Abstract
Although colorectal cancer (CRC) treatment with 5-fluorouracil (5-FU) is the first line of therapy for this debilitating disease, treatment effectiveness is often hampered by the development of drug resistance and toxicity at high doses. ER-β can play an important role in CRC development and possibly in its response to therapy. Pterostilbene (PT) possesses antioxidant and anticancer effects that are mediated by ER-β. In the current study, we test the hypothesis that PT sensitizes colon cancer cells to 5-FU and we examine the underlying mechanism(s) by which PT exerts its cytotoxic effects in CRC cells. Our data indicate that PT exhibited a more potent cytotoxic effect in Caco-2 compared to HCT-116 cells. PT/5-FU co-treatment was more effective in Caco-2 cells. Our data indicate that ER-β is expressed at higher levels in Caco-2 cells and its levels are further boosted with PT treatment. PT significantly suppressed Akt and ERK phosphorylations and enhanced FOXO-1 and p27kip1 levels in Caco-2 cells. PT also induced a significant increase in Caco-2 cells at pre-G phase coupled with increased Bax/Bcl-2 ratio and PARP cleavage. These results provide a rationale for novel combination treatment strategies, especially for patients with 5-FU-resistant tumors expressing ER-β protein.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is one of the most commonly diagnosed solid tumors worldwide. It is ranked as the second cause of cancer-related death in males and the third cause of cancer-death in females in developed countries1. The chemotherapeutic agent 5-fluorouracil (5-FU) is the first line of therapy for this debilitating disease. Treatment with 5-FU represses the growth of cancer cells by acting as a false substrate to thymidylate synthase enzyme that incorporates its metabolites into DNA and RNA leading to defective synthesis and subsequent induction of apoptosis. However, treatment effectiveness is hampered by resistance to therapy and toxicity that develops at high doses2. Estrogen receptor(ER) status is suggested to be implicated in the pathogenesis of CRC. The ER-β is the predominant ER in the colorectal epithelium and studies indicated that ER-β is expressed at higher levels in normal colon mucosa compared to adenomatous polyps. Importantly, ER-β expression is significantly reduced in CRC compared with normal colon tissue3. The expression of ER-β is directly correlated with apoptosis and inversely correlated with cell proliferation4. Treatment of MC38 colon cancer cell line with diaryl-propionitrile, which acts as ER-β agonist, reduced cell proliferation rate5. Likewise, transfection of ER-β into SW480 colon cancer cells suppressed cell proliferation3. ER-β is associated with stage and grade of the disease and an inverse relationship between ER-β expression and tumor progression has been reported in cell lines and clinical samples3,6,7. As such, it is hypothesized that estrogen-mediated signaling exerts a protective role in CRC and its modulation could provide another therapeutic option for the disease8.
Stilbenes, including resveratrol and pterostilbene (PT), are a class of naturally occurring phenolic compounds that exhibit a wide spectrum of biological functions including anticancer activity9,10,11. Berries are considered a rich source for PT and its abundance varies between different types of berries. Some varieties of blueberries contain up to 15 μg PT per 100 gm (1 cup) of berries12. PT is a structural analogue to resveratrol and is characterized by the presence of 2 methoxy groups instead of the hydroxyl groups of resveratrol13. PT was reported to be superior to resveratrol in suppressing the formation of aberrant foci in a mouse model of azoxymethane-induced colon carcinogenesis14. Moreover, PT surpasses resveratrol in its inhibition for the DNA synthesis as well as suppressing pro-inflammatory mediators (iNOS and COX-2) in colon cancer cells15. In vitro studies showed that PT possesses cytotoxic activity against CRC cells16,17 and that it is more potent compared to resveratrol in inhibiting CRC cell proliferation18. Furthermore, PT strongly inhibits colon cancer tumors growth in nude mice carrying human colorectal carcinoma COLO 205 tumor xenografts17. The growth inhibitory effects of PT were demonstrated to be through an ER-β-mediated mechanism19. As such, PT could constitute a promising therapeutic candidate for CRC by acting as a chemosensitizer to conventional therapy of the disease. The chemosensitizing effect of PT in CRC has not been investigated before. In the current study, we test the hypothesis that PT sensitizes colon cancer cells to 5-FU. We also examine the underlying mechanism(s) by which PT exerts its cytotoxic effects on colon cancer cells.
