Abstract
Background
Higher prefrontal cortex (PFC) activation while walking may indicate reduced gait automaticity.
Aim
We examine whether PFC activation during walking improves after training in older adults at risk for mobility disability.
Methods
Forty-two adults aged ≥ 65 participated in a randomized clinical trial (NCT026637780) of a 12-week timing and coordination physical therapy intervention to improve walking (n = 20 intervention, n = 22 active control). PFC activation was measured by functional near-infrared spectroscopy (fNIRS) during four walking tasks over 15 m, each repeated 4 times: even surface walking, uneven surface walking, even dual-task, uneven dual-task; dual-task was reciting every other letter of the alphabet while walking. Gait speed and rate of correct letter generation were recorded. Linear mixed models tested between arm differences in change of fNIRS, gait speed, and letter generation from baseline to follow-up (12-week, 24-week, and 36-week).
Results
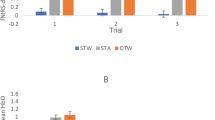
Intervention arms were similar in mean age (74.3 vs. 77.0) and baseline gait speed (0.96 vs. 0.93 m/s). Of 24 comparisons of between arm differences in the fNIRS signals, only two were significant which were not supported by differences at other follow-up times or on other tasks. Gait speed, particularly during dual-task conditions, and correct letter generation did improve post-intervention but improvements did not differ by arm.
Discussion and Conclusions
After training, PFC activation during walking generally did not improve and did not differ by intervention arm. Improvements in gait speed without increased PFC activation may point toward more efficient neural control of walking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mobility limitations affect one-quarter to one-half of community-dwelling older adults [1, 2] with an associated $42 billion in annual health care costs [3]. Current exercise and therapy recommendations for older adults typically target the musculoskeletal and cardiopulmonary systems and overlook the brain’s role in mobility. In response, we developed a motor skill training approach using goal-oriented, task-specific timing, and coordination exercises [4, 5]. The goal of this motor skill training is to challenge the brain to adapt and relearn the sequence of movements and timing with the postures and phases of gait to improve walking. Thereby, improvements in walking may occur by restoring the pattern of brain and neuromuscular activation that optimizes the use of physiologic capacity to meet the demands of walking [5].
The automaticity of walking decreases with age, leading to a greater dependence on attention for motor control [6]. The timing and coordination training is a task-oriented intervention with the goal of returning the older adult to a skilled “expert” walker; in other words, restoring more automatic motor control during walking. The extent of automatic vs. attentional motor control can be detected through functional near-infrared spectroscopy (fNIRS) of the PFC during complex walking tasks [7]. Attentional motor control relies on prefrontal–parietal pathways; with training, motor control shifts toward striatal–cerebellar pathways [8]. Walking in healthy adults is an automatic process with little reliance on the PFC. However, as automatic motor control diminishes in older adults due to impairments in the brain and other systems [5, 7], activation of the PFC during walking tasks increases, particularly under challenging walking conditions [9, 10]. The left dorsolateral PFC (dlPFC) is thought to be particularly relevant for challenging walking conditions in older adults [11, 12].
Current evidence for the plasticity of PFC involvement in walking is limited. A motor learning dance video game intervention resulted in a decrease in PFC activation during treadmill walking in older adults as they became more trained, resulting in a more “youthful” fNIRS signature [13]. Transcranial direct stimulation of the PFC in older adults may also improve walking performance, particularly during challenging walking conditions [14, 15]. Studies in individuals with Parkinson’s disease also demonstrate that behavioral interventions can lead to more automatic motor control as evidenced by lower PFC activation after the intervention [16,17,18].
Here, we examine the effects of a 12-week timing and coordination training program intended to improve walking in older adults at risk for mobility disability compared to a standard physical therapy intervention on PFC activation. The primary results of this randomized clinical trial found that there were clinically meaningful improvements in gait speed but that improvements were no greater in the timing and coordination training compared to the standard physical therapy arm [19]. We proposed that the timing and coordination training would improve automaticity of motor control during walking, resulting in decreased PFC activation by fNIRS during challenged walking, compared to usual care. We further hypothesized that these differences would be driven by changes in activation specifically in the left dorsolateral PFC (dlPFC) and that the intervention would result in better performance defined by faster gait speed and better cognitive performance during dual-task walking. Differential changes in PFC activation could occur in the absence of between arm changes in gait speed, indicating improved automaticity and more efficient walking with the timing and coordination training.
