Abstract
Purpose of Review
To evaluate the effectiveness of psychostimulant augmentation of antidepressants in the treatment of depression in adults.
Recent Findings
In our analysis of 13 RCTs involving 2478 participants, psychostimulant augmentation demonstrated a statistically significant reduction in depressive symptom severity compared to placebo augmentation (N = 1827; SMD = − 0.18; 95% CI (− 0.36, − 0.01); p = 0.04, I2 = 65%). However, we did not observe a significant increase in remission rates (N = 1709; OR = 1.30; 95% CI (0.97, 1.75); p = 0.08, I2 = 32%).
Summary
Depression, a pressing global health issue, is typically treated with antidepressant monotherapy, offering limited relief. Our study introduces a potential breakthrough: psychostimulant augmentation of antidepressants, which significantly alleviates depressive symptoms. Nevertheless, larger high-quality trials evaluating a wider range of drugs are necessary to further explore and strengthen our findings.
Similar content being viewed by others
Introduction
Depressive disorders remain one of the leading causes of disease burden worldwide. The most recent and comprehensive statistics available, dating to 2019, rank these disorders as the second and 13 leading causes of years lost due to disability and disability-adjusted life years, respectively. Furthermore, from 1990 to 2019, their prevalence has remained fundamentally unchanged [12•].
Despite extensive research, the exact etiology and pathophysiology of depression remain elusive. Multiple theories have been proposed, including the monoamine hypothesis, which suggests dysregulation of serotonin, dopamine, and noradrenaline pathways in depressed individuals [14].
Currently, pharmacological treatment of depression primarily relies on antidepressants, particularly selective serotonin reuptake inhibitors (SSRIs), serotonin-noradrenaline reuptake inhibitors (SNRIs), and tricyclic antidepressants. It is thought that these drugs work first and foremost by increasing the synaptic levels of monoamines [5], thus falling within the framework of the monoamine hypothesis.
The exact definition of what does and does not constitute a psychostimulant is not widely agreed upon; namely, atomoxetine (included in this review as a psychostimulant) is usually considered to be a non-stimulant ADHD treatment [30]. However, for the purposes of this review, this class encompasses several drugs that, in increasing synaptic dopamine and noradrenaline levels [4, 7, 11, 21, 30], produce overlapping effects, such as aiding attention, reducing sleepiness and fatigue, and increasing alertness, and therefore share therapeutic indications [25].
Having been established that monoamine dysregulation is significantly implicated in the pathogenesis of depression and that psychostimulants influence the metabolic pathways of monoamines, not unlike most antidepressants, it would follow that treatment of depressed patients with psychostimulants might constitute an effective approach. Moreover, on a symptomatic level, the mood-elevating, cognitive-enhancing, and wake-inducing effects of these drugs are in stark contrast with common depressive symptoms, which further corroborates a potential therapeutic use for these drugs.
As previously expanded on, MDD is a prevalent and burdensome disease, which makes having effective treatment options of special importance. Nevertheless, antidepressants have been shown to only be moderately more effective than placebo [5] and even to be comparable in efficacy to “active” placebos [20]. This paradigm allows for two approaches: either novel, more effective, drugs are developed, or older drugs are repurposed. This review explores the latter option.
Experimental Procedures (Methods)
Criteria for considering studies
Type of Studies
We included randomized controlled trials with at least double blinding, in which participants have been randomly allocated to an experimental group and a placebo control group. Only studies published in English and after the year 2000 were considered.
Participants
Studies were included if they involved adult (> 18 years old) men and women, with a primary diagnosis of major depressive disorder, as defined by the DSM (any edition) or the ICD (any edition). A decision was made to exclude studies which included participants with a diagnosis of bipolar disorder, because it is not within the objectives of this review to assess the efficacy of augmentation in this subset of depressed patients, as the distinct nature of bipolar disorder would merit its own review. It was also decided that studies focusing on depressed patients diagnosed with diseases for which psychostimulant therapy is formally indicated (ADHD, narcolepsy, and obstructive sleep apnea, for example) or with severe fatigue or apathy-causing diseases (notably, AIDS, cancer, Parkinson’s disease, and dementia) should be excluded, as any benefit of psychostimulant therapy cannot necessarily be attributed to its effect on the pathophysiology of depression.
No restrictions were placed regarding location, setting, and number of participants.