Results
Effect of PT on the cytotoxicity of 5-FU in colon cancer cells
To investigate the effect of PT on the cytotoxicity of 5-FU, concentration- response curves of 5-FU in both Caco-2 and HCT-116 cell lines were assessed and compared to those obtained after co-treatment with PT. Treatment with PT alone produced a significant inhibition of cell viability with median inhibitory concentration (IC50) values of 31.2 ± 0.42 μM and 84.4 ± 1.14 μM in Caco-2 and HCT-116 cells, respectively (Fig. 1A). 5-FU exerted a concentration-dependent growth inhibition of colon cancer cells with IC50 value of 46.8 ± 2.5 μM and 4.3 ± 0.85 μM in Caco-2 and HCT-116 cells, respectively. Co-treatment with PT at a ratio of 10:1 of PT: 5-FU significantly reduced 5-FU IC50 to 2.44 ± 0.16 μM and 1.07 ± 0.01 μM in Caco-2 cells and HCT-116 cells, respectively (P < 0.05; Fig. 1B). The reduction in 5-FU IC50 after co-treatment with PT was more pronounced in Caco-2 cells (19- fold) compared to HCT-116 cells (4-fold). Drug interactions were analyzed by Calcusyn software and the combination index (CI) as well as dose reduction index (DRI) values for both cell lines are summarized in Tables 1 and 2.
Effect of PT on the viability of normal cells
The normal villous cytotrophoblast placental cells (CRL-1584, ATCC, Manassas, VA) were used to evaluate the effect of PT treatment on normal cells viability. Exposure to PT at concentrations 40 and 80 μM resulted in percent cell viability of 38.2 ± 0.36 and 27.8 ± 1.54 with reductions of about 61.8% and 72.2% compared to control (Supplementary Fig. 1).
Effect of PT on ER-β protein expression
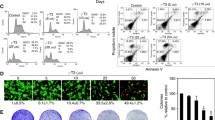
Evaluation of ER-β status in both Caco-2 and HCT-116 cell lines using immunocytochemistry showed that ER-β abundance is higher in Caco-2 cells and that it is boosted after PT treatment (Fig. 2A,B). The results were confirmed using ER-β ELSA assay which indicated that the expression of ER-β is higher in Caco-2 cells compared to HCT-116 cells by about 8.2 fold (P < 0.001). Treatment of Caco-2 cells with PT caused significant elevation in ER-β protein expression levels by 1.29 fold (P < 0.001) compared to untreated control cells. Exposure of HCT-116 cells to PT resulted in 1.1 fold increase in ER-β expression compared to untreated cells (Fig. 2C). Furthermore, single treatment of Caco-2 with 5-FU reduced ER-β expression by 20.5% (P < 0.001). Combined treatment with PT improved 5-FU mediated suppression of ER-β by 8.9%.
Effect of PT on the protein expression of ER-β in Caco-2 and HCT-116 colon cancer cell lines using immunocytochemistry (A,B) and ELISA assay (C).Data are means ± SD (n = 3). The experiment was done in triplicates. a indicates significant difference from Caco-2 control at p < 0.05. b indicates significant difference from Caco-2/PT group at p < 0.05.
Molecular docking into ER-β active site
The docking study revealed that PT docked into ER- β (1 × 7R) active site with a very high affinity value (−42.723 Kcal/mol) that is comparable to its ligand 17-β estradiol (−51.803 Kcal/mol). The docking poses for both ligands are shown in Fig. 3.
The docking poses of 17-β estradiol and PT into ER-β (1 × 7R) active site (PDB; http://www.rcsb.org/pdb).
