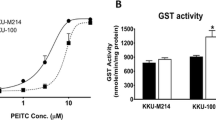
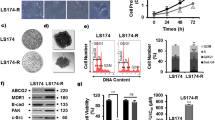
Abstract
Purpose of Review
This short review is aimed at summarizing our recent findings about the regulating role of PI3K in the benzyl isothiocyanate (BITC)–induced drug-resistant mechanisms in human colorectal cancer cells and identification of potential components to overcome this resistance by combinatory utilization.
Recent Findings
Benzyl isothiocyanate (BITC), the organosulfur compounds derived from cruciferous vegetables, exerts anti-proliferative effects in various human cancer cells. BITC also enhances the activation of the phosphoinositide 3-kinase (PI3K)/Akt survival pathway, possibly through the inhibition of the protein tyrosine phosphatase 1B. Since the PI3K/Akt pathway as well as drug efflux mediates resistance against anti-cancer drugs, it is important to find an agent to improve the anti-cancer effects of BITC without enhancing the side effects. We have shown that inhibition of PI3K or depletion of the plasma membrane cholesterol significantly enhanced the BITC-induced apoptosis, coinciding with inhibition of the survival pathway. Furthermore, BITC induced autophagy concomitantly with the up-regulation of the nuclear factor–erythroid 2 (NF-E2)–related factor 2 (Nrf2) and Nrf2-dependent cytoprotective genes in human colorectal cancer cells. Experiments using PI3K inhibitors implicated that PI3K is involved not only in the accumulation of autophagic molecules but also the up-regulation of Nrf2 with p62/sequestosome-1. PI3K might play pivotal roles in the resistant mechanisms, such as the activation of cell survival signaling and autophagy-dependent drug metabolism in human colon cancer cells.
Summary
Our studies suggest that combinatory treatment of PI3K inhibitors or cholesterol-depleting agents is a promising strategy to improve the BITC-induced anti-cancer effects in human colorectal cancer cells.


Similar content being viewed by others
References
Nakamura Y, Miyoshi N. Electrophiles in foods: the current status of isothiocyanates and their chemical biology. Biosci Biotechnol Biochem. 2010;74:242–55. https://doi.org/10.1271/bbb.90731.
Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. https://doi.org/10.1016/s0031-9422(00)00316-2.
Nakamura T, Abe-Kanoh N, Nakamura Y. Physiological relevance of covalent protein modification by dietary isothiocyanates. J Clin Biochem Nutr. 2018;62:11–9. https://doi.org/10.3164/jcbn.17-91.
Nakamura T, Murata Y, Nakamura Y. Characterization of benzyl isothiocyanate extracted from mashed green papaya by distillation. Food Chem. 2019 Nov 30;299:125118. https://doi.org/10.1016/j.foodchem.2019.125118.
Tian S, Liu X, Lei P, Zhang X, Shan Y. Microbiota: a mediator to transform glucosinolate precursors in cruciferous vegetables to the active isothiocyanates. J Sci Food Agric. 2018;98:1255–60. https://doi.org/10.1002/jsfa.8654.
Zhang Y. Cancer-preventive isothiocyanates: measurement of human exposure and mechanism of action. Mutat Res. 2004;555:173–90. https://doi.org/10.1016/j.mrfmmm.2004.04.017.
Yu R, Mandlekar S, Harvey KJ, Ucker DS, Kong AN. Chemopreventive isothiocyanates induce apoptosis and caspase-3-like protease activity. Cancer Res. 1998;58:402–8.
Wu X, Kassie F, Mersch-Sundermann V. Induction of apoptosis in tumor cells by naturally occurring sulfur-containing compounds. Mutat Res. 2005;589:81–102. https://doi.org/10.1016/j.mrrev.2004.11.001.
Qin CZ, Zhang X, Wu LX, Wen CJ, Hu L, Lv QL, et al. Advances in molecular signaling mechanisms of β-phenethyl isothiocyanate antitumor effects. J Agric Food Chem. 2015;63:3311–22. https://doi.org/10.1021/jf504627e.
Nakamura Y. Chemoprevention by isothiocyanates: molecular basis of apoptosis induction. Forum Nutr. 2009;61:170–81. https://doi.org/10.1159/000212749.
Danielsen SA, Eide PW, Nesbakken A, Guren T, Leithe E, Lothe RA. Portrait of the PI3K/AKT pathway in colorectal cancer. Biochim Biophys Acta. 1855;2015:104–21. https://doi.org/10.1016/j.bbcan.2014.09.008.