Intervention
This review only included trials in which antidepressant therapy (with antidepressants (ADs) of any class and in any regimen) was augmented with a psychostimulant and a placebo (as a control intervention). Thus, the comparison can be formulated as: “Psychostimulant vs placebo augmentation of antidepressant therapy.”
Psychostimulants considered for the purpose of this review were atomoxetine, armodafinil, dextroamphetamine, lisdexamfetamine, methylphenidate, and modafinil.
Outcome Measures
Change in Depression Severity from Baseline to End-of-Study
Continuous measures of change in symptom severity, assessed by adequately validated scales specific to depression, were included. As such, measures determined by assessment instruments not specific to this disease, such as the CGI scales, were excluded. Whenever a study measured the outcome using more than one appropriate scale, this review used the values derived from the scale most frequently used in the included studies. If the scales were equally frequent, a decision was made to select the most appropriate option.
Remission at End-of-Study
This review also included dichotomous measures of remission, defined by the categorization of the scores of the scales. As with the previous outcome, if a study included different definitions of remission, this review considered the definition most frequently applied in the included studies. A decision aiming to select the most appropriate option was made whenever the definitions were equally frequent.
Search Methods for Identification of Studies
Electronic Searches
Searches were carried out from 11th February to 6th July 2023 on PubMed, Web of Science Core Collection, and ClinicalTrials.gov. On PubMed, the query used was as follows: (“depression” [MeSH Terms] OR “depression” OR “depressive disorder” [MeSH Terms] OR “depressive disorder” OR “depressive disorders” OR “depressive disorder, major” [MeSH Terms] OR “major depressive disorder”) AND (“modafinil” [MeSH Terms] OR “modafinil” OR “armodafinil” OR “atomoxetine hydrochloride” [MeSH Terms] OR “lisdexamfetamine dimesylate” [MeSH Terms] OR “lisdexamfetamine dimesylate” OR “lisdexamfetamine” OR “dextroamphetamine” [MeSH Terms] OR “dextroamphetamine” OR “atomoxetine hydrochloride” OR “atomoxetine” OR “methylphenidate” [MeSH Terms] OR “methylphenidate”). On Web of Science Core Collection, the query used was as folllows: (ALL = “depression” OR ALL = “depressive disorder” OR ALL = “depressive disorders” OR ALL = “major depressive disorder”) AND (ALL = “modafinil” OR ALL = “armodafinil” OR ALL = “lisdexamfetamine dimesylate” OR ALL = “lisdexamfetamine” OR ALL = “dextroamphetamine” OR ALL = “atomoxetine” OR ALL = “atomoxetine hydrochloride” OR ALL = “methylphenidate”). ClinicalTrials.gov was searched using “depression” as the “Condition or disease term” and the name of each psychostimulant as the “Intervention/treatment” term.
Searching Other Resources
No other searches were made.
Data Collection and Analysis
Selection of Studies
In accordance with the inclusion criteria, the identified studies were initially screened based on the information provided in the title and in the abstract. Following screening, the full text of the initially included studies was assessed for eligibility and reasons for exclusion were provided. This process was carried out by two authors (DJ and FN).
Data Extraction and Management
Using a data extraction form, the information was extracted solely by two authors (DJ and FN). For every included study, whenever possible, the following data was retrieved: methodological characteristics of the trial, total number of participants, number of male and female participants, inclusion and exclusion criteria, diagnostic criteria applied in diagnosing depression, characteristics of the intervention and the comparison, outcomes assessed, and treatment effects for the relevant outcomes.
Assessment of Risk of Bias in Included Studies
Risk of bias was accessed using the Revised Cochrane risk-of-bias tool for randomized trials [31]. Five domains were taken into consideration: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Each domain was assessed as either low-risk, some concerns, or high-risk of bias. Based on these judgements, an overall assessment of bias for each included study was made.
Measures of Treatment Effect
Continuous Data
As different scales were used to measure the same outcome, the standardized mean difference was used, along with its 95% confidence interval.
Dichotomous Data
Odds ratios and their 95% confidence intervals were used.
Unit of Analysis Issues
Studies with Multiple Treatment Groups
If a study had more than two arms, with one of them being placebo control and the other interventions with different doses of a same psychostimulant, then the formulae presented in Sect. 6.5.2.10 of the Cochrane Handbook for Systematic Reviews of Interventions [13•] were used in order to combine treatment groups and create a single “Psychostimulant vs placebo augmentation” comparison.
