Abstract
Purpose
This study investigated the association between childhood eating behaviors and cortical morphology, in relation to sex and age, in a community sample.
Methods
Neuroimaging data of 71 children (mean age = 9.9 ± 1.4 years; 39 boys/32 girls) were obtained from the Nathan Kline Institute-Rockland Sample. Emotional overeating, food fussiness, and emotional undereating were assessed using the Children’s Eating Behavior Questionnaire. Cortical thickness was obtained at 81,924 vertices covering the entire cortex. Generalized Linear Mixed Models were used for statistical analysis.
Results
There was a significant effect of sex in the association between cortical thickness and emotional overeating (localized at the right postcentral and bilateral superior parietal gyri). Boys with more emotional overeating presented cortical thickening, whereas the opposite was observed in girls (p < 0.05). Different patterns of association were identified between food fussiness and cortical thickness (p < 0.05). The left rostral middle frontal gyrus displayed a positive correlation with food fussiness from 6 to 8 years, but a negative correlation from 12 to 14 years. Emotional undereating was associated with cortical thickening at the left precuneus, left middle temporal gyrus, and left insula (p < 0.05) with no effect of sex or age.
Conclusions
Leveraging on a community sample, findings support distinct patterns of associations between eating behaviors and cortical thickness, depending on sex and age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Various studies have shown that eating disorders (EDs) have early developmental origins [1,2,3,4,5]. Throughout childhood, maladaptive eating patterns (i.e. overeating, fussy eating and undereating) have been associated with an increased risk of ED symptoms or diagnoses in adolescence [2, 3]. Children with overeating are at risk of binge-eating behaviors in adolescence [3], while those with high levels of fussy eating are at risk of anorexia nervosa (AN)[3]. The preceding suggests that childhood eating patterns might predict ED symptoms development. Yet, neural mechanisms involved in the association between childhood eating behaviors and later-life ED symptoms are unknown.
According to their mother’s reports, 39% of the children in the Quebec Longitudinal Study of Child Development (QLSCD) had exhibited overeating behaviors by age 5 [6]. Further, food fussiness and undereating can be considered as “picky-eating” behaviors [7, 8], which has a prevalence of 22% among preschoolers [7]. Picky-eating eventually fade for most children [7,8,9,10]. Little is known about the persistence of picky-eating later in life; however, there is evidence that in some children, picky-eating could be a long-standing behavior with adverse consequences for growth and neurodevelopment [7, 11, 12]. This strengthens the need to better understand such eating behaviors over time, as well as their underlying neurodevelopmental mechanisms.
To date, most studies on brain correlates of childhood eating behaviors concern food choices and preferences [13,14,15] or overweight and obesity [16,17,18]. For example, researchers have been using various functional magnetic resonance imaging (fMRI) tasks to assess children’s food preferences, attempting to identify cognitive pathways (e.g., reward response) involved in food choices [13, 17, 19]. One multimodal neuroimaging study using data from the Adolescent Brain Cognitive Development cohort found that differences in brain structure (i.e., surface area, cortical thickness, subcortical volume, fractional anisotropy and mean diffusivity) predicted significant weight gain over a year in children aged 9–10 years [16]. Other studies have found an inverted U-shaped association between body mass index (BMI) and brain gyrification in school-age children [18]. Specifically, both children with low and high BMI had lower brain gyrification [18]. One study examined the association between food-approach eating behaviors during childhood (at 4 or 10 years old) and brain volume at 13 years old, and reported a positive association between the two [20]. However, this earlier study did not consider restrictive eating behaviors, such as fussy eating or undereating behaviors and it did not include brain measurements during childhood.
In addition, sex differences in ED symptoms presentations are well known, with girls and women being considered at higher risk of AN and bulimia nervosa (BN) than boys and men [21,22,23]. Yet, cultural and social factors such as gendered stereotypes, as well as the lack of inclusion of males in eating disorders research likely have negatively impacted our understanding of eating disorders in boys and men [24]. Still, it is thought that sex differences are less pronounced (and often absent) in binge-eating disorder (BED) and avoidant/restrictive food intake disorder (ARFID) [25]. A study in a Canadian pediatric population found that the clinical presentation of ARFID often differs between sexes, as typically, girls more often present with symptoms related to undereating, whereas boys more often refuse to eat due to sensory characteristics of aliments [26]. Overall, the neurodevelopmental patterns that may help explain the differences and similarities between sexes have not yet been identified.