Methods
We follow the CONSORT reporting guidelines [20].
PRIMA study
The Program to Improve Mobility in Aging (PRIMA) is described in detail elsewhere [4] (NCT02663778; https://clinicaltrials.gov/ct2/show/NCT02663778). PRIMA was a randomized clinical trial of motor skill training to improve timing and coordination of gait. The primary outcome was gait speed, which improved significantly but improvements did not differ by intervention arm [19]. The parent study enrolled 249 participants who were randomized to either standard physical therapy or to standard-plus timing and coordination training, referred to as standard and standard-plus below. Participants were recruited from the greater Pittsburgh, USA area using the Pittsburgh Claude D. Pepper Older Americans Independence Center Research Registry. Inclusion criteria included age 65 years or older and walking speed between 0.6 and 1.2 m/s, indicating increased risk for negative outcomes but ability to participate in the intervention [21]. Persons who were unable to participate in testing had medical conditions which would make participation unsafe, or who had plans to leave the area during the study were excluded (for detailed exclusion criteria [4]). Recruitment flow of the main trial and the randomization process are detailed elsewhere [19].
This study complies with standards of the Declaration of Helsinki. The University of Pittsburgh Institutional Review Board approved the protocol and all participants provided written informed consent prior to participation.
PRIMA-NIRS ancillary study
Recruitment for the PRIMA-NIRS ancillary study began after the parent study with the goal to recruit at least 50 participants. Recruitment started in 2018, paused in March 2020 due to COVID-19 restrictions, and ended without restart in September 2020. Follow-up visits were conducted in parallel with the parent study at 12 (immediately post-intervention), 24, and 36 weeks. The first seven participants were enrolled as part of a pilot feasibility study and were only invited to return for PRIMA-NIRS visits at 12 and 24 weeks.
Interventions
Both intervention arms included 2 clinic visits/week for 12 weeks. Intervention arms included a standard physical therapy and a standard-plus timing and coordination training. Intervention protocols are described briefly here; details are reported elsewhere [4].
Standard: The standard intervention included progressive lower extremity strength training and endurance training. Strength training included hip extensor and hip abductor muscle strengthening, plus strengthening of any 2 other lower extremity muscle groups based on individual needs. The endurance training consisted of treadmill walking at a submaximal workload with a self-reported rate of perceived exertion (RPE) of 10–13, somewhat hard.
Standard Plus: The active intervention arm included the standard intervention plus timing and coordination training. Time spent in the standard intervention components was reduced to keep total intervention time equal between groups. The timing and coordination program used goal-oriented, progressively more difficult stepping and walking patterns to promote proper timing and coordination of stepping integrated with the phases of the gait cycle [22]. The goal was to improve the motor skill of walking by realigning biomechanical and neuromotor programs and improving feedback for adjusting movements.
Behavioral: Both intervention groups received a physical activity behavioral change intervention based on the Group Lifestyle Balance™ program, [23] totaling 16 sessions.
Data collection
All data were collected by assessors who were blind to intervention arm.
Challenged walking protocol
Participants were asked to complete both cognitive and mobility tasks along a track with 15 m straightaways (for detailed description see [24]). The mobility tasks consisted of four combinations of two surfaces, even and uneven, and two task conditions, single- and dual-task. Participants completed four repetitions of each of the four combinations of surfaces and tasks in pseudo-randomized order to ensure that walking always progressed continuously around the track, alternating even and uneven surfaces. The even surface consisted of level flooring without obstacles or perturbations. The uneven surface consisted of 1.5 cm high wood prisms arranged randomly at a density of 26 pieces/m2 underneath carpeting [25]. A 20 s quiet standing period was included before each task to act as the baseline condition for fNIRS recordings. The single task involved the participant walking at their comfortable walking pace while not speaking. The dual-task required participants to recite every other letter of the alphabet out loud starting with the letter “B” [26] while walking. Participants were instructed to return to the beginning of the alphabet and continue if they reached the end of the alphabet before completing the walk.