Miyoshi N, Takabayashi S, Osawa T, Nakamura Y. Benzyl isothiocyanate inhibits excessive superoxide generation in inflammatory leukocytes: implication for prevention against inflammation-related carcinogenesis. Carcinogenesis. 2004;25:567–75. https://doi.org/10.1093/carcin/bgh051.
Nakamura Y, Morimitsu Y, Uzu T, Ohigashi H, Murakami A, Naito Y, et al. A glutathione S-transferase inducer from papaya: rapid screening, identification and structure-activity relationship of isothiocyanates. Cancer Lett. 2000;157:193–200. https://doi.org/10.1016/s0304-3835(00)00487-0.
Kawakami M, Harada N, Hiratsuka M, Kawai K, Nakamura Y. Dietary isothiocyanates modify mitochondrial functions through their electrophilic reaction. Biosci Biotechnol Biochem. 2005;69:2439–44. https://doi.org/10.1271/bbb.69.2439.
Abe N, Hou DX, Munemasa S, Murata Y, Nakamura Y. Nuclear factor-kappaB sensitizes to benzyl isothiocyanate-induced antiproliferation in p53-deficient colorectal cancer cells. Cell Death Dis. 2014;5:e1534. https://doi.org/10.1038/cddis.2014.495.
Miyoshi N, Uchida K, Osawa T, Nakamura Y. Selective cytotoxicity of benzyl isothiocyanate in the proliferating fibroblastoid cells. Int J Cancer. 2007;120:484–92. https://doi.org/10.1002/ijc.22350.
Stark LA, Dunlop MG. Nucleolar sequestration of RelA (p65) regulates NF-kappaB-driven transcription and apoptosis. Mol Cell Biol. 2005;25:5985–6004. https://doi.org/10.1128/mcb.25.14.5985-6004.2005.
Liu X, Takano C, Shimizu T, Yokobe S, Abe-Kanoh N, Zhu B, et al. Inhibition of phosphatidylinositide 3-kinase ameliorates antiproliferation by benzyl isothiocyanate in human colon cancer cells. Biochem Biophys Res Commun. 2017;491:209–16. https://doi.org/10.1016/j.bbrc.2017.07.078.
Yang Q, Miyagawa M, Liu X, Zhu B, Munemasa S, Nakamura T, et al. Methyl-β-cyclodextrin potentiates the BITC-induced anti-cancer effect through modulation of the Akt phosphorylation in human colorectal cancer cells. Biosci Biotechnol Biochem. 2018;82:2158–67. https://doi.org/10.1080/09168451.2018.1514249.
Mueller A, Bachmann E, Linnig M, Khillimberger K, Schimanski CC, Galle PR, et al. Selective PI3K inhibition by BKM120 and BEZ235 alone or in combination with chemotherapy in wild-type and mutated human gastrointestinal cancer cell lines. Cancer Chemother Pharmacol. 2012;69:1601–15. https://doi.org/10.1007/s00280-012-1869-z.
Chen PJ, Cai SP, Huang C, Meng XM, Li J. Protein tyrosine phosphatase 1B (PTP1B): a key regulator and therapeutic target in liver diseases. Toxicology. 2015;337:10–20. https://doi.org/10.1016/j.tox.2015.08.006.
Lai KC, Huang AC, Hsu SC, Kuo CL, Yang JS, Wu SH, et al. Benzyl isothiocyanate (BITC) inhibits migration and invasion of human colon cancer HT29 cells by inhibiting matrix metalloproteinase-2/-9 and urokinase plasminogen (uPA) through PKC and MAPK signaling pathway. J Agric Food Chem. 2010;58:2935–42. https://doi.org/10.1021/jf9036694.
Boreddy SR, Pramanik KC, Srivastava SK. Pancreatic tumor suppression by benzyl isothiocyanate is associated with inhibition of PI3K/AKT/FOXO pathway. Clin Cancer Res. 2011;17:1784–95. https://doi.org/10.1158/1078-0432.ccr-10-1891.
Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18. https://doi.org/10.1038/sj.cr.7290105.
Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 1802;2010:396–405. https://doi.org/10.1016/j.bbadis.2009.12.009.
Xu C, Shen G, Yuan X, Kim JH, Gopalkrishnan A, Keum YS, et al. ERK and JNK signaling pathways are involved in the regulation of activator protein 1 and cell death elicited by three isothiocyanates in human prostate cancer PC-3 cells. Carcinogenesis. 2006;27:437–45. https://doi.org/10.1093/carcin/bgi251.
Miyoshi N, Uchida K, Osawa T, Nakamura Y. A link between benzyl isothiocyanate-induced cell cycle arrest and apoptosis: involvement of mitogen-activated protein kinases in the Bcl-2 phosphorylation. Cancer Res. 2004;64:2134–42. https://doi.org/10.1158/0008-5472.can-03-2296.