The aim of the present study was to investigate the link between cortical morphology and child and adolescent eating behaviors that have previously been identified as potential predictors of EDs (i.e., overeating, food fussiness and undereating), in a community-based sample. Furthermore, we studied the moderating role of sex and age in the preceding associations.
Methods
Participants
Participants were selected from the Nathan-Kline Institute Rockland sample (NKI-RS) [27]. The cohort was established with the aim of advancing knowledge in psychiatric neuroscience, particularly the developmental factors associated with risk and resilience to mental disorders across the lifespan. As specified in the original study [27], written informed consent and assent were obtained from every child participant and their legal guardian, and the study was approved by the respective Institutional Review Boards and no new data were collected in the context of the present study. The NKI-RS dataset is an open-access database. A data usage agreement was signed prior to the use of the data. Children aged 6 to 15 years were included in this study if complete data on age, sex, eating behaviors were available, and if they had participated in at least one MRI session. For individuals with more than one follow-up, MRI sessions and clinical assessments were at 15-month interval. The sample included scans from N = 71 participants, 39 of whom were boys (54.9%), with a mean age of 9.9 ± 1.4 years. A summary of participants’ characteristics can be found in Table 1.
Measures
Children’s Eating Behavior Questionnaire (CEBQ)
Eating behaviors were assessed using the Children’s Eating Behavior Questionnaire (CEBQ) [28], completed by the participant’s primary caregiver. The CEBQ contains 35 items assessing eight eating behaviors. The present study focused on three subscales of the CEBQ: emotional overeating, food fussiness and emotional undereating. This was based on previous studies reporting these behaviors as potential predictors of ED symptoms later in life [2, 3, 5]. Items from each subscale were rated on a 5-point Likert scale from 1 (never) to 5 (always) and a mean was calculated (see Table 2). Previous studies on the psychometric properties of the CEBQ showed Cronbach alpha’s for the different subscales of 0.75 or higher [29].
Body mass index (BMI)
Study staff measured height (cm) and weight (kg), and BMI was automatically calculated from height and weight during each study visits.
Image acquisition, pre-processing and cortical thickness measurements
Information on data acquisition can be find elsewhere (http://fcon-1000.projects.nitrc.org/indi/enhanced/mri-protocol.html; [27]). Structural MRI data were pre-processed with the CIVET pipeline (http://www.bic.mni.mcgill.ca/ServicesSoftware/CIVET), and cortical thickness measurements were obtained at 81,924 vertices covering the entire cortex. The T1-weighted image were first corrected for non-uniformity, and linearly registered to a standard space (Talairach-like MNI152 template, established from the ICBM152 dataset). Brain tissues were segmented into grey matter (GM), white matter (WM), and cerebrospinal fluid based on non-linear registration and the artificial neural network using priors defined in the MNI152 template. Inner and outer GM surfaces were extracted using the Constrained Laplacian-based Automated Segmentation with Proximities (CLASP) algorithm. Then, the Laplacian distance between the two surfaces at 81,924 vertices were used to measure cortical thickness. To force a normal distribution on the corticometric data and to increase the signal to noise ratio, each individual’s map of cortical thickness was blurred using a 30-mm full width at half maximum surface-based diffusion smoothing kernel.
Quality control (QC) of the pre-processed data was performed by two independent experts with scores (0 = failed, 1 = questionable, 2 = passed), and only the scans with consensus were included. QC steps included exclusion of data with low signal to noise ratio, motion artifacts, artifacts due to hyperintensities from blood vessels, surface-surface intersections or poor placement of the GM or WM surface. In total, 530 MRI scans from 162 participants were downloaded from the NKI-RS website (**htttp***://fcon_1000.projects.nitrc.org/indi/enhanced/). Of these, 85 scans failed the QC process and 150 scans were questionable, resulting in 380 scans from 141 participants (see NKI_QC at https://github.com/bkhundrakpam/Project-on-Childhood-Eating-Behaviours). Filtering for CEBQ scores resulted in the final study sample of N = 155 scans from 71 participants (Table 1).
Statistical analyses
Data were analyzed using mixed effect generalized linear models (GLM)s at vertex-level (79,950 vertices covering the entire cortex without ventricule region) and were quantified using t-statistics. Significant associations were assessed with multiple comparisons corrected p-statistic using random field theory (RFT) [30]. Statistical analyses were conducted using MATLAB (R2019b).