Time to walk, 15 m segments, was recorded by stopwatch and converted to gait speed in m/s. The rate of correct letter generation was calculated as the number of correct letters voiced divided by the number of seconds during each task period (i.e., time to walk the m segment).
Functional near-infrared spectroscopy
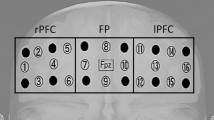
fNIRS data collection, analysis, and reporting are in line with consensus recommendations [27]. Participants wore an eight-channel continuous wave fNIRS headband (Octa-Mon; Artinis Medical Systems; Netherlands) to estimate changes in activity in the right and left prefrontal cortical regions. The fNIRS instrument measured oxygenated (HbO) and deoxygenated (Hbr) hemoglobin concentrations at 850 and 760 nm, respectively, with four sources and one detector on each side of the head. Source to detector distance was 35 mm. For consistent placement, the center of the optodes was aligned with the center of the nose and the bottom of probe was placed just above the eyebrow. The probe covered approximately bilateral Brodmann areas BA9, BA44, BA45, and BA46 (Fig. 1). Optical data were collected at 10 Hz and stored using OxySoft software (Artinis Medical Systems; Netherlands).
fNIRS processing
Raw signals recorded by the fNIRS device were exported to Matlab (MATLAB and Statistics Toolbox Release 2021b, The MathWorks, Inc., Natick, MA) and processed using the NIRS Brain AnalyzIR Toolbox [28]. The start time and the end time of each walking task were recorded and used to label each trial of the fNIRS signals. Trials were trimmed to include 2 s of data before and after labeled tasks to reduce noise. Flat channels due to saturation or equipment error were removed from analysis. Visual checks were performed to confirm no time overlap across tasks and for data quality. Observations were excluded when fNIRS or time data were missing or for protocol deviations (e.g., participant walked during a standing task). Excluded observations from any one visit ranged from 1.8 to 8.9% of available data. Because all walking tasks were completed four times, exclusion of some observations did not impact overall data availability or quality.
The fNIRS light intensity signals were converted to optical density and resampled to 4 Hz to reduce computational burden. The modified Beer–Lambert law was applied to calculate concentration of HbO and Hbr (both in µM) across time from optical density data [29]. In first-level modeling, a canonical model with autoregressive pre-whitening approach using iteratively reweighted least-squares (AR-IRLS) was implemented to model changes in HbO and Hbr for each task relative to a global baseline [30]. We conducted channel-wise Student’s t tests comparing the level of HbO change for each task from the quiet standing baseline state. A mixed effects model with fixed effect of tasks was applied to combine the results of the four tests for each visit. The mixed effects model used robust weighted least-square regression with the covariance matrix derived from the first-level model. We obtained a single t-statistics for each channel and task per visit. These channel-specific t-statistical values were averaged over the right and left hemispheres of the PFC for all primary analyses. Secondary analyses focused on the left dlPFC using channel-specific values from D2S6 and D2S8 (Fig. 1).
Descriptive variables
Demographic characteristics, including age, gender, race, marital status, living arrangement, and educational attainment, were self-reported at screening. The Duke comorbidity index was used to determine whether 8 different physiologic systems were affected by chronic conditions at screening. The Modified Mini-Mental State Exam (3MS) [31] was also administered at screening to assess overall cognitive function. Depressive symptoms were determined by the Geriatric Depression Scale [32]. Speed of processing and switching ability were assessed by Trails A and B tests [8]. Height and weight were measured using standard clinical protocols. Falls in the prior 12 months as well as fear of falling were determined with single-item questions. Gait speed was averaged over six passes of a 14-foot instrumented walkway (Protokinetics LLC, Havertown, PA).
Sample size calculation
At the time, this study was proposed, there was limited data from which to conduct accurate power analyses for fNIRS outcomes. In addition, our recruitment was based on a convenience sample from a larger clinical trial and our analyses were considered exploratory. As such, we did not conduct separate power analyses for this research question. However, other published studies have found significant differences in fNIRS measures with as little as 14–19 participants per group [13, 33]. Our primary outcome was differences at the 12-week time period and our number in each intervention arm with data at that time period is within this range.