Simons K, Ehehalt R. Cholesterol, lipid rafts, and disease. J Clin Invest. 2002;110:597–603. https://doi.org/10.1172/jci16390.
Lundbaek JA, Andersen OS, Werge T, Nielsen C. Cholesterol-induced protein sorting: an analysis of energetic feasibility. Biophys J. 2003;84:2080–9. https://doi.org/10.1016/s0006-3495(03)75015-2.
Miersch S, Espey MG, Chaube R, Akarca A, Tweten R, Ananvoranich S, et al. Plasma membrane cholesterol content affects nitric oxide diffusion dynamics and signaling. J Biol Chem. 2008;283:18513–21. https://doi.org/10.1074/jbc.m800440200.
Li YC, Park MJ, Ye SK, Kim CW, Kim YN. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol. 2006;168:1107–18. https://doi.org/10.2353/ajpath.2006.050959.
Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3:1023–35. https://doi.org/10.1038/nrd1576.
Morrison PW, Connon CJ, Khutoryanskiy VV. Cyclodextrin-mediated enhancement of riboflavin solubility and corneal permeability. Mol Pharm. 2013;10:756–62. https://doi.org/10.1021/mp3005963.
Reis-Sobreiro M, Roué G, Moros A, Gajate C, de la Iglesia-Vicente J, Colomer D, et al. Lipid raft-mediated Akt signaling as a therapeutic target in mantle cell lymphoma. Blood Cancer J. 2013;3:e118. https://doi.org/10.1038/bcj.2013.15.
Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim Biophys Acta. 1768;2007:1311–24. https://doi.org/10.1016/j.bbamem.2007.03.026.
Calay D, Vind-Kezunovic D, Frankart A, Lambert S, Poumay Y, Gniadecki R. Inhibition of Akt signaling by exclusion from lipid rafts in normal and transformed epidermal keratinocytes. J Invest Dermatol. 2010;130:1136–45. https://doi.org/10.1038/jid.2009.415.
Elamin KM, Yamashita Y, Higashi T, Motoyama K, Arima H. Supramolecular complex of methyl-β-cyclodextrin with adamantane-grafted hyaluronic acid as a novel antitumor agent. Chem Pharm Bull (Tokyo). 2018;66:277–85. https://doi.org/10.1248/cpb.c17-00824.
Li W, Liu X, Yang Q, Zhang N, Du Y, Zhu H. Preparation and characterization of inclusion complex of benzyl isothiocyanate extracted from papaya seed with β-cyclodextrin. Food Chem. 2015;184:99–104. https://doi.org/10.1016/j.foodchem.2015.03.091.
Upadhyay AK, Singh S, Chhipa RR, Vijayakumar MV, Ajay AK, Bhat MK. Methyl-beta-cyclodextrin enhances the susceptibility of human breast cancer cells to carboplatin and 5-fluorouracil: involvement of Akt, NF-kappaB and Bcl-2. Toxicol Appl Pharmacol. 2006;216:177–85. https://doi.org/10.1016/j.taap.2006.05.009.
Sarkar P, Chakraborty H, Chattopadhyay A. Differential membrane dipolar orientation induced by acute and chronic cholesterol depletion. Sci Rep. 2017;30(7):4484. https://doi.org/10.1038/s41598-017-04769-4.
Franke TF. PI3K/Akt: getting it right matters. Oncogene. 2008;27:6473–88. https://doi.org/10.1038/onc.2008.313.
Chen YR, Wang W, Kong AN, Tan TH. Molecular mechanisms of c-Jun N-terminal kinase-mediated apoptosis induced by anticarcinogenic isothiocyanates. J Biol Chem. 1998;273:1769–75. https://doi.org/10.1074/jbc.273.3.1769.
Qin S, Hou DX. Multiple regulations of Keap1/Nrf2 system by dietary phytochemicals. Mol Nutr Food Res. 2016;60:1731–55. https://doi.org/10.1002/mnfr.201501017.
Panieri E, Saso L. Potential applications of NRF2 inhibitors in cancer therapy. Oxidative Med Cell Longev. 2019;2019:8592348–34. https://doi.org/10.1155/2019/8592348.
Tong KI, Katoh Y, Kusunoki H, Itoh K, Tanaka T, Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol Cell Biol. 2006;26:2887–900. https://doi.org/10.1128/mcb.26.8.2887-2900.2006.
Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–26.
Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. https://doi.org/10.1016/j.cell.2007.12.018.