Dealing with Missing Data
For continuous data, whenever the standard deviation was not provided, but instead a standard error of the mean was indicated, the standard deviation was calculated by multiplying the standard error of the mean by the square root of the sample size. When possible, in the cases in which a standard error of the mean was also not provided, the standard deviation was obtained through the calculations described in the Cochrane Handbook for Systematic Reviews of Interventions [13•].
For dichotomous data, an intention to treat analysis was performed.
Assessment of Heterogeneity
Statistical heterogeneity was assessed using the I-squared statistic, which indicates the percentage of the variability in effect estimates that is due to heterogeneity rather than chance [13•].
Assessment of Reporting Biases
Reporting biases were assessed through funnel plot inspection and, if the meta-analysis included ten or more studies, a Begg's Rank correlation test of funnel plot asymmetry [3]. The decision to not test for funnel plot asymmetry if the meta-analysis included less than ten studies stems from the fact that the power of this test is significantly lower in meta-analyses with few studies [13•].
Data Synthesis
Statistical analysis was done using Review Manager 5.4.1.
In the analysis of both continuous and dichotomous outcome measures a random-effects model was used. For the continuous outcome, the meta-analysis reported the standardized mean difference and its 95% confidence interval. In the case of the dichotomous outcome, the odds ratio and its 95% confidence interval were presented.
Results
Description of Studies
Results of the Search
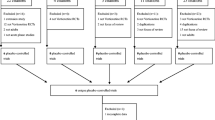
The electronic search produced a total of 3152 references. Of these, 929 were eliminated based on being duplicates. The remaining 2223 were analyzed by title and abstract and, at this point, 2182 references were excluded. Next, the 41 articles’ full texts were assessed for eligibility and 12 of them were included. Richards et al. [28] describes two separate trials, hence the study was divided into Richards et al. [28] and Richards et al. [29], raising the number of included studies to 13. This selection process is schematically represented in Fig. 1. Reasons for exclusion are detailed in the “Excluded studies” section.
Included Studies
Thirteen studies were included [1, 6, 8, 9, 15, 17, 19, 23, 26, 28, 29, 32] (Table 1).
All included studies were prospective, double-blind, placebo-controlled, parallel-group randomized trials. Two were single-center [15, 17], while the other 11 were multi-center. Publication years ranged between 2003 [6] and 2017 [29].
All studies included male and female patients with a diagnosis of MDD according to the DSM-IV [1, 6, 8, 9, 15, 19, 23] or the DSM-IV-TR. Only two trials exclusively included elderly participants [15, 17].
A majority of studies (7/13) only included patients with a partial-response to antidepressant therapy [6, 8, 9, 19, 28, 29, 32], and one study had a population of both non- and partial-responders to antidepressant therapy [23]. The other five trials did not account for previous therapeutic response.
A minority of studies (2/13) restricted inclusion to patients who displayed significant fatigue and sleepiness [8, 9].
Regarding interventions, of the thirteen trials, four evaluated modafinil [1, 6, 8, 9], four evaluated methylphenidate [15, 17, 23, 26], one evaluated atomoxetine [19], and another four evaluated lisdexamfetamine [28, 29, 32].
Nearly all studies had two conditions, antidepressant plus psychostimulant and antidepressant plus placebo. The exceptions were Lavretsky et al. [17], which added a third condition, psychostimulant plus placebo, and Richards et al. [29], which established five conditions, one of them being antidepressant plus placebo and the other four being antidepressant plus different doses of the same psychostimulant.
The duration of the intervention ranged from 4 [23] to 16 weeks [17].
Three studies did not present data for the mean change in depression severity from baseline to end-of-trial using the assessment instruments deemed pertinent [8, 17, 19]. Of those that did present such data, six used MADRS [9, 26, 28, 29, 32], one used Ham-D-24 [15], one used Ham-D-21 [23], one used Ham-D-17 [1], and one used both Ham-D-21 and Ham-D-17 [6].
All but three studies [6, 26, 29] assessed remission rates. Of the ten studies which considered remission, two defined it as a Ham-D-24 score of less than 7 [15, 17], two defined it as a Ham-D-21 score of less than 8 [8, 23], two defined it as a Ham-D-17 score of less than 8 [1, 9], two defined it as a MADRS score of less than 11 [28], one defined it as a Maier and Philipp core mood severity subscale score of less than 5 with no single item scored upwards of 1 [19], and one gave two definitions, either a MADRS score of less than 11, or a Ham-D-17 score of less than 8, [32].