Data analysis
We used independent t-samples, chi-square, and Fisher’s exact tests, as appropriate, to compare participant characteristics and baseline measurements between the two groups. We fitted a series of linear mixed models using the SAS® MIXED procedure (SAS Institute, Inc., Cary, NC) with the dependent variable being change from baseline in each of the fNIRS and performance measures. Also included in the models were intervention arm (standard/standard-plus), follow-up time point (at 12/24/36 weeks) and their interaction as fixed effects of interest, baseline value of the outcome as a fixed-effect covariate, and a participant random effect. We used appropriately constructed means contrasts to estimate the difference between intervention-related changes at 12 (immediate effect), 24, and 36 weeks (effect sustained over the longer term).
Results
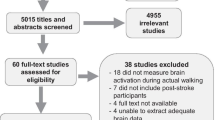
Seventy-one PRIMA participants were contacted to participate in the PRIMA-NIRS ancillary (Fig. 2). Of those contacted, 17 could not be reached within the designated time window, 6 could not be scheduled within the window, 4 were not interested in participating, and 1 dropped out of the parent study. Forty-three participants were enrolled in the PRIMA-NIRS ancillary study. One enrolled participant did not complete baseline fNIRS testing, resulting in 42 participants who are included in these analyses. Of these, 20 were randomized to the standard-plus arm and 22 to the standard arm (Fig. 2). At the 12-week, post-intervention visit, 17 participants in the standard-plus and 17 participants in the standard arm completed the visit. At 24 weeks, 11 in the standard-plus and 15 in the standard arms completed the visit and at 36 weeks, 9 in the standard-plus, and 12 in the standard arm completed the visit (Fig. 2).
Participants had a mean age in the mid-seventies and were predominantly female and White (Table 1). On average, participants had comorbidities in 3 physiologic systems, low depressive symptoms by the GDS, and performed well on the 3MS (Table 1). The average usual pace gait speed at baseline was slightly below 1.0 m/s in both arms (Table 1).
There were no between arm differences in the fNIRS signal or gait speeds at baseline; those in the standard-plus arm had better alphabet task performance (Table 2). Mean values for fNIRS signal (both HbO and Hbr) and performance measures at all time points are provided in Supplemental Tables 1 and 2.
The only significant pre- to post-intervention change in fNIRS was a decrease in HbO for the left PFC during dual-task walking on the even surface (even ABC) from baseline to 24 weeks in the standard-plus group (−1.05 ± 0.39; p = 0.02). The between arm differences in change of fNIRS HbO signal from baseline to follow-up visits were generally small and nonsignificant (Table 3, adjusted difference column). There were only two significant findings indicating a greater decrease in fNIRS HbO signal for both right and left PFC during uneven ABC at week 24 compared to baseline (right: −1.72 ± 0.80, p = 0.04; left: −1.79 ± 0.78, p = 0.03; Table 3) for the standard-plus compared to the standard arm. There was only one between intervention arm comparison that was significant for Hbr values (not shown in tables); this was for the right PFC during even ABC at 24 weeks indicating a greater decrease in Hbr in the standard-plus compared to the standard arm (between arm difference = −2.22, p = 0.003).
There were several significant changes from baseline for the channels D2S6 and D2S8 centered over the left dlPFC, but results did not indicate a consistent pattern (data not shown). D2S6 significantly increased in the standard-plus group from baseline to 12 weeks during even walking (0.72 ± 0.30, p = 0.03). D2S8 significantly decreased in the standard group from baseline to 24 weeks during even (−1.03 ± 0.45, p = 0.04) and even ABC (−0.96 ± 0.41, p = 0.04) and from baseline to 36 weeks during even (−1.43 ± 0.62, p = 0.04) walking. None of the between arm comparisons for change in D2S6 or D2S8 were significant (data not shown). While not significant, magnitudes of between-groups differences in these channel-specific results indicate that the greater decrease in left PFC HbO in the standard-plus group at 24 weeks during the uneven ABC task may be driven by D2S6 (upper left dlPFC) (−1.30 ± 0.67; p = 0.06) rather than D2S8 (lower left dlPFC; −0.65 ± 0.63; p = 0.3).
There were significant improvements in gait speed, particularly during even ABC and uneven ABC tasks, observed in both groups at week 12. These improvements persisted for the standard-plus group on ABC tasks at week 24 (Table 4). Rate of correct letter generation significantly improved at weeks 24 and 36 for both arms (Table 4). There were no significant between arm differences observed in the change in gait speed or letter generation over time (Table 4).