Ichimura Y, Kominami E, Tanaka K, Komatsu M. Selective turnover of p62/A170/SQSTM1 by autophagy. Autophagy. 2008;4:1063–6. https://doi.org/10.4161/auto.6826.
Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–45. https://doi.org/10.1074/jbc.m702824200.
Jiang T, Harder B, Rojo de la Vega M, Wong PK, Chapman E, Zhang DD. p62 links autophagy and Nrf2 signaling. Free Radic Biol Med. 2015;88:199–204. https://doi.org/10.1016/j.freeradbiomed.2015.06.014.
Nakamura Y, Ohigashi H, Masuda S, Murakami A, Morimitsu Y, Kawamoto Y, et al. Redox regulation of glutathione S-transferase induction by benzyl isothiocyanate: correlation of enzyme induction with the formation of reactive oxygen intermediates. Cancer Res. 2000;60:219–25.
Liu Y, Yamanaka M, Abe-Kanoh N, Liu X, Zhu B, Munemasa S, et al. Benzyl isothiocyanate ameliorates acetaldehyde-induced cytotoxicity by enhancing aldehyde dehydrogenase activity in murine hepatoma Hepa1c1c7 cells. Food Chem Toxicol. 2017;108:305–13. https://doi.org/10.1016/j.fct.2017.08.016.
Xiao D, Bommareddy A, Kim SH, Sehrawat A, Hahm ER, Singh SV. Benzyl isothiocyanate causes FoxO1-mediated autophagic death in human breast cancer cells. PLoS One. 2012;7:e32597. https://doi.org/10.1371/journal.pone.0032597.
Liu X, Abe-Kanoh N, Liu Y, Zhu B, Munemasa S, Nakamura T, et al. Inhibition of phosphatidylinositide 3-kinase impairs the benzyl isothiocyanate-induced accumulation of autophagic molecules and Nrf2 in human colon cancer cells. Biosci Biotechnol Biochem. 2017;81:2212–5. https://doi.org/10.1080/09168451.2017.1374830.
Itakura E, Kishi C, Inoue K, Mizushima N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell. 2008;19:5360–72. https://doi.org/10.1091/mbc.e08-01-0080.
Zhou XY, Luo Y, Zhu YM, Liu ZH, Kent TA, Rong JG, et al. Inhibition of autophagy blocks cathepsins-tBid-mitochondrial apoptotic signaling pathway via stabilization of lysosomal membrane in ischemic astrocytes. Cell Death Dis. 2017;8:e2618. https://doi.org/10.1038/cddis.2017.34.
Rojo de la Vega M, Chapman E, Zhang DD. NRF2 and the hallmarks of cancer. Cancer Cell. 2018;34:21–43. https://doi.org/10.1016/j.ccell.2018.03.022.
Bai X, Chen Y, Hou X, Huang M, Jin J. Emerging role of NRF2 in chemoresistance by regulating drug-metabolizing enzymes and efflux transporters. Drug Metab Rev. 2016;48:541–67. https://doi.org/10.1080/03602532.2016.1197239.
Telang U, Ji Y, Morris ME. ABC transporters and isothiocyanates: potential for pharmacokinetic diet-drug interactions. Biopharm Drug Dispos. 2009;30:335–44. https://doi.org/10.1002/bdd.668.
Yu C, Jiao Y, Xue J, Zhang Q, Yang H, Xing L, et al. Metformin sensitizes non-small cell lung Cancer cells to an epigallocatechin-3-gallate (EGCG) treatment by suppressing the Nrf2/HO-1 signaling pathway. Int J Biol Sci. 2017;13:1560–9. https://doi.org/10.7150/ijbs.18830.
Zou X, Feng Z, Li Y, Wang Y, Wertz K, Weber P, et al. Stimulation of GSH synthesis to prevent oxidative stress-induced apoptosis by hydroxytyrosol in human retinal pigment epithelial cells: activation of Nrf2 and JNK-p62/SQSTM1 pathways. J Nutr Biochem. 2012 Aug;23(8):994–1006. https://doi.org/10.1016/j.jnutbio.2011.05.006.
Funding
This study was partly supported by MEXT KAKENHI Grant Numbers 17H03818 and 20H02933 (YN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Molecular Targets, mechanisms, pharmacology and in vivo efficacy
Rights and permissions
About this article
Cite this article
Liu, X., Yang, Q. & Nakamura, Y. Inhibition of Drug Resistance Mechanisms Improves the Benzyl Isothiocyanate–Induced Anti-Proliferation in Human Colorectal Cancer Cells. Curr Pharmacol Rep 6, 306–314 (2020). https://doi.org/10.1007/s40495-020-00227-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40495-020-00227-4