.
Coste I, Judlin P, Lepargneur J-P, Bou-Antoun S. Safety and efficacy of an intravaginal prebiotic gel in the prevention of recurrent bacterial vaginosis: a randomized double-blind study. Obstetrics and gynecology international. 2012;2012.
Collins SL, McMillan A, Seney S, van der Veer C, Kort R, Sumarah MW, Reid G. Evaluation of lactitol, lactulose, raffinose, and oligofructose for maintenance of a Lactobacillus-dominated vaginal microbiota establishes a promising prebiotic candidate. Appl Environ Microbiol. 2017;84(8):AEM. 02200-AEM. 17.
Rousseau V, Lepargneur J, Roques C, Remaud-Simeon M, Paul F. Prebiotic effects of oligosaccharides on selected vaginal lactobacilli and pathogenic microorganisms. Anaerobe. 2005;11(3):145–53.
Lanzafame RJ, de la Torre S, Leibaschoff GH. The rationale for photobiomodulation therapy of vaginal tissue for treatment of genitourinary syndrome of menopause: An analysis of its mechanism of action, and current clinical outcomes. Photobiomodul Photomed Laser Surg. 2019;37(7):395–407.
• Bicknell B, Laakso E-L, Liebert A, Kiat H. Modifying the microbiome as a potential mechanism of photobiomodulation: A case report. Photobiomodul Photomed Laser Surg. 2022;40(2):88–97. (Suggesting a potential therapeutic avenue (PBM) for chronic diseases with limited treatment options.)
Lev-Sagie A, Goldman-Wohl D, Cohen Y, Dori-Bachash M, Leshem A, Mor U, et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat Med. 2019;25(10):1500–4.
DeLong K, Bensouda S, Zulfiqar F, Zierden HC, Hoang TM, Abraham AG, et al. Conceptual design of a universal donor screening approach for vaginal microbiota transplant. Front Cell Infect Microbiol. 2019;9:306.
• Lehtoranta L, Ala-Jaakkola R, Laitila A, Maukonen J. Healthy vaginal microbiota and influence of probiotics across the female life span. Front Microbiol. 2022;13:819958. (Undergoes dynamic changes influenced by hormonal shifts, with probiotic lactobacilli emerging as a promising approach for maintaining balance and well-being across different life stages.)
• Heravi FS, Naseri K, Hu H. Gut Microbiota Composition in Patients with Neurodegenerative Disorders (Parkinson’s and Alzheimer’s) and Healthy Controls: A Systematic Review. Nutrients. 2023;15(20):4365. (Examines gut microbiota composition in Parkinson’s disease (PD) and Alzheimer’s disease (AD), finding consistent differences between cases and controls, with specific microbial signatures associated with each condition.)
Stone L. A vagina on a chip to model microbiome–host interactions. Nature Reviews Urology. 2023;20(2):64-.
•• Mahajan G, Doherty E, To T, Sutherland A, Grant J, Junaid A, et al. Vaginal microbiome-host interactions modeled in a human vagina-on-a-chip. Microbiome. 2022;10(1):201. (Establishing a human model that accurately replicates the vaginal epithelial microenvironment.)
•• Mirzayi C, Renson A, Consortium GS, Analysis M, 84 QCSFCSS-A, Zohra F, et al. Reporting guidelines for human microbiome research: the STORMS checklist. Nature medicine. 2021;27(11):1885–92. Dddressing the unique challenges of interdisciplinary human microbiome research, providing guidance for comprehensive and clear reporting across various scientific domains
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
FSH conceptualized the review manuscript, conducted the literature review and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Human and Animal Rights and Informed Consent
This article contains no studies with human or animal subjects performed by the author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sadeghpour Heravi, F. Host-vaginal microbiota interaction: shaping the vaginal microenvironment and bacterial vaginosis. Curr Clin Micro Rpt (2024). https://doi.org/10.1007/s40588-024-00227-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s40588-024-00227-8