Discussion
In this ancillary study to a randomized controlled trial of a timing and coordination physical therapy program to improve walking speed in older adults, we found that PFC activation as measured by fNIRS generally did not change from pre- to post-intervention and activation did not significantly differ by intervention arm. The lack of consistent change in PFC activation was observed for both left and right hemispheres and for channels specific to the left dlPFC. There was a significant decrease in HbO for the right PFC during dual-task walking on the uneven surface from baseline to 24 weeks in the standard-plus group, consistent with our hypothesis that the training would improve gait automaticity and, therefore, result in decreased PFC activation during walking. However, this change is not supported by similar changes on other tasks or on this task at other time points which suggests that it is spurious. There were also no between arm differences observed for changes in performance for either gait speed or correct letter generation on any of the tasks. Both arms did experience an improved gait speed after the intervention which was most pronounced for the cognitive dual-task conditions. This is consistent with the findings from the main trial which found significant and clinically meaningful improvements in usual pace gait speed in both study arms but no difference between arms [19].
The extent of automatic versus attentional motor control may be assessed through fNIRS of the PFC during complex walking tasks. Walking in healthy adults is an automatic process with little reliance on the PFC. However, as automaticity of motor control diminishes in older adults due to impairments in the brain and other systems [5], activation of the PFC during walking tasks increases [9]. It’s possible that our results of an improvement in gait speed, particularly during dual-task conditions, coupled with no increase in PFC activation indicates an improved efficiency or automaticity in neural control of walking. Only one prior study was identified that assessed changes in PFC activation in older adults without neurologic disease during a motor skill training intervention [13]. In that study, older adults who completed 8 weeks of training on an integrated cognitive–motor dance video game compared to a balance and stretching program had significantly reduced PFC activation of left and right hemispheres after training, regardless of intervention arm. The dance game resulted in larger reductions of left PFC activation during the final 10 s of 30 s fast-paced treadmill walking compared to the balance program [13]. We only assessed overall activation during our overground walking tasks and not the temporal dynamics of the fNIRS signal. It is unknown whether changes in the temporal dynamics of the fNIRS signal have clinical significance.
Our study had several limitations, most notably we did not meet our enrollment goals due to COVID-19-related shutdowns of research operations. However, we were sufficiently close to our aim to enroll at least 50 participants. Also, there was no evidence for the presence of trends that did not reach significance due to insufficient sample size. Further, the large standard errors in our fNIRS estimates may suggest high variability in PFC response. This could have limited our ability to detect significance in changes, particularly if they occurred only in a subgroup of participants. Further research is needed to understand the variability of fNIRS signals and gait performance across older individuals. In addition, fNIRS measurements are limited to the cortical surface of the brain. As a result, we were unable to assess activation of additional relevant regions of the brain, such as the basal ganglia. We chose not to control multiple comparisons as this we considered to be an exploratory subsample analyses rather than a definitive trial and as a result, we were more concerned with missing a potential effect than with detecting one that wasn’t there. As our findings were largely negative, we did not believe this unduly affected the interpretation of our results. Finally, the sample included here was likely not representative of the general population of older adults in the US. Particularly notable was the high educational attainment of this sample with nearly 80% of the sample having a college degree or greater.
Our study had several notable strengths. Our study was a randomized clinical trial of a theory-based motor skill physical therapy intervention [5]. While the evidence for PFC involvement in dual-task walking in older adults is quite robust [9], few randomized clinical trials have assessed whether PFC activation during walking can be modified in older adults without overt neurological conditions [13, 14]. We assessed PFC function under both physically and cognitively demanding walking conditions to better capture the range of challenges experienced in daily mobility [34] and to distinguish between possible differences due to types of challenges. While generalizability of our sample based on demographics is limited, we did include a sample with a wide range of gait speeds and a representative burden of chronic conditions and falls. Finally, while we did not find significant between-groups differences, it is important to nonetheless document our findings from a rigorously conducted randomized trial.
