Abstract
Objective
This meta-analysis assessed the efficacy and safety of 5 mg/day vortioxetine compared to placebo for adult major depressive disorder.
Methods
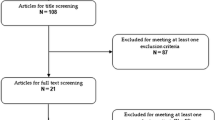
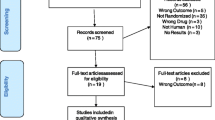
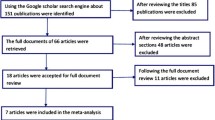
We performed a meta-analysis of the double-blind, randomized, controlled clinical trials involving 5 mg/day vortioxetine in adult patients with major depressive disorder published on PubMed, EBSCO, and PsycINFO, and the Clinical Trials databases were searched from 2000 through October 2013. The abstracts for the Annual Meetings of the American Psychiatric Association (APA) and previous reviews were searched to identify additional studies. Results were expressed with odds ratios (ORs) with their 95 % confidence interval (CI). The effect size (ES) for the four studies was derived by computing the standardized mean difference (SMD). The data were pooled with a random effects model.
Results
Five RCTs met the selection criteria. Results of the meta-analysis showed the following: (1) The treatment response of 5 mg/day vortioxetine group was greater than placebo group (OR = 1.84, 95 % CI = 1.16–2.93, Z = 2.59, P = 0.010), and there was a significant antidepressant effect of vortioxetine (ES = 2.98, P = 0.001). However, there was no significant difference in remission (OR = 1.47, 95 % CI = 0.95–2.30, Z = 1.71, P = 0.090). (2) The common adverse effects included nausea, dizziness, headache, dry mouth, and diarrhea. There was a significant difference for nausea between the two groups (OR = 3.01, 95 % CI = 2.22–4.09, Z = 7.08, P = 0.00001), but no significant differences were observed for the other four adverse effects.
Conclusions
For the treatment of major depressive disorder, our results show that a dose of 5 mg/day vortioxetine was more effective, but more easily induced nausea, compared to placebo.







Similar content being viewed by others
References
Alvarez E, Perez V, Dragheim M et al (2012) A double-blind, randomized, placebo-controlled, active reference study of Lu AA21004 in patients with major depressive disorder. Int J Neuropsychopharmacol 15(5):589–600
Baldwin DS, Loft H, Dragheim M (2012) A randomised, double-blind, placebo controlled, duloxetine-referenced, fixed-dose study of three dosages of Lu AA21004 in acute treatment of major depressive disorder (MDD). Eur Neuropsychopharmacol 22(7):482–491
Bang-Andersen B, Ruhland T, Jorgensen M, Smith G et al (2011) Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J Med Chem 54(9):3206–3221
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Bidzan L, Mahableshwarkar AR, Jacobsen P et al (2012) Vortioxetine (Lu AA21004) in generalized anxiety disorder: results of an 8-week, multinational, randomized, double-blind, placebo-controlled clinical trial. Eur Neuropsychopharmacol 22(12):847–857
Bijlsma EY, Chan JS, Olivier B, et al. (2013) Sexual side effects of serotonergic antidepressants: mediated by inhibition of serotonin on central dopamine release? Pharmacol Biochem Behav
Boulenger JP, Loft H, Florea I (2012) A randomized clinical study of Lu AA21004 in the prevention of relapse in patients with major depressive disorder. J Psychopharmacol 26(11):1408–1416
Chen G, Lee R, Højer AM et al (2013) Pharmacokinetic drug interactions involving vortioxetine (Lu AA21004), a multimodal antidepressant. Clin Drug Investig 33(10):727–736
Di Florio A, Forty L, Gordon-Smith K et al (2013) Perinatal episodes across the mood disorder spectrum. JAMA Psychiatry 70(2):168–175
Egger M, Davey Smith G, Schneider M et al (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62
Hatta S, Duni A, Ng CG et al (2013) Effects of selective serotonin reuptake inhibitors (SSRIs) therapy on the female sexual response cycle of women with major depression. Clin Ter 164(1):11–15
Henigsberg N, Mahableshwarkar AR, Jacobsen P et al (2012) A randomized, double-blind, placebo-controlled 8-week trial of the efficacy and tolerability of multiple doses of Lu AA21004 in adults with major depressive disorder. J Clin Psychiatry 73(7):953–959
Herman JB, Brotman AW, Pollack MH et al (1990) Fluoxetine-induced sexual dysfunction. J Clin Psychiatry 51(1):25–27
Higgins JP, Thompson SG, Deeks JJ et al (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Jadad AR, Moore RA, Carroll D et al (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17(1):1–12
Jain R, Mahableshwarkar AR, Jacobsen PL et al (2013) A randomized, double-blind, placebo-controlled 6-wk trial of the efficacy and tolerability of 5 mg vortioxetine in adults with major depressive disorder. Int J Neuropsychopharmacol 16(2):313–321
Katona C, Hansen T, Olsen CK (2012) A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol 27(4):215–223
Mahableshwarkar AR, Jacobsen PL, Chen Y (2013) A randomized, double-blind trial of 2.5 mg and 5 mg vortioxetine (Lu AA21004) versus placebo for 8 weeks in adults with major depressive disorder. Curr Med Res Opin 29(3):217–226
Montgomery SA, Asberg M (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389
Mørk A, Pehrson A, Brennum LT et al (2012) Pharmacological effects of vortioxetine: a novel multimodal compound for the treatment of major depressive disorder. J Pharmacol Exp Ther 340(3):666–675
Murray CJ, Lopez AD (1997) Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet 349(9064):1498–1504
Musher JS (1990) Anorgasmia with the use of fluoxetine. Am J Psychiatry 147(7):948
Penninx BW, Beekman AT, Honig A et al (2001) Depression and cardiac mortality: results from a community-based longitudinal study. Arch Gen Psychiatry 58(3):221–227
Robinson, Robert G, Spalletta et al (2010) Poststroke depression: a review. Can J Psychiatry 55(6):341–349
Rothschild AJ, Mahableshwarkar AR, Jacobsen P et al (2012) Vortioxetine (Lu AA21004) 5 mg in generalized anxiety disorder: results of an 8-week randomized, double-blind, placebo-controlled clinical trial in the United States. Eur Neuropsychopharmacol 22(12):858–866
Schlienger JL (2013) Type 2 diabetes complications. Presse Med 42(5):839–848
Ustün TB, Ayuso-Mateos JL, Chatterji S et al (2004) Global burden of depressive disorders in the year 2000. Br J Psychiatry 184:386–392
World Health Organization.Mental Health.Depression.http://www.who.int/mental_health/management/depression/definition/en/Accessed 2-15-2012.
Acknowledgments
This systematic review was supported by the National Natural Science Foundation of China (No.81171225).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fu, J., Chen, Y. The efficacy and safety of 5 mg/d Vortioxetine compared to placebo for major depressive disorder: A meta-analysis. Psychopharmacology 232, 7–16 (2015). https://doi.org/10.1007/s00213-014-3633-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-014-3633-z