Abstract
Aims
Previous studies have suggested that high mobility group box-1 protein (HMGB1) binds to the toll-like receptor 4 (TLR4) signaling mediates the progression of various inflammatory diseases. But the roles of HMGB1 and TLR4 in aging remain poorly unknown. In this study, we aimed to investigate the serum levels of HMGB1 and myeloid differentiation factor 88 (MyD88), which is one of TLR4’s intracellular adaptor proteins during human aging process and their relevance with cathepsin B (CTSB).
Methods
This research was conducted using the blood samples provided by healthy people (n = 90, 63 men and 27 women). Subjects were subdivided into groups with respect to age: young (about 25 years old, n = 30), middle age (about 40 years old, n = 30), and aged (above 65 years old, n = 30). Altered serum levels of HMGB1, MyD88 and CTSB were measured using an enzyme-linked immunosorbent assay.
Results
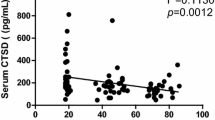
The serum levels of HMGB1 and MyD88 were significantly decreased in the aged group compared with those in the young group. Linear regression analysis showed that HMGB1 and MyD88 positively correlated with CTSB among the whole healthy people. A negative correlation was determined between MyD88 and age.
Conclusions
The serum levels of HMGB1 and MyD88 significantly decreased with age. MyD88, but not HMGB1, was negatively correlated with age.


Similar content being viewed by others
References
Barkauskaite V, Ek M, Popovic K et al (2007) Translocation of the novel cytokine HMGB1 to the cytoplasm and extracellular space coincides with the peak of clinical activity in experimentally UV induced lesions of cutaneous lupus erythematosus. Lupus 16:794–802
Andersson U, Erlandsson-Harris H, Yang H et al (2002) HMGB1 as a DNAbinding cytokine. J Leukoc Biol 72:1084–1091
Gaini S, Pedersen SS, Koldkaer OG et al (2008) New immunological serum markers in bacteraemia: anti-inflammatory soluble CD163, but not proinflammatory high mobility group-box 1 protein, is related to prognosis. Clin Exp Immunol 151:423–431
Bruchfeld A, Qureshi AR, Lindholm B et al (2008) High mobility group box protein-1 correlates with renal function in chronic kidney disease (CKD). Mol Med 14:109–115
Chaichalotornkul S, Nararatwanchai T, Narkpinit S et al (2015) Secondhand smoke exposure-induced nucleocytoplasmic shuttling of HMGB1 in a rat premature skin aging model. Biochem Biophys Res Commun 456(1):92–97
Salminen A, Ojala J, Kaarniranta K et al (2012) Mitochondrial dysfunction and oxidative stress activate inflammasomes: impact on the aging process and age-related diseases. Cell Mol Life Sci 69(18):2999–3013
Huebener P, Pradere JP, Hernandez C et al (2015) The HMGB1/RAGE axis triggers neutrophil-mediated injury amplification following necrosis. J Clin Invest 125(2):539–550
Kaczorowski DJ, Nakao A, Vallabhaneni R et al (2009) Mechanisms of toll-like receptor 4 (TLR4)-mediated inflammation after cold ischemia/reperfusion in the heart. Transplantation 87(10):1455–1463
Fulop T, Larbi A, Douziech N et al (2004) Signal transduction and functional changes in neutrophils with aging. Aging Cell 3:217–226
Vasto S, Candore G, Balistreri CR et al (2007) Inflammatory networks in ageing, age related diseases and longevity. Mech Ageing Dev 128:83–91
Krabbe KS, Pedersen M, Bruunsgaard H (2004) Inflammatory mediators in the elderly. Exp Gerontol 39:687–699
Balistreri CR, Colonna-Romano G, Lio D et al (2009) TLR4 polymorphisms and ageing: implications for the pathophysiology of age-related diseases. J Clin Immunol 29(4):406–415
Tang AH, Brunn GJ, Cascalho M et al (2007) Pivotal advance: endogenous pathway to SIRS, sepsis, and related conditions. J Leukoc Biol 82:282–285
Park JS, Gamboni-Robertson F, He Q et al (2006) High mobility group box 1 protein interacts with multiple toll-like receptors. Am J Physiol Cell Physiol 290:C917–C924