First, the associations between cortical thickness and the emotional overeating, food fussiness and emotional undereating subscales of the CEBQ were assessed separately. For each three CEBQ subscales, cortical thickness was included as outcome and one CEBQ subscale, age, sex, and an age*sex interaction were included as predictors. Participant was added as a random factor in the GLM models to control for having some participants with one, two or three measurement times.
To investigate sex-specific patterns in the association between eating behaviors and cortical thickness, the models were re-estimated separately for boys and girls. Re-estimation was only calculated for the CEBQ subscales showing an interaction of sex in step one.
To clarify the role of age in the association between childhood eating behaviors and cortical thickness, an age-centered approach was used, again only for the CEBQ subscales displaying an interaction of age in step one. An age-centered approach has previously been used for investigating age-specific associations between behavior and brain measures [31, 32]. In this approach, linear mixed models were computed at different centered ages. For example, for age 10 years, 10 was subtracted from the age at data acquisition and this value was put in as the age term. Then, the GLM computed the effect of CEBQ scores on cortical thickness at each age based on values estimated from developmental curves modelled on all data.
Finally, because BMI is typically related to eating behaviors [6, 33, 34], a sensitivity analysis including BMI allowed us to assess its contribution to the preceding associations.
Results
Emotional overeating
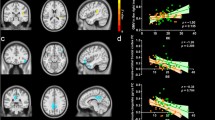
Table 3 presents the associations between cortical thickness and the emotional overeating, food fussiness and emotional undereating subscales of the CEBQ. Whereas there was no overall association between cortical thickness and emotional overeating, there was a moderating role of sex in the association. In girls, cortical thickness in the right postcentral and bilateral superior parietal gyri was negatively associated with emotional overeating (p < 0.05, RFT corrected; Fig. 1). Thus, girls with more emotional overeating had a thinner cortex in these regions. The preceding associations were in the opposite direction for boys (p < 0.05, RFT corrected; Fig. 1), indicating that boys with more emotional overeating had a thicker cortex in these brain regions. No age effect was observed for the association between emotional overeating and cortical thickness.
Interaction of sex and emotional overeating on cortical thickness. The interaction of sex and emotional overeating scores on cortical thickness is represented by t-statistics (left), while the right panel shows the cortical regions with significant interaction (p < 0.05). At the peak vertex of interaction, scatter plots are shown separately for boys and girls. Notably, girls showed thinning with increasing emotional overeating while boys showed thickening with increasing emotional overeating
Food fussiness
There was a negative association between cortical thickness and food fussiness in the right inferior parietal gyrus, suggesting that children with more fussy eating showed cortical thinning in this brain region (p < 0.05, RFT corrected; Fig. 2). Further, an age-centered approach highlighted different patterns of association between food fussiness and cortical thickness according to age (p < 0.05, RFT corrected; Fig. 3). The left rostral middle frontal gyrus displayed a significant positive correlation with food fussiness from 6 until 8 years (i.e., thickening in children with more fussy eating), and there was no significant association at 10 years old. Notably, between 12 and 14 years, the association was negative, indicating that young adolescents who had more fussy eating presented cortical thinning.
Association of food fussiness and cortical thickness. The association of food fussiness and cortical thickness is represented by t-statistics (left), while the right panel shows the cortical regions with significant interaction (p < 0.05). We observed significant thinning with food fussiness localized at the right inferior parietal cortex
Association of food fussiness and cortical thickness with age using age-centering approach. The association of food fussiness and cortical thickness with age is represented by t-statistics (upper panel), while the lower panel shows the cortical regions with significant association (p < 0.05). During childhood (6 years and 8 years), we observed significant positive associations between food fussiness and cortical thickness, while during adolescence (12 and 14 years), we observed significant negative associations between food fussiness and cortical thickness
Emotional undereating
Children with more emotional undereating had a greater cortical thickness in the left precuneus, left middle temporal gyrus and left insula (p < 0.05, RFT corrected; Fig. 4). No interaction of sex or age was observed on this subscale.
Association of emotional undereating and cortical thickness. The association of emotional overeating and cortical thickness is represented by t-statistics (left), while the right panel shows the cortical regions with significant interaction (p < 0.05). We observed significant thickening with emotional overeating localized at the left precuneus, the left insula and the left superior temporal gyrus
Sensitivity analyses
Including BMI in the models did not change interpretation (i.e., effect size/direction of effect) of the preceding findings.