In conclusion, we did not find that participation in a 12-week timing and coordination physical therapy intervention improved gait automaticity as measured by PFC activation from fNIRS. There was evidence for improvements in gait speed, particularly under cognitive dual-task conditions, which did not differ by intervention arm. The role of exercise interventions in improving automaticity of gait in older adults is unclear, and more direct interventions targeting neural control of mobility, such as inclusion of transcranial direct stimulation, may be needed.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Cummings SR, Studenski S, Ferrucci L (2014) A diagnosis of dismobility—giving mobility clinical visibility: a Mobility Working Group recommendation. JAMA 311:2061–2062
Satariano WA, Guralnik JM, Jackson RJ, Marottoli RA, Phelan EA, Prohaska TR (2012) Mobility and aging: new directions for public health action. Am J Public Health 102:1508–1515
Hardy SE, Kang Y, Studenski SA, Degenholtz HB (2011) Ability to walk 1/4 mile predicts subsequent disability, mortality, and health care costs. J Gen Intern Med 26:130–135
Brach JS, VanSwearingen JM, Gil A, Nadkarni NK, Kriska A, Cham R, Perera S (2020) Program to improve mobility in aging (PRIMA) study: methods and rationale of a task-oriented motor learning exercise program. Contemp Clin Trials 89:105912
VanSwearingen JM, Studenski SA (2014) Aging, motor skill, and the energy cost of walking: implications for the prevention and treatment of mobility decline in older persons. J Gerontol A Biol Sci Med Sci 69:1429–1436
Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB (2010) Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev 34:721–733
Clark DJ (2015) Automaticity of walking: functional significance, mechanisms, measurement and rehabilitation strategies. Front Hum Neurosci 9:246
Reitan RM, Wolfson D (1985) The Halstead-Reitan neuropsychological test battery: theory and clinical interpretation, vol 15. Neuropsychology Press, Tucson, AZ, p 486
Udina C, Avtzi S, Durduran T, Holtzer R, Rosso AL, Castellano-Tejedor C, Perez LM, Soto-Bagaria L, Inzitari M (2019) Functional near-infrared spectroscopy to study cerebral hemodynamics in older adults during cognitive and motor tasks: a review. Front Aging Neurosci 11:367
Herold F, Wiegel P, Scholkmann F, Thiers A, Hamacher D, Schega L (2017) Functional near-infrared spectroscopy in movement science: a systematic review on cortical activity in postural and walking tasks. Neurophotonics 4:041403
Manor B, Zhou J, Jor'dan A, Zhang J, Fang J, Pascual-Leone A (2016) Reduction of dual-task costs by noninvasive modulation of prefrontal activity in healthy elders. J Cogn Neurosci 28:275–281
Schneider N, Dagan M, Katz R, Thumm PC, Brozgol M, Giladi N, Manor B, Mirelman A, Hausdorff JM (2021) Combining transcranial direct current stimulation with a motor-cognitive task: the impact on dual-task walking costs in older adults. J Neuroeng Rehabil 18:23
Eggenberger P, Wolf M, Schumann M, de Bruin ED (2016) Exergame and balance training modulate prefrontal brain activity during walking and enhance executive function in older adults. Front Aging Neurosci 8:66
Clark DJ, Chatterjee SA, Skinner JW, Lysne PE, Sumonthee C, Wu SS, Cohen RA, Rose DK, Woods AJ (2021) Combining frontal transcranial direct current stimulation with walking rehabilitation to enhance mobility and executive function: a pilot clinical trial. Neuromodulation 24:950–959
Zhou J, Hao Y, Wang Y, Jor'dan A, Pascual-Leone A, Zhang J, Fang J, Manor B (2014) Transcranial direct current stimulation reduces the cost of performing a cognitive task on gait and postural control. Eur J Neurosci 39:1343–1348
Maidan I, Nieuwhof F, Bernad-Elazari H, Bloem BR, Giladi N, Hausdorff JM, Claassen J, Mirelman A (2018) Evidence for differential effects of 2 forms of exercise on prefrontal plasticity during walking in Parkinson’s disease. Neurorehabil Neural Repair 32:200–208
Thumm PC, Maidan I, Brozgol M, Shustak S, Gazit E, Shema Shiratzki S, Bernad-Elazari H, Beck Y, Giladi N, Hausdorff JM, Mirelman A (2018) Treadmill walking reduces pre-frontal activation in patients with Parkinson’s disease. Gait Posture 62:384–387