Effect of PT on Akt phosphorylation/activation
Evaluation of Akt phophorylation in colon cancer cell lines indicated that treatment of Caco-2 cells with PT at its IC50 significantly reduced Akt phosphorylation/ activation by about 19.6% (P < 0.001) compared to untreated cells (Fig. 4A). Akt activation was reduced by 5% and 15.6% (P < 0.001) after treatment with either 5-FU-alone or PT/5-FU combination.
Effect of PT on Akt1/2/3 activation (A), ERK1/2 (B), FOXO1 (C) and p27kip1(D) expression in Caco-2 and HCT-116 colon cancer cell lines using ELISA assay. Data are means ± SD (n = 3). The experiment was done in triplicates. a indicates significant difference from Caco-2 control at p < 0.05. b indicates significant difference from Caco-2/PT group at p < 0.05. c indicates significant difference from Caco-2/5-FU group at p < 0.05. d indicates significant difference from HCT-116 control at p < 0.05.
Effect of PT on ERK phosphorylation/activation
ERK phophorylation in HCT-116 was 28% (P < 0.001) higher compared to Caco-2. The activation of ERK in Caco-2 was reduced by 20.9% (P < 0.05) and 14% after single treatment with PT or 5-FU at their IC50 and by 20% (P < 0.05) in cells treated with PT/5-FU combination. Treatment of HCT-116 with PT at its IC50 reduced ERK activation by 14% (P < 0.05) (Fig. 4B).
Effect of PT on FOXO-1 protein expression
The basal levels of FOXO-1 were higher in Caco-2 by about 1.6 folds (P < 0.001) compared to HCT-116. Treatment of Caco-2 cells with PT boosted FOXO-1 expression by 25% (P < 0.001) compared to control group (Fig. 4C). Furthermore, while 5-FU enhanced FOXO-1 by 9% (P < 0.01), co-treatment with PT boosted FOXO-1 by 36% (P < 0.001) versus control. The increase in FOXO-1 was 27% higher in the presence of PT which is a modulator for ER-β.
Effect of PT on cell cycle phases of colon cancer cells
Treatment of Caco-2 or HCT-116 cells with PT induced significant alterations in cell cycle phases (Fig. 5). Treatment of Caco-2 with low concentration of PT (3 μM) increased the accumulation of cells at pre-G phase by 12 fold (P < 0.05). Exposure of Caco-2 cells to 30 μM PT increased the percentage of cells at G2/M phase by 52% (P < 0.001) with concomitant increase in cells at pre-G phase by 17.5 fold (P < 0.001). Moreover, PT (30 μM) treatment led to reduction in the percentage of cells at G0/G1 and S-phases by 99% and 69% (P < 0.001), respectively compared to control. Treatment with PT/5-FU combination (10/1 μM) completely abolished cells at S-phase with concurrent increase in cells at pre-G by 27.5 fold (P < 0.001). High concentration of PT/5-FU (100/10 μM) produced complete abolishment of cells at G0/G1 and S-phases with concomitant increase in cells at G2/M by 42% (P < 0.001) as well as Pre-G phase by 34 fold (P < 0.001). Treatment of HCT-116 cells with 8 μM PT resulted in 2.2 folds (P < 0.05) arrest of cells at the S-phase coupled with 9.8 fold (P < 0.05) and 2.3 fold (P < 0.001) increase in cells at pre-G and G2/M phases. A subsequent reduction in percent of cells at G0/G1 by 29% (P < 0.01) was observed. It is noteworthy that cells at G2/M were eliminated by 80 μM PT treatment. Treatment with 80 μM PT also reduced the accumulation of cells at G0/G1 phase by 29.5% (P < 0.01). A significant increase in cells at S-phase and pre-G phase by 15.7(P < 0.01) and 3.5 (P < 0.001) folds was observed with the same treatment.
Effect of PT on P27kip1 protein expression
Exposure of Caco-2 cells to PT significantly increased P27kip1 protein expression levels by 1.36 fold (P < 0.05) compared to control. The expression levels of P27kip1 were boosted by 1.34 fold (P < 0.05) and 2.28 fold (P < 0.001) after treatment with 5-FU alone or combined with PT. No change was observed in the levels of P27kip1 in HCT-116 cells (Fig. 4D).