Discussion
The present study reported an association between eating behaviors and cortical thickness in a community-based sample of children aged 6 to 15 years. The association between cortical morphology partly varied according to sex and age.
Our results suggest that the association between brain structure and eating patterns might already be, at least in part, established early in development. However, it remains unknown whether this association reflects a differential risk, or a consequence of maladaptive eating patterns. To date, developmental studies support the association between early eating behaviors and disordered eating in adolescence [3, 5], and brain differences have been identified in EDs [35, 36]. The current study contributes to the literature because most existing studies to date have focused on the link between brain processes and BMI [16,17,18] or food preferences/food choices [13,14,15, 17], but not on specific eating patterns linked to ED symptoms across development.
A novel finding from the present study is the identification of sex differences in the association between cortical morphology and emotional overeating. Girls presented cortical thinning with increasing emotional overeating, while boys showed the opposite. The direction of effects in girls in the right postcentral and bilateral superior parietal gyri is consistent with some studies in women with BN showing cortical thinning in similar brain regions [37,38,39]. Findings related to emotional overeating are also in line with evidence of sex differences in children’s food choices and preferences [8]. Parental practices and differences in attitude toward girls and boys could play a role in these sex differences [8]. In the future, it would be of interest to consider gendered factors related to eating behaviors and brain processes, given that gender has been associated with differences in the clinical presentation of EDs [24, 40, 41].
Uncontrolled eating (an umbrella term for a few psychological constructs related to loss-of-control overeating and emotional eating) has been associated with EDs [42,43,44], and particularly EDs involving binge-eating symptoms [42]. Uncontrolled eating has been associated with temperament traits (e.g., self-directedness) and cognitive processes (e.g., reward sensitivity, cognitive control) that have been linked to EDs [43, 44]. The present study complemented previous studies by identifying brain correlates of emotional overeating in childhood, which may be relevant to the development of EDs later in life.
Children with fussy eating displayed cortical thinning in the right inferior parietal gyrus, and those with undereating behaviors presented cortical thickening in the left precuneus, left middle temporal gyrus and left insula. Both behaviors are components of picky-eating, which can be considered a precursor to ARFID [25]. The lack of sex interaction between cortical thickness and the food fussiness and emotional undereating subscales is consistent with lack of pronounced sex differences typically reported in picky-eating and ARFID [26, 45].
Notably, the age-centered approach revealed a dynamic pattern of association between food fussiness and cortical morphology. The region of the left rostral middle frontal gyrus was thicker in younger children with high levels of food fussiness but thinner in older children with high levels of food fussiness. This finding is of interest in relation to previous research on ARFID and neurodevelopmental disorders. Given that most children grow out of picky-eating [7,8,9,10], it is possible that the persistence of picky-eating behaviors might be related to an atypical neurodevelopmental pattern. Further, the neurodevelopmental trajectory of children who continue to exhibit food fussiness in adolescence may, in part, be similar to what is observed in youth with some neurodevelopmental disorders. Supporting this hypothesis, neurodevelopmental disorders are frequently comorbid with ARFID [25, 45,46,47,48], and autism spectrum disorder has been associated with the chronicity of problematic eating patterns in children [45, 49]. In the present study, we found a reduction in cortical thickness in the left rostral middle frontal gyrus in children who continued to exhibit food fussiness in adolescence. This cortical thinning was located in a brain region previously highlighted in individuals with neurodevelopmental disorders, including autism spectrum disorder, attention deficit/hyperactivity disorder and schizophrenia [50, 51]. These results suggest that there may be a neurobiological link between certain maladaptive childhood eating behaviors and neurodevelopmental disorders, although more work is needed to test this hypothesis.
Lastly, the positive association between emotional undereating and cortical thickness in the left precuneus, left middle temporal gyrus and left insula is in line with previous studies on brain correlates of restrictive eating behaviors in women [52, 53]. These brain regions have been related to emotion perception and social cognition [52], two important cognitive aspects in EDs. Our results indicate that at least some neural differences might be observable during childhood in individuals with undereating behaviors. Overall, future studies should consider brain processes and eating behaviors from childhood onward to determine whether neurobiological differences could be involved in the transition from early maladaptive eating patterns to long-term risk of EDs, as few (if any) studies have investigated the link between early eating behaviors, neurodevelopment and risk of EDs altogether.