Hoang I, Ranchet M, Cheminon M, Derollepot R, Devos H, Perrey S, Luaute J, Danaila T, Paire-Ficout L (2022) An intensive exercise-based training program reduces prefrontal activity during usual walking in patients with Parkinson’s disease. Clin Park Relat Disord 6:100128
Brach JS, Perera S, Shuman V, Gil AB, Kriska A, Nadkarni NK, Rockette-Wagner B, Cham R, VanSwearingen JM (2022) Effect of timing and coordination training on mobility and physical activity among community-dwelling older adults: a randomized clinical trial. JAMA Netw Open 5:e2212921
Schulz KF, Altman DG, Moher D, Group C (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 340:c332
Cesari M, Kritchevsky SB, Penninx BW, Nicklas BJ, Simonsick EM, Newman AB, Tylavsky FA, Brach JS, Satterfield S, Bauer DC, Visser M, Rubin SM, Harris TB, Pahor M (2005) Prognostic value of usual gait speed in well-functioning older people—results from the Health, Aging and Body Composition Study. J Am Geriatr Soc 53:1675–1680
Gentile A (1987) Skill acquisition: action, movement, and neuromotor processes. In: Carr J, Shepherd R, Gordon J, Gentile A, Held J (eds) Movement sciences. Aspen Publishers, Rockville, pp 93–154
Kramer MK, Kriska AM, Venditti EM, Miller RG, Brooks MM, Burke LE, Siminerio LM, Solano FX, Orchard TJ (2009) Translating the Diabetes Prevention Program: a comprehensive model for prevention training and program delivery. Am J Prev Med 37:505–511
Hoppes CW, Huppert TJ, Whitney SL, Dunlap PM, DiSalvio NL, Alshebber KM, Furman JM, Kwon YH, Rosso AL (2020) Changes in cortical activation during dual-task walking in individuals with and without visual vertigo. J Neurol Phys Ther 44:156–163
Thies SB, Richardson JK, Demott T, Ashton-Miller JA (2005) Influence of an irregular surface and low light on the step variability of patients with peripheral neuropathy during level gait. Gait Posture 22:40–45
Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J (2011) fNIRS study of walking and walking while talking in young and old individuals. J Gerontol A Biol Sci Med Sci 66:879–887
Menant JC, Maidan I, Alcock L, Al-Yahya E, Cerasa A, Clark DJ, de Bruin ED, Fraser S, Gramigna V, Hamacher D, Herold F, Holtzer R, Izzetoglu M, Lim S, Pantall A, Pelicioni P, Peters S, Rosso AL, St George R, Stuart S, Vasta R, Vitorio R, Mirelman A (2020) A consensus guide to using functional near-infrared spectroscopy in posture and gait research. Gait Posture 82:254–265
Santosa H, Zhai X, Fishburn F, Huppert T (2018) The NIRS Brain AnalyzIR Toolbox Algorithms 11:73
Scholkmann F, Wolf M (2013) General equation for the differential pathlength factor of the frontal human head depending on wavelength and age. J Biomed Opt 18:105004
Huppert TJ (2016) Commentary on the statistical properties of noise and its implication on general linear models in functional near-infrared spectroscopy. Neurophotonics 3:010401
Teng EL, Chui HC (1987) The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 48:314–318
Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO (1982) Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 17:37–49
Ono Y, Noah JA, Zhang X, Nomoto Y, Suzuki T, Shimada S, Tachibana A, Bronner S, Hirsch J (2015) Motor learning and modulation of prefrontal cortex: an fNIRS assessment. J Neural Eng 12:066004
Patla AE, Shumway-Cook A (1999) Dimensions of mobility: defining the complexity and difficulty associated with community mobility. J Aging Phys Act 7:7–19
Funding
This work was supported by grants from the National Institutes of Health (R01 AG057671, R01 AG045252A, K24 AG057728, P30 AG024827, K01 AG053431). The funders had no role in the conduct of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest related to this research.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Institutional Review Board of the University of Pittsburgh prior to the start of the study.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rosso, A.L., Baillargeon, E.M., Perera, S. et al. Prefrontal cortex activation while walking did not change but gait speed improved after a randomized physical therapy intervention. Aging Clin Exp Res 36, 43 (2024). https://doi.org/10.1007/s40520-023-02666-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40520-023-02666-7