Excluded Studies
Of the forty-one full-text articles assessed for eligibility, twenty-nine articles were excluded. Of these, thirteen were excluded on the basis of not being full articles, but instead publications related to poster sessions. Nine articles were not included for being ClinicalTrials.gov Study Registers of other studies being assessed for eligibility, and therefore duplicates. Other reasons for exclusion were concurrent treatment of participants with sleep deprivation [2], being a retrospective pooled analysis of two already included studies [10], being a secondary analysis of already included studies [16, 22, 27], including patients in full remission [18], and not defining the diagnostic criteria applied in diagnosing depression [24].
Risk of Bias in Included Studies
Risk of Bias Arising from the Randomization Process
Given that few studies published detailed information on allocation sequence generation and concealment, judgements were necessary. It was decided, based on each trial’s context, that all studies randomly generated and adequately concealed allocation sequences. No studies showed relevant baseline differences between groups. All studies were therefore judged to be at low risk of bias arising from the randomization process (Fig. 2).
Risk of Bias Due to Deviations from Intended Interventions
All publications included were, per inclusion criteria, double-blinded. However, nearly half of the studies (6/13) used per-protocol analysis instead of the more appropriate intention-to-treat or modified intention-to treat analyses. In each of these studies, only a small fraction of participants were excluded, thereby lowering the potential for substantial impact on the results. They [8, 9, 28, 29, 32] were therefore judged to raise some concerns of bias due to deviations from intended interventions, while the other seven studies were assessed as low risk (Fig. 2).
Risk of Bias Due to Missing Outcome Data
Only in Abolfazli et al. [1] was data available for nearly all participants (defined as availability of data from at least 95% of participants). Two studies [23, 32] imputed missing data using the inappropriate last-observation-carried-forward approach and another two studies [6, 19] made no mention of strategies for dealing with missing data. These four studies were judged to be at high risk of bias, as the nature of depression makes it probable that drop-outs are tied to disease severity, thus requiring an analysis that adequately accounts for bias. Since nearly all data was available in Abolfazli et al. [1], it was deemed as low risk, as were the remaining eight studies, which accounted for bias using mixed-effects models (Fig. 2).
Risk of Bias in Measurement of the Outcome
Symptom scales used for measuring outcomes were adequately validated across all studies and there was no evidence that measurement procedures differed between groups or that outcome assessors were unblinded. Hence, all studies were assessed as low risk of bias in measurement of the outcome (Fig. 2).
Risk of Bias in Selection of the Reported Result
Through a non-systematic online search, only five analysis plans previous to the study start date were retrieved. As no relevant differences existed between these plans and their respective studies [17, 28, 29, 32], they were considered to be at low risk of bias. The other eight studies raised some concerns because no such plans were found (Fig. 2).
Effects of Interventions
Change in Depression Severity from Baseline to End-of-Study
Ten trials were found to present data for the mean change in depression scores between the beginning and the end of the trial. In spite of this, only nine were included in the meta-analysis, as Patkar et al. [23] provided neither standard deviations, nor information which could be used to ascertain standard deviations.
Data pooled from the nine trials indicates that augmentation of antidepressant therapy with psychostimulants significantly improves depression scores when compared to placebo augmentation (N = 1827; SMD = − 0.18; 95% CI (− 0.36, − 0.01); p = 0.04, I2 = 65%) (Fig. 3).
Patkar et al. [23] reported a greater mean decrease in depression scores in the psychostimulant augmented group (− 6.9 vs − 4.7), but the effect was deemed not significant (between-group effect: F1,47 = 1.24, p = 0.22).
Remission at End-of-Study
All of the ten trials that published remission rates were included in the meta-analysis. Psychostimulant augmentation was found to be somewhat better than placebo augmentation at achieving remission, but the effect was not significant (N = 1709; OR = 1.30; 95% CI (0.97, 1.75); p = 0.08, I2 = 32%) (Fig. 4).
Discussion
This review set out to evaluate the efficacy of psychostimulant augmentation of standard antidepressant therapy in depressed patients by focusing on its effects on two distinct, yet related outcomes, change in depression scores and remission rates.