The strengths of the current study include the inclusion of both boys and girls, and the use of a standardized imaging protocol in a well-characterized sample. Still, results should be considered in light of some limitations. The CEBQ is a parent-reported questionnaire, which can induce a social desirability bias. Also, the number of brain scans was not the same for all individuals (which was controlled for by adding participants as a random factor in the statistical analyses) and the sample size was relatively small, possibly limiting statistical power.
In conclusion, the present study provides evidence for an association between cortical morphology and childhood eating behaviors. We found that sex was a moderator of the association between cortical thickness and emotional eating, while age moderated the association between cortical thickness and food fussiness. Findings may have implications for the early detection and prevention of EDs, as they may contribute to the understanding of early brain differences associated with childhood eating behaviors, which may be present before the development of full-blown EDs and could represent behavioral and neurobiological risk factors.
Availability of data and materials
Scripts are available at https://github.com/bkhundrakpam/Project-on-Childhood-Eating-Behaviours
References
Berkowitz SA, Witt AA, Gillberg C, Råstam M, Wentz E, Lowe MR (2016) Childhood body mass index in adolescent-onset anorexia nervosa. Int J Eat Disord 49:1002–1009. https://doi.org/10.1002/eat.22584
Breton É, Côté SM, Dubois L, Vitaro F, Boivin M, Tremblay RE, Booij L (2023) Childhood overeating and disordered eating from early adolescence to young adulthood: a longitudinal study on the mediating role of BMI, victimization and desire for thinness. J Youth Adolesc 52(8):1582–1594. https://doi.org/10.1007/s10964-023-01796-5
Herle M, Stavola BD, Hübel C, Abdulkadir M, Ferreira DS, Loos RJF, Bryant-Waugh R, Bulik CM, Micali N (2020) A longitudinal study of eating behaviours in childhood and later eating disorder behaviours and diagnoses. Br J Psychiatry 216:113–119. https://doi.org/10.1192/bjp.2019.174
Kotler LA, Cohen P, Davies M, Pine DS, Walsh BT (2001) Longitudinal relationships between childhood, adolescent, and adult eating disorders. J Am Acad Child Adolesc Psychiatry 40:1434–1440. https://doi.org/10.1097/00004583-200112000-00014
Van Eeden AE, Oldehinkel AJ, van Hoeken D, Hoek HW (2021) Risk factors in preadolescent boys and girls for the development of eating pathology in young adulthood. Int J Eat Disord 54:1147–1159. https://doi.org/10.1002/eat.23496
Dubois L, Farmer AP, Girard M, Peterson K (2007) Preschool children’s eating behaviours are related to dietary adequacy and body weight. Eur J Clin Nutr 61:846–855. https://doi.org/10.1038/sj.ejcn.1602586
Cole NC, An R, Lee S-Y, Donovan SM (2017) Correlates of picky eating and food neophobia in young children: a systematic review and meta-analysis. Nutr Rev 75:516–532. https://doi.org/10.1093/nutrit/nux024
Keller KL, Kling SMR, Fuchs B, Pearce AL, Reigh NA, Masterson T, Hickok K (2019) A biopsychosocial model of sex differences in children’s eating behaviors. Nutrients 11:682. https://doi.org/10.3390/nu11030682
Cano SC, Tiemeier H, Hoeken DV, Tharner A, Jaddoe VWV, Hofman A, Verhulst FC, Hoek HW (2015) Trajectories of picky eating during childhood: a general population study. Int J Eat Disord 48:570–579. https://doi.org/10.1002/eat.22384
Herle M, Stavola BD, Hübel C, Ferreira DLS, Abdulkadir M, Yilmaz Z, Loos RJF, Bryant-Waugh R, Bulik CM, Micali N (2020) Eating behavior trajectories in the first 10 years of life and their relationship with BMI. Int J Obes 44:1766–1775. https://doi.org/10.1038/s41366-020-0581-z
Gibson EL, Cooke L (2017) Understanding food fussiness and its implications for food choice, health, weight and interventions in young children: the impact of professor Jane Wardle. Curr Obes Rep 6:46–56. https://doi.org/10.1007/s13679-017-0248-9