Effect of PT on Bax and Bcl-2 protein expression
Treatment of Caco-2 cells with PT resulted in an increase in Bax level (pro-apoptotic) by about 15% together with a significant decrease in Bcl-2 (anti-apoptotic) level by 31% (P < 0.05) compared to untreated cells (Fig. 6A,B). Co-treatment with PT/5-FU increased Bax level by 2.14 fold (P < 0.001) and reduced Bcl-2 level by 87.7% (P < 0.001). Bax/Bcl-2 ratio was significantly boosted by 9.4 fold (P < 0.001) compared to 5-FU alone treatment. Likewise, treatment of HCT-116 with PT produced a slight increase in Bax level (5%) and decrease in Bcl-2 level (6%) compared to control. PT treatment resulted in increased Bax/Bcl-2 ratio by 14% in HCT-116 cells (Fig. 6A,B).
Effect of PT on the protein levels of (A) Bax, (B) Bcl-2, (C) Bax/Bcl-2 ratio and (D) PARP in Caco-2 and HCT-116 colon cancer cell lines using ELISA assay. Data are means ± SD (n = 3). The experiment was done in triplicates. a indicates significant difference from Caco-2 control at p < 0.05. b indicates significant difference from Caco-2/PT group at p < 0.05. c indicates significant difference from Caco-2/5-FU group at p < 0.05.
Effect of PT on the level of cleaved PARP
Treatment of Caco-2 or HCT-116 cells with PT produced an increase in the levels of cleaved PARP by 1.22 and 1.1 fold, respectively compared to untreated controls (Fig. 6D). Treatment of Caco-2 with 5-FU alone or in combination with PT boosted PARP level by 2.9(P < 0.05) and 11 fold (P < 0.001). Combined treatment boosted the level of cleaved PARP by 3.8 fold (P < 0.001) compared to 5-FU alone.
Discussion
In the current investigation, the cytotoxicity of PT alone and in combination with 5-FU was tested and compared in Caco-2 and HCT-116 colon cancer cells. ER-β is reported to be essential for PT beneficial effects in mammalian cells19. To test the hypothesis that PT cytotoxicity in colon cancer cells is mediated by ER-β, we assessed the cytotoxicity of PT in two colon cancer cell lines that exhibit differential ER-β expression. Previous reports indicated that ER-β is constitutively expressed in Caco-2 cells20 while HCT-116 cells have lower expression level of ER-β21,22 which was confirmed by our data. Cytotoxicity studies indicated that PT exhibited a more potent cytotoxic effect in Caco-2 compared to HCT-116 cells as indicated by its IC50 in both cells. These results are in line with the work of Nutakul et al.18 who indicated that PT is more potent in inhibiting the colony formation of Caco-2 compared to HCT-116. Additional support for the role of ER-β in this process stems from our data indicating that treatment of Caco-2 with PT boosted the expression of ER-β in these cells while only inducing a slight increase in ER-β expression in HCT-116. Treatment of both cell lines with 5-FU showed a concentration-dependent cytotoxicity, with Caco-2 being less sensitive to treatment than HCT-116 cells. Co-treatment with PT/5-FU was more effective in Caco-2 as indicated by Calcusyn® Synergy analyses showing lower CI values and higher dose-reduction indices compared to HCT-116 cells. Treatment with 5-FU alone reduced ER-β expression by 20.5% (P < 0.001) which is consistent with the work of Yu-Jing Fang et al.23. Combined treatment with PT/5-FU improved 5-FU- mediated suppression of ER-β by 8.9%.
Testing the cytotoxicity of PT in normal non-cancer cells indicated that normal cells are more sensitive to its toxicity which is in line with PT previously reported antiproliferative effects in normal C2C12 myoblasts19. This finding indicates the need for a method of targeted delivery of PT to cancer cells to mitigate its potential toxic effects to normal cells.