The main findings indicate a statistically significant improvement in depression scores among individuals who received psychostimulants in conjunction with antidepressants, suggesting that this therapeutic approach may lead to a notable reduction in depressive symptoms. However, in terms of remission rates, this review failed to demonstrate a statistically significant increase in the psychostimulant augmentation group.
These findings imply that while the intervention may be beneficial in reducing depressive symptoms, it does not necessarily lead to a greater proportion of individuals achieving remission from the disease. As such, these results, albeit limited, suggest a role for psychostimulants in the symptomatic relief of depressed patients.
Some limitations must be considered when interpreting the completeness and applicability of the evidence presented due to the following reasons: (1) the population samples used in the included studies differed, notably in regards to response to previous antidepressant therapy; (2) studies aimed at patients with certain comorbidities were excluded; (3) only six psychostimulants were considered for this review and no eligible studies were found to evaluate the use of two of them (armodafinil and dextroamphetamine); (4) there were no studies assessing the augmentation of antidepressants not belonging to the SSRI and SNRI classes; (5) the duration of the intervention differed considerably across studies; (6) the scales used for measuring the continuous outcome were inconsistent, as were the definitions of remission; and (7) only studies published in English and after the year 2000 were considered.
Quality of the Evidence
The quality of the evidence was assessed using the GRADE approach. The grading of each outcome is presented in the summary of findings table (Table 2).
Potential Biases in the Review Process
As with all systematic reviews, there is the possibility of bias, particularly reporting bias. The present review focused exclusively on studies published in English after the year 2000, without reaching out to authors to address missing data or incorporating unpublished data. These methodological decisions make this review particularly susceptible to reporting bias. Notwithstanding, there was no indication of significant asymmetry through funnel plot inspection for both outcomes, which suggests the absence of reporting bias. The test for funnel plot asymmetry [3] conducted for the “Remission at end-of-study” outcome corroborated this (p = 0.1797).
Conclusions
In the present context of high depression prevalence, relatively low efficacy of classic therapeutic strategies, and lack of new drugs targeting the disease, the application of novel alternative regimens is more relevant than it has ever been. There is evidence to suggest that, as with other diseases, successfully treating depression, particularly in its early stages, leads to better outcomes and, as such, a case can be made for the use of augmentation strategies earlier on in the disease and not only in patients with treatment resistant depression.
This review showed that there is significant evidence that psychostimulant augmentation relieves the overall symptomatic burden of depression and that its impact on remission rates remains inconclusive. Therefore, the findings support the clinical use of certain psychostimulants for disease severity reduction.
However, due to the limitations found in the literature, it is fair to say that not only are more trials necessary, but also that these newer trials should aim to study, possibly give priority to, psychostimulants less researched in this context. Moreover, it is important that further research be in the form of larger trials with longer intervention and follow-up periods.
Additionally, outcome assessment instruments and the definitions for remission varied substantially between studies, which raised important difficulties in pooling data. For this reason, moving forward, it is important that some degree of uniformity be achieved, ideally through the adoption of a predefined set of pertinent scales and definitions to be used across studies.
Data Availability
The data used was extracted from the different studies considered in the review process. The authors have ensured the accuracy and validity of the data extracted. For replication or further investigation of our findings, interested researchers can refer to the original sources provided. Any additional data or clarifications required can be obtained by contacting the corresponding author directly.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Abolfazli R, Hosseini M, Ghanizadeh A, Ghaleiha A, Tabrizi M, Raznahan M, Golalizadeh M, Akhondzadeh S. Double-blind randomized parallel-group clinical trial of efficacy of the combination fluoxetine plus modafinil versus fluoxetine plus placebo in the treatment of major depression. Depress Anxiety. 2011;28(4):297–302. https://doi.org/10.1002/da.20801.
Beck J, Hemmeter U, Brand S, Muheim F, Hatzinger M, Holsboer-Trachsler E. Modafinil reduces microsleep during partial sleep deprivation in depressed patients. J Psychiatr Res. 2010;44(13):853–64. https://doi.org/10.1016/j.jpsychires.2010.01.008.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088. https://doi.org/10.2307/2533446.
Blick SKA, Keating GM. Lisdexamfetamine. Pediatr Drugs. 2007;9(2):129–35. https://doi.org/10.2165/00148581-200709020-00007.
Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JPT, Egger M, Takeshima N, Hayasaka Y, Imai H, Shinohara K, Tajika A, Ioannidis JPA, Geddes JR. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357–66. https://doi.org/10.1016/s0140-6736(17)32802-7.