Mascola AJ, Bryson SW, Agras WS (2010) Picky eating during childhood: a longitudinal study to age 11 years. Eat Behav 11:253–257. https://doi.org/10.1016/j.eatbeh.2010.05.006
Adise S, Geier CF, Roberts NJ, White CN, Keller KL (2018) Is brain response to food rewards related to overeating? A test of the reward surfeit model of overeating in children. Appetite 128:167–179. https://doi.org/10.1016/j.appet.2018.06.014
English LK, Masterson TD, Fearnbach SN, Tanofsky-Kraff M, Fisher J, Wilson SJ, Rolls BJ, Keller KL (2019) Increased brain and behavioural susceptibility to portion size in children with loss of control eating. Pediatr Obes 14:e12436. https://doi.org/10.1111/ijpo.12436
Reichelt AC, Rank MM (2017) The impact of junk foods on the adolescent brain. Birth Defects Res 109:1649–1658. https://doi.org/10.1002/bdr2.1173
Adise S, Allgaier N, Laurent J, Hahn S, Chaarani B, Owens M, Yuan D, Nyugen P, Mackey S, Potter A, Garavan HP (2021) Multimodal brain predictors of current weight and weight gain in children enrolled in the ABCD study ®. Dev Cogn Neurosci 49:100948. https://doi.org/10.1016/j.dcn.2021.100948
Bruce AS, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR, Butler MG, Savage CR (2010) Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obes 34:1494–1500. https://doi.org/10.1038/ijo.2010.84
Steegers C, Blok E, Lamballais S, Jaddoe V, Bernardoni F, Vernooij M, van der Ende J, Hillegers M, Micali N, Ehrlich S, Jansen P, Dieleman G, White T (2021) The association between body mass index and brain morphology in children: a population-based study. Brain Struct Funct. https://doi.org/10.1007/s00429-020-02209-0
van Meer F, van der Laan LN, Eiben G, Lissner L, Wolters M, Rach S, Herrmann M, Erhard P, Molnar D, Orsi G, Viergever MA, Adan RAH, Smeets PAM, I. Family Consortium (2019) Development and body mass inversely affect children’s brain activation in dorsolateral prefrontal cortex during food choice. Neuroimage 201:116016. https://doi.org/10.1016/j.neuroimage.2019.116016
Dmitrichenko O, Mou Y, Voortman T, White T, Jansen PW (2022) Food-approach eating behaviors and brain morphology: the generation R study. Front Nutr 9:846148. https://doi.org/10.3389/fnut.2022.846148
Lavender JM, Brown TA, Murray SB (2017) Men, muscles, and eating disorders: an overview of traditional and muscularity-oriented disordered eating. Curr Psychiatry Rep 19:32. https://doi.org/10.1007/s11920-017-0787-5
Strother E, Lemberg R, Stanford SC, Turberville D (2012) Eating disorders in men: underdiagnosed, undertreated, and misunderstood. Eat Disord 20:346–355. https://doi.org/10.1080/10640266.2012.715512
Timko CA, DeFilipp L, Dakanalis A (2019) Sex differences in adolescent anorexia and bulimia nervosa: beyond the signs and symptoms. Curr Psychiatry Rep 21:1. https://doi.org/10.1007/s11920-019-0988-1
Breton É, Juster R-P, Booij L (2023) Gender and sex in eating disorders: a narrative review of the current state of knowledge, research gaps, and recommendations. Brain Behav 13:e2871. https://doi.org/10.1002/brb3.2871
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Association, Washington
Katzman DK, Spettigue W, Agostino H, Couturier J, Dominic A, Findlay SM, Lam P-Y, Lane M, Maguire B, Mawjee K, Parikh S, Steinegger C, Vyver E, Norris ML (2021) Incidence and age- and sex-specific differences in the clinical presentation of children and adolescents with avoidant restrictive food intake disorder. JAMA Pediatr 175:e213861. https://doi.org/10.1001/jamapediatrics.2021.3861
Nooner K, Colcombe S, Tobe R, Mennes M, Benedict M, Moreno A, Panek L, Brown S, Zavitz S, Li Q, Sikka S, Gutman D, Bangaru S, Schlachter RT, Kamiel S, Anwar A, Hinz C, Kaplan M, Rachlin A, Adelsberg S, Cheung B, Khanuja R, Yan C, Craddock C, Calhoun V, Courtney W, King M, Wood D, Cox C, Kelly C, DiMartino A, Petkova E, Reiss P, Duan N, Thompsen D, Biswal B, Coffey B, Hoptman M, Javitt D, Pomara N, Sidtis J, Koplewicz H, Castellanos F, Leventhal B, Milham M (2012) The NKI-rockland sample: a model for accelerating the pace of discovery science in psychiatry. Front Neurosci 6:152. https://doi.org/10.3389/fnins.2012.00152