Phosphatidylinositol-4,5-biphosphate 3-kinase (PI3K)/Akt pathway is known to play an important role in the pathogenesis and aggressiveness of many types of cancers including CRC24. Expression of ER-β was reported to repress PI3K/Akt signaling in glioma25, ovarian cancer26 and breast cancer cells27. Therefore, we tested the effect of PT on Akt phosphorylation in colon cancer cells. Treatment of Caco-2 with PT at a concentration equal to its IC50 significantly suppressed Akt phosphorylation, while there was no change in the Akt phosphorylation in HCT-116. Co-treatment of Caco-2 with PT/5-FU suppressed Akt activation by 10% greater than that observed with 5-FU-alone treatment. PT was previously reported to downregulate Akt signaling in COLO 205 colon cancer cells17. Previous reports indicated that ER-β expression downregulates ERK1/2 activation in breast cancer cells28. The tested cells Caco-2 and HCT-116 are known to be different in ERK1/2 activation status because Caco-2 is KRAS wild type while HCT-116 is KRAS mutant29. Therefore, ERK1/2 constitutive activation is higher in HCT-116 which was confirmed in the current work. Furthermore, PT treatment suppressed ERK1/2 activation in both cell lines with greater suppression of ERK1/2 phosporylation in Caco-2. This is in line with PT previously reported ability to suppress ERK1/2 activity in lung cancer cells30.
Forkhead-Box Class-O1 (FOXO-1) transcription factor is a downstream to ER-β that mediates ER-β pro-apoptotic functions31,32. Moreover, the activity of FOXO-1 is inhibited by Akt phosphorylation33,34. Our data indicate that the baseline level of FOXO-1 was higher in Caco-2 compared to HCT-116 cells. This could be partly explained by our data which indicated higher expression levels of ER-β in Caco-2. ER-β maintains FOXO-1 expression through tethering on FOXO-1 promoter31. Treatment of Caco-2 with PT enhanced FOXO-1 expression compared to untreated cells which can be explained at least partly by PT effect on ER-β expression in Caco-2. This finding is supported by previous studies with prostate cancer cells, where CAPE treatment enhanced ER-β as well as FOXO-1 protein abundance in PC-3 cells32. While 5-FU enhanced FOXO-1 by 9% (P < 0.01), co-treatment with PT boosted FOXO-1 by 36% (P < 0.001) versus control. Treatment with 5-FU was previously reported to enhance FOXO-1 levels in breast cancer cells35. The increase in FOXO-1 in our study was 27% higher in the presence of PT which is an ER-β modulator. This gains support by our molecular docking studies which revealed that PT exhibits strong interaction with the receptor active site that is comparable to its ligand 17-β estradiol.
Upregulation of FOXO-1 signaling is associated with induction of cell cycle arrest and apoptosis36. We therefore evaluated the effect of PT on those cellular processes. Cell cycle analysis indicated a significant G2/M arrest in Caco-2 coupled with an increase in the percentage of cells in pre-G phase, which is indicative of apoptosis, after treatment of cells with PT IC50. These findings are suggestive of the induction of mitotic catastrophe37. Co-treatment of Caco-2 with PT/5-FU (10/1 μM) increased the percent of cells at pre-G. Combined treatment at a higher concentration of PT/5-FU (100/10 μM) resulted in a complete abolishment for the cells at G0/G1 or S-phases with significant increase in the percent of cells at G2/M or pre-G phases. Reduction of the percent of dormant cell population at G0 phase by increasing the percent of cells in S- or G2/M phases was previously shown to sensitize Caco-2 to 5-FU cytotoxicity38. Therefore, the increased abundance of cells at G2/M together with suppression of cells at G0/G1 by PT can also justify its chemosensitizing effect. Although pre-G phase was also elevated after treatment of HCT-116 with PT, the main cell cycle arrest was at S-phase. PT-induced arrest at S-phase was previously reported in bladder cancer39 and leukemia cells40. The increased number of cells at pre-G after PT treatment was also reported in COLO 205 colon cancer cells17.