DeBattista C, Doghramji K, Menza MA, Rosenthal MH, Fieve RR. Adjunct modafinil for the short-term treatment of fatigue and sleepiness in patients with major depressive disorder. J Clin Psychiatry. 2003;64(9):1057–64. https://doi.org/10.4088/jcp.v64n0911.
del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiat. 2011;69(12):e145–57. https://doi.org/10.1016/j.biopsych.2011.02.036.
Dunlop BW, Crits-Christoph P, Evans DL, Hirschowitz J, Solvason HB, Rickels K, Garlow SJ, Gallop RJ, Ninan PT. Coadministration of modafinil and a selective serotonin reuptake inhibitor from the initiation of treatment of major depressive disorder with fatigue and sleepiness. J Clin Psychopharmacol. 2007;27(6):614–9. https://doi.org/10.1097/jcp.0b013e31815abefb.
Fava M, Thase ME, DeBattista C. A multicenter, placebo-controlled study of modafinil augmentation in partial responders to selective serotonin reuptake inhibitors with persistent fatigue and sleepiness. J Clin Psychiatry. 2005;66(01):85–93. https://doi.org/10.4088/jcp.v66n0112.
Fava M, Thase ME, DeBattista C, Doghramji K, Arora S, Hughes RJ. Modafinil augmentation of selective serotonin reuptake inhibitor therapy in mdd partial responders with persistent fatigue and sleepiness. Ann Clin Psychiatry. 2007;19(3):153–9. https://doi.org/10.1080/10401230701464858.
Garnock-Jones KP, Dhillon S, Scott LJ. Armodafinil. CNS Drugs. 2009;23(9):793–803. https://doi.org/10.2165/11203290-000000000-00000.
• GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9(2):137–150. https://doi.org/10.1016/s2215-0366(21)00395-3. Recent study on the epidemiology of depression.
• Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from https://www.training.cochrane.org/handbook. Provided guidance throughout our study.
Jesulola E, Micalos P, Baguley IJ. Understanding the pathophysiology of depression: From monoamines to the neurogenesis hypothesis model - are we there yet? Behav Brain Res. 2018;341:79–90. https://doi.org/10.1016/j.bbr.2017.12.025.
Lavretsky H, Park S, Siddarth P, Kumar A, Reynolds CF. Methylphenidate-enhanced antidepressant response to citalopram in the elderly: a double-blind, placebo-controlled pilot trial. Am J Geriatr Psychiatry. 2006;14(2):181–5. https://doi.org/10.1097/01.jgp.0000192503.10692.9f.
Lavretsky H, Siddarth P, Kumar A, Reynolds CF. The effects of the dopamine and serotonin transporter polymorphisms on clinical features and treatment response in geriatric depression: a pilot study. Int J Geriatr Psychiatry. 2007;23(1):55–9. https://doi.org/10.1002/gps.1837.
Lavretsky H, Reinlieb M, St. Cyr N, Siddarth P, Ercoli LM, Senturk D. Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2015;172(6):561–569. https://doi.org/10.1176/appi.ajp.2014.14070889.
Madhoo M, Keefe RS, Roth RM, Sambunaris A, Wu J, Trivedi MH, Anderson CS, Lasser R. Lisdexamfetamine dimesylate augmentation in adults with persistent executive dysfunction after partial or full remission of major depressive disorder. Neuropsychopharmacology. 2013;39(6):1388–98. https://doi.org/10.1038/npp.2013.334.
Michelson D, Adler LA, Amsterdam JD, Dunner DL, Nierenberg AA, Reimherr FW, Schatzberg AF, Kelsey DK, Williams DW. Addition of atomoxetine for depression incompletely responsive to sertraline. J Clin Psychiatry. 2007;68(04):582–7. https://doi.org/10.4088/jcp.v68n0414.
Moncrieff J, Wessely S, Hardy R. Active placebos versus antidepressants for depression. Cochrane Database Syst Rev. 2004;2012(10). https://doi.org/10.1002/14651858.cd003012.pub2.
Murillo-Rodríguez E, Barciela Veras A, Barbosa Rocha N, Budde H, Machado S. An overview of the clinical uses, pharmacology, and safety of modafinil. ACS Chem Neurosci. 2017;9(2):151–8. https://doi.org/10.1021/acschemneuro.7b00374.