Imai Y, Kuba K, Neely GG et al (2008) Identification of oxidative stress and toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133:235–249
Harris HE, Andersson U, Pisetsky DS (2012) HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol 8(4):195–202
Lin Y, Lin LJ, Jin Y et al (2015) Correlation between serum levels of high mobility group box-1 protein and pancreatitis: a meta-analysis. Biomed Res Int 2015:430185
Boya P, Kroemer G (2008) Lysosomal membrane permeabilization in cell death. Oncogene 27(50):6434–6451
Guicciardi ME, Leist M, Gores GJ (2004) Lysosomes in cell death. Oncogene 23(16):2881–2890
Bobek D, Grčević D, Kovačić N et al (2014) The presence of high mobility group box-1 and soluble receptor for advanced glycation end-products in juvenile idiopathic arthritis and juvenile systemic lupus erythematosus. Pediatr Rheumatol Online J 12:50. doi:10.1186/1546-0096-12-50 (eCollection 2014)
Tang D, Kang R, Zeh HJ 3rd et al (2011) High-mobility group box 1, oxidative stress, and disease. Antioxid Redox Signal 14(7):1315–1335
Davalos AR, Kawahara M, Malhotra GK et al (2013) p53-dependent release of alarmin HMGB1 is a central mediator of senescent phenotypes. J Cell Biol 201(4):613–629
Flynn MG, McFarlin BK, Phillips MD et al (2003) Toll-like receptor 4 and CD14 mRNA expression are lower in resistive exercise-trained elderly women. J Appl Physiol 95:1833–1842
McFarlin BK, Flynn MG, Campbell WW et al (2004) TLR4 is lower in resistance-trained older women and related to inflammatory cytokines. Med Sci Sports Exerc 36:1876–1883
Stewart LK, Flynn MG, Campbell WW et al (2005) Influence of exercise training and age on CD141 cell-surface expression of toll-like receptor 2 and 4. Brain Behav Immun 19:389–397
McFarlin BK, Flynn MG, Campbell WW et al (2006) Physical activity status, but not age, influences inflammatory biomarkers and toll-like receptor 4. J Gerontol A Biol Sci Med Sci 61A:388–393
Biragyn A, Coscia M, Nagashima K et al (2008) Murine beta-defensin 2 promotes TLR-4/MyD88-mediated and NF-kappaB-dependent atypical death of APCs via activation of TNFR2. J Leukoc Biol 83(4):998–1008
van Duin D, Shaw AC (2007) Toll-like receptors in older adults. J Am Geriatr Soc 55(9):1438–1444
Repnik U, Stoka V, Turk V et al (2012) Lysosomes and lysosomal cathepsins in cell death. Biochim Biophys Acta 24(1):22–33
McGuire KA, Barlan AU, Griffin TM et al (2011) Adenovirus type 5 rupture of lysosomes leads to cathepsin B-dependent mitochondrial stress and production of reactive oxygen species. J Virol 85:10806–10813
Wyczałkowska-Tomasik A, Pączek L (2012) Cathepsin B and L activity in the serum during the human aging process: cathepsin B and L in aging. Arch Gerontol Geriatr 55(3):735–738
Morinaga Y, Yanagihara K, Nakamura S et al (2010) Legionella pneumophila induces cathepsin B-dependent necrotic cell death with releasing high mobility group box1 in macrophages. Respir Res 11:158
Goto M, Sugimoto K, Hayashi S et al (2012) Aging-associated inflammation in healthy Japanese individuals and patients with Werner syndrome. Exp Gerontol 47(12):936–939
Acknowledgments
This study was supported by the National Natural Science Fund (No: 81401158).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The protocol of the study was approved by the ethics committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital (Shanghai, China). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
All authors certify that they have not had any financial and personal relationships with other people or organizations that could inappropriately influence or bias this work. There is no conflict of interest for any of the authors.
Rights and permissions
About this article
Cite this article
Fu, GX., Chen, A.F., Zhong, Y. et al. Decreased serum level of HMGB1 and MyD88 during human aging progress in healthy individuals. Aging Clin Exp Res 28, 175–180 (2016). https://doi.org/10.1007/s40520-015-0402-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-015-0402-8