Reduced level of p27kip1, a cyclin-dependent kinase inhibitor, is linked to poor prognosis and elevated rate of tumor relapse in patients with CRC41,42. We therefore evaluated the effect of PT on its level. Treatment of Caco-2 but not HCT-116 with PT resulted in a significant upregulation of p27kip1 protein. Co-treatment of Caco-2 with PT/5-FU significantly boosted p27kip1expression. This finding further support the data generated from cell cycle analysis. It is noteworthy that p27kip1 is a transcriptional target for FOXO-143. Therefore, PT-mediated increase in p27kip1expression in Caco-2 can be attributed to its effects on FOXO-1.
The proapoptotic effects of PT, evidenced by increased accumulation of cells at pre-G phase, were further investigated. Both Bax and Bcl-2 proteins are key regulators for the intrinsic apoptosis pathway. The balance between the proapoptotic protein Bax and the antiapoptotic protein Bcl-2 is a critical factor for the cells’ threshold to undergo apoptosis44,45. This ratio is also an important determinant for the sensitivity of cancer cells to chemotherapy including 5-FU46. Previous studies indicated that PT downregulated Bcl-239,47,48 and upregulated Bax48,49 in several cancer cell types. In the current study, PT treatment significantly increased Bax/Bcl-2 ratio in Caco-2 compared to control. Combined treatment of Caco-2 with PT/5-FU increased Bax/Bcl-2 ratio by 17 fold compared to control and 10 fold compared to 5-FU-alone treatment. On the other hand, HCT-116 treatment with PT led only to a slight increase in the ratio. These findings support our DNA-ploidy data indicating that the pro-apoptotic effects of PT were more evident in Caco-2 cells. Additionally, the percent of Caco-2 cells at pre-G after combined treatment was significantly higher compared to single treatments of either PT or 5-FU. ER-β expression in colon cancer cells21,50 and other tumor types51,52 is directly correlated with apoptosis. Our data for PT effects in Caco-2 versus HCT-116 cells, are concordant with these observations. Activation of Akt leads to tumor resistance to apoptosis53. The significant enhancement of Bax/Bcl-2 ratio in PT alone and combined treatments can be linked to the effect of PT on Akt activation in Caco-2 cells. Since, treatment with PT inhibited Akt activation in Caco-2 only and combined PT/5-FU treatment inhibited Akt activation to a greater extent compared to 5-FU-alone treatment. Poly (ADP-ribose) polymerase (PARP) is a crucial enzyme for DNA repair. This enzyme is inactivated (cleaved) via caspase-3 at the end of apoptosis cascade leading to accumulation of unrepaired DNA with subsequent cell death54. Treatment of cells with PT increased cleaved PARP levels in Caco-2 to a greater extent compared to HCT-116. Combined treatment significantly increased cleaved PARP levels in Caco-2 compared to 5-FU-alone. This is concordant with the observed effects of PT on the apoptosis markers (Bax/Bcl-2 ratio and the percent of cells at the pre-G phase) in Caco-2 cells.
In summary, it could be concluded from these studies that, PT synergizes the cytotoxic effects of 5-FU in Caco-2 colon cancer cells. PT exhibited higher potency in Caco-2 cells, which express higher levels of ER-β, compared to HCT-116. These results provide a rationale for novel combination treatment strategies, especially for patients with 5-FU-resistant tumors expressing ER-β protein.
Materials and Methods
Chemicals
Pterostilbene (PT), sulforhodamine-B (SRB) and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO). RPMI-1640 medium, fetal bovine serum and other cell culture materials were purchased from Lonza (Basel, Switzerland).
Cell culture
The human colon cancer cell lines Caco-2 and HCT-116 were obtained from the National Cancer Institute (Cairo, Egypt). Cells were maintained in RPMI-1640 medium supplemented with 100 μg/mL of streptomycin, 100 units/mL of penicillin and 10% of heat-inactivated fetal bovine serum in a humidified 5% (v/v) CO2 atmosphere at 37 °C.