Pae CU, Marks DM, Masand PS, Peindl K, Hooper-Wood C, Han C, Mannelli P, Ciccone P, Patkar AA. Methylphenidate extended release (OROS MPH) for the treatment of antidepressant-related sexual dysfunction in patients with treatment-resistant depression. Clin Neuropharmacol. 2009;32(2):85–8. https://doi.org/10.1097/wnf.0b013e31817e559b.
Patkar AA, Masand PS, Pae CU, Peindl K, Hooper-Wood C, Mannelli P, Ciccone P. A randomized, double-blind, placebo-controlled trial of augmentation with an extended release formulation of methylphenidate in outpatients with treatment-resistant depression. J Clin Psychopharmacol. 2006;26(6):653–6. https://doi.org/10.1097/01.jcp.0000246212.03530.fd.
Paulus MP, Kuplicki R, Victor TA, Yeh HW, Khalsa, SS. Methylphenidate augmentation of escitalopram to enhance adherence to antidepressant treatment: a pilot randomized controlled trial. BMC Psychiatry. 2021;21(1). https://doi.org/10.1186/s12888-021-03583-7.
Plumber N, Majeed M, Ziff S, Thomas SE, Bolla SR, Gorantla VR. Stimulant usage by medical students for cognitive enhancement: a systematic review. Cureus. 2021. https://doi.org/10.7759/cureus.15163.
Ravindran AV, Kennedy SH, O’Donovan MC, Fallu A, Camacho F, Binder CE. Osmotic-release oral system methylphenidate augmentation of antidepressant monotherapy in major depressive disorder. J Clin Psychiatry. 2008;69(1):87–94. https://doi.org/10.4088/jcp.v69n0112.
Reimherr F, Amsterdam J, Dunner D, Adler L, Zhang S, Williams D, Marchant B, Michelson D, Nierenberg A, Schatzberg A, Feldman P. Genetic polymorphisms in the treatment of depression: speculations from an augmentation study using atomoxetine. Psychiatry Res. 2010;175(1–2):67–73. https://doi.org/10.1016/j.psychres.2009.01.005.
Richards C, McIntyre RS, Weisler R, Sambunaris A, Brawman-Mintzer O, Gao J, Geibel B, Dauphin M, Madhoo M. Lisdexamfetamine dimesylate augmentation for adults with major depressive disorder and inadequate response to antidepressant monotherapy: results from 2 phase 3, multicenter, randomized, double-blind, placebo-controlled studies. J Affect Disord. 2016;206:151–60. https://doi.org/10.1016/j.jad.2016.07.006.
Richards C, Iosifescu DV, Mago R, Sarkis E, Reynolds J, Geibel B, Dauphin M. A randomized, double-blind, placebo-controlled, dose-ranging study of lisdexamfetamine dimesylate augmentation for major depressive disorder in adults with inadequate response to antidepressant therapy. J Psychopharmacol. 2017;31(9):1190–203. https://doi.org/10.1177/0269881117722998.
Sauer JM, Ring BJ, Witcher JW. Clinical Pharmacokinetics of atomoxetine. Clin Pharmacokinet. 2005;44(6):571–90. https://doi.org/10.2165/00003088-200544060-00002.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, . . . Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;l4898. https://doi.org/10.1136/bmj.l4898.
Trivedi MH, Cutler AJ, Richards C, Lasser R, Geibel BB, Gao J, Sambunaris A, Patkar AA. A randomized controlled trial of the efficacy and safety of lisdexamfetamine dimesylate as augmentation therapy in adults with residual symptoms of major depressive disorder after treatment with escitalopram. J Clin Psychiatry. 2013;74(08):802–9. https://doi.org/10.4088/jcp.13m08360.
Acknowledgements
The authors wish to thank Dr. Licínia Isabel Ferreira Da Costa Ganança for reviewing their manuscript.
Funding
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Contributions
DJ designed the study, reviewed the literature, and wrote the initial draft. FN was the second reviewer. JJ, JR, DTC, and FN all provided substantial revisions and comments to the manuscript. All authors have approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Janela, D., Jerónimo, J., Rema, J. et al. Psychostimulant Augmentation of Antidepressant Therapy in Depression: a Systematic Review and Meta-Analysis. Curr Treat Options Psych 10, 492–510 (2023). https://doi.org/10.1007/s40501-023-00307-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40501-023-00307-4