Wardle J, Guthrie CA, Sanderson S, Rapoport L (2001) Development of the children’s eating behaviour questionnaire. J Child Psychol Psychiatry 42:963–970. https://doi.org/10.1111/1469-7610.00792
Njardvik U, Klar EK, Thorsdottir F (2018) The factor structure of the Children’s Eating Behaviour Questionnaire: a comparison of four models using confirmatory factor analysis. Health Sci Rep 1:e28. https://doi.org/10.1002/hsr2.28
Worsley KJ, Taylor JE, Tomaiuolo F, Lerch J (2004) Unified univariate and multivariate random field theory. Neuroimage 23(Suppl 1):S189-195. https://doi.org/10.1016/j.neuroimage.2004.07.026
Khundrakpam B, Choudhury S, Vainik U, Al-Sharif N, Bhutani N, Jeon S, Gold I, Evans A (2020) Distinct influence of parental occupation on cortical thickness and surface area in children and adolescents: Relation to self-esteem. Hum Brain Mapp 41:5097–5113. https://doi.org/10.1002/hbm.25169
Nguyen T-V, McCracken J, Ducharme S, Botteron KN, Mahabir M, Johnson W, Israel M, Evans AC, Karama S, Brain Development Cooperative Group (2013) Testosterone-related cortical maturation across childhood and adolescence. Cereb Cortex 23:1424–1432. https://doi.org/10.1093/cercor/bhs125
Kininmonth A, Smith A, Carnell S, Steinsbekk S, Fildes A, Llewellyn C (2021) The association between childhood adiposity and appetite assessed using the Child Eating Behavior Questionnaire and Baby Eating Behavior Questionnaire: a systematic review and meta-analysis. Obes Rev 22:e13169. https://doi.org/10.1111/obr.13169
Stice E, Gau JM, Rohde P, Shaw H (2017) Risk factors that predict future onset of each DSM-5 eating disorder: predictive specificity in high-risk adolescent females. J Abnorm Psychol 126:38–51. https://doi.org/10.1037/abn0000219
Frank GKW (2019) Neuroimaging and eating disorders. Curr Opin Psychiatry 32:478–483. https://doi.org/10.1097/YCO.0000000000000544
Frank GKW, Shott ME, DeGuzman MC (2019) The neurobiology of eating disorders. Child Adolesc Psychiatr Clin N Am 28:629–640. https://doi.org/10.1016/j.chc.2019.05.007
Berner LA, Wang Z, Stefan M, Lee S, Huo Z, Cyr M, Marsh R (2019) Subcortical shape abnormalities in bulimia nervosa. Biol Psychiatry Cogn Neurosci Neuroimaging 4:1070–1079. https://doi.org/10.1016/j.bpsc.2018.12.011
Marsh R, Stefan M, Bansal R, Hao X, Walsh BT, Peterson BS (2015) Anatomical characteristics of the cerebral surface in bulimia nervosa. Biol Psychiatry 77:616–623. https://doi.org/10.1016/j.biopsych.2013.07.017
Westwater ML, Seidlitz J, Diederen KMJ, Fischer S, Thompson JC (2018) Associations between cortical thickness, structural connectivity and severity of dimensional bulimia nervosa symptomatology. Psychiatry Res Neuroimaging 271:118–125. https://doi.org/10.1016/j.pscychresns.2017.11.006
Coelho JS, Suen J, Clark BA, Marshall SK, Geller J, Lam P-Y (2019) Eating disorder diagnoses and symptom presentation in transgender youth: a scoping review. Curr Psychiatry Rep 21:107. https://doi.org/10.1007/s11920-019-1097-x
Simone M, Hazzard VM, Askew A, Tebbe EA, Lipson SK, Pisetsky EM (2022) Variability in eating disorder risk and diagnosis in transgender and gender diverse college students. Ann Epidemiol S1047–2797(22):00057–00066. https://doi.org/10.1016/j.annepidem.2022.04.007
Reichenberger J, Schnepper R, Arend A-K, Richard A, Voderholzer U, Naab S, Blechert J (2021) Emotional eating across different eating disorders and the role of body mass, restriction, and binge eating. Int J Eat Disord. https://doi.org/10.1002/eat.23477
Rotella F, Fioravanti G, Godini L, Mannucci E, Faravelli C, Ricca V (2015) Temperament and emotional eating: a crucial relationship in eating disorders. Psychiatry Res 225:452–457. https://doi.org/10.1016/j.psychres.2014.11.068
Vainik U, García-García I, Dagher A (2019) Uncontrolled eating: a unifying heritable trait linked with obesity, overeating, personality and the brain. Eur J Neurosci 50:2430–2445. https://doi.org/10.1111/ejn.14352
Watts R, Archibald T, Hembry P, Howard M, Kelly C, Loomes R, Markham L, Moss H, Munuve A, Oros A, Siddall A (2023) The clinical presentation of avoidant restrictive food intake disorder in children and adolescents is largely independent of sex, autism spectrum disorder and anxiety traits. EClinicalMedicine. https://doi.org/10.1016/j.eclinm.2023.102190
Bourne L, Mandy W, Bryant-Waugh R (2022) Avoidant/restrictive food intake disorder and severe food selectivity in children and young people with autism: a scoping review. Dev Med Child Neurol 64:691–700. https://doi.org/10.1111/dmcn.15139
Farag F, Sims A, Strudwick K, Carrasco J, Waters A, Ford V, Hopkins J, Whitlingum G, Absoud M, Kelly VB (2022) Avoidant/restrictive food intake disorder and autism spectrum disorder: clinical implications for assessment and management. Dev Med Child Neurol 64:176–182. https://doi.org/10.1111/dmcn.14977
Nygren G, Linnsand P, Hermansson J, Dinkler L, Johansson M, Gillberg C (2021) Feeding problems including avoidant restrictive food intake disorder in young children with autism spectrum disorder in a multiethnic population. Front Pediatr 9:780680. https://doi.org/10.3389/fped.2021.780680
Dumont E, Jansen A, Duker PC, Seys DM, Broers NJ, Mulkens S (2022) Feeding/eating problems in children who refrained from treatment in the past: who did (not) recover? Front Pediatr 10:860785. https://doi.org/10.3389/fped.2022.860785
Dumontheil I, Burgess PW, Blakemore S-J (2008) Development of rostral prefrontal cortex and cognitive and behavioural disorders. Dev Med Child Neurol 52:168–181. https://doi.org/10.1111/j.1469-8749.2008.02026.x
Rasser PE, Ehlkes T, Schall U (2024) Fronto-temporal cortical grey matter thickness and surface area in the at-risk mental state and recent-onset schizophrenia: a magnetic resonance imaging study. BMC Psychiatry 24(1):33
Finch JE, Palumbo IM, Tobin KE, Latzman RD (2021) Structural brain correlates of eating pathology symptom dimensions: a systematic review. Psychiatry Res Neuroimaging 317:111379. https://doi.org/10.1016/j.pscychresns.2021.111379
Walton E, Bernardoni F, Batury VL, Bahnsen K, Larivière S, Abbate-Daga G, Andres-Perpiña S, Bang L, Bischoff-Grethe A, Brooks SJ, Campbell IC (2022) Brain structure in acutely underweight and partially weight-restored individuals with anorexia nervosa: a coordinated analysis by the ENIGMA eating disorders working group. Biol Psychiatry 92(9):730–738. https://doi.org/10.1016/j.biopsych.2022.04.022
Funding
EB was supported by a doctoral research award from the FRQS. AE is grateful to CIHR for a Project Grant (254573). BK was supported by the Brain & Behavior Research Foundation (BBRF) through a NARSAD 2020 Young Investigator Grant (29492). SJ is supported by the National Research Foundation of Korea grant funded by the Korea government (MSIT; 2022R1C1C2011227).
Author information
Authors and Affiliations
Contributions
EB, BK and LB designed the specific research question described in this manuscript. BK, SJ and AE analyzed the data. EB, BK, and LB interpreted the data. EB, BK and LB generated the figures and table and drafted the manuscript. LB supervised the work described in this manuscript. All authors (EB, BK, SJ, AE, and LB) revised, edited and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Breton, E., Khundrakpam, B., Jeon, S. et al. Cortical thickness and childhood eating behaviors: differences according to sex and age, and relevance for eating disorders. Eat Weight Disord 29, 47 (2024). https://doi.org/10.1007/s40519-024-01675-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40519-024-01675-3