Cytotoxicity Assay and Synergy Analysis
PT was dissolved in DMSO and kept at a stock concentration of 100 mM. Initially, single-drug concentration– effect curves of PT and 5-FU were assessed. Seeding was done at a density of 3,000 cells/well in 96-well plates. Cells were exposed to different treatments for 72 h during which 5 different drug concentrations (0–103 μM) were tested. Cytotoxicity was assessed at the end of drug exposure using the sulforhodamine-B (SRB) assay as described previously55. The optical density was measured at 545 nm using a microplate reader (ChroMate- 4300, FL, USA). Results were expressed as the relative percentage of absorbance compared to control. Experimental conditions were tested using three replicates (three wells of the 96-well plate per experimental condition) and all experiments were performed in triplicates. Control wells were exposed to the same concentration of DMSO (1%) used in the highest concentration of PT series. Cell viability was >99% compared to untreated cells. Half-maximal inhibitory concentration (IC50), the drug concentration at which 50% growth inhibition is achieved, was calculated according to the equation for Boltzman sigmoidal concentration–response curve using the nonlinear regression fitting models (Graph Pad, Prism Version 5). Drug interactions were analyzed by CalcuSyn program, Version 2.1 (Biosoft, Cambridge, UK) based on the analytical method of Chou and Talalay56. The analytical method of Chou and Talalay57 yields two parameters that describe the interactions in a given combination: the combination index (CI) and the dose reduction index (DRI). The combination index (CI) equation is based on the multiple drug-effect equation of Chou–Talalay derived from enzyme kinetic models, where CI of <1 indicates synergism, CI of 1 or close to 1 indicates additive effects and CI of >1 indicates antagonism. The DRI denotes the factor by which the dose of each drug in a combination may be reduced at a given effect level compared with the dose when each drug is used alone. As such, the DRI is important in clinical situations, where dose reduction can lead to reduced toxicity while retaining the therapeutic efficacy. DRI >1 is beneficial and the greater the DRI value, the higher the dose reduction is for a given therapeutic effect.
Cytotoxicity testing in normal cells
Normal placental trophoblasts were utilized to assess the selective cytotoxicity of PT. The villous 3A cytotrophoblast first trimester placental cell line (CRL-1584, ATCC, Manassas, VA) was used. The cells were cultured in EMEM medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin in a humidified incubator at 37 °C supplemented with 5% CO2. The cells were seeded at density of 75 × 103 cells/well in 24-well plates and incubated overnight to allow for optimum attachment. The following day, cells were exposed to PT at a concentration close to its IC50 observed in Caco-2 and HCT-116 cancer cells (40 and 80 μM) and the incubation was continued for 72 h. At the end of the exposure period the cells were stained using SRB as described previously55. Results were expressed as the relative percentage of optical density at 545 nm compared to control.
Molecular Docking
Docking of PT was performed using Discovery Studio 4/CDOCKER protocol (Accelrys Software Inc.). Protein crystallographic structures of human ER-β active site (PDB code 1×7R) were obtained from Protein Data Bank (PDB; http://www.pdb.org/pdb/search/structidSearch.do?structureId=http://www.rcsb.org/pdb. The proteins were prepared for docking process according to the standard protein preparation procedure integrated in Accelry’s Discovery Studio 4. The 3D structures of 17-β estradiol and PT were drawn and refined using CHARMM force field with full potential. Docking study of PT was perfumed on ER-β active site. All the docking simulations were run using CDOCKER protocol. The binding energy was calculated as a score to rank the docking poses. Ten docking poses were calculated for each ligand. Docking poses were ranked according to their –CDOCKER interaction energy and the top poses were chosen for analysis of interactions.
Assessment of ER-β and FOXO-1 expression
The protein abundance ER-β in both Caco-2 and HCT-116 was assessed using immunocyotochemistry as previously described by Tolba et al.32, after culturing the cells on ibidi® μ-Chamber 12-well slides (Munich, Germany). ELISA kit for ER-β (AMS Biotechnology Ltd, UK) was used to determine the expression level of ER-β in cell lysates exposed or unexposed to PT treatment. Total FOxO-1 Cell-Based Colorimetric ELISA Kit (Ameritech Biomedicines, Houston, Tx) was used to determine the levels of FOXO-1 in the fixed cells according to the manufacturer’s instructions. The cells were treated with PT at a concentration equal to its IC50 (30 μM for Caco-2 and 80 μM for HCT-118) for 48 h. Caco-2 cells were treated with 5-FU 40 μM and PT/5-FU combination 10/1 μM.
Assessment of Akt and ERK activation/phosphorylation
The effect of PT on AKT activation in the tested colon cancer cell lines was assessed using phospho-AKT 1/2/3 (Ser473) and phospho-ERK1/2 InstantOne™ ELISA kit (eBiosciences, San Diego, CA). This kits were developed to detect phosphorylation at AKT1/2/3(Ser473) and ERK1/2(Thr202/Tyr204, Thr185/Tyr187) respectively. Caco-2 and HCT-116 cells were seeded into 96- well plates at a density of 3 × 104 cells/well and incubated overnight to allow for attachment. PT was added at concentrations equal to the IC50 and half of the IC50 value for each cell line. After 48 h of exposure, the medium was discarded and the wells were washed twice with Hank’s buffered salt solution. Akt activity was determined in the cell lysates as previously described by Tolba et al.32.
DNA-flow cytometry
Caco-2 or HCT-116 cells at a density of 3 × 105 cells were exposed to different treatments for 48 h. Treatments of Caco-2 were done at 3, 30 μM PT, 4 μM 5-FU, 10/1 and 100/10 μM of PT/5-FU. Treatments of HCT-116 were done using 8 and 80 μM PT. The cells were collected by trypsinization, washed in PBS and then fixed in ice-cold absolute alcohol. The cells were then stained using CycleTESTTM PLUS DNA Reagent Kit (BD Biosciences, San Jose, CA) according to the manufacturer’s instructions. Cell-cycle distribution was determined using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA).
Assessment of p27kip1 and apoptosis markers
The levels of p27kip1, Bax, Bcl-2 and cleaved PARP in the cell lysates collected at 48 h exposure were detected using specific ELISA kits. Human p27kip1 simple step ELISA kit was purchased from Abcam (Cambridge, MA), while kits for Bax and Bcl-2 were obtained from Abnova (Taiwan). ELISA kit for cleaved PARP was purchased from Invitrogen (Camarillo, CA). All the assay procedures were conducted according to the manufacturer’s recommendations.
Statistical Analysis
Data are presented as means ± SD. Individual groups were compared using the two-tailed independent Student’s t-test. Multiple group comparisons were carried out using one-way analysis of variance (ANOVA) followed by the Tukey-Kramer test for post-hoc analysis. Statistical significance was accepted at a level of P < 0.05. All statistical analyses were performed using GraphPad InStat software, version 3.05 (GraphPad Software, Inc. La Jolla, CA, USA). Graphs were sketched using GraphPad Prism software, version 5.00 (GraphPad Software, Inc. La Jolla, CA, USA).
Additional Information
How to cite this article: Tolba, M. F. and Abdel-Rahman, S. Z. Pterostilbine, an active component of blueberries, sensitizes colon cancer cells to 5-fluorouracil cytotoxicity. Sci. Rep. 5, 15239; doi: 10.1038/srep15239 (2015).
References
American Cancer Society. Global Cancer Facts & Figures 3rd Edition. (Atlanta: American Cancer Society, 2015).
Ohtsu, A. Chemotherapy for metastatic gastric cancer: past, present and future. J Gastroenterol 43, 256–264 (2008).
Hartman, J. et al. Tumor repressive functions of estrogen receptor beta in SW480 colon cancer cells. Cancer Res. 69, 6100–6106 (2009).
Barone, M. et al. ERbeta expression in normal, adenomatous and carcinomatous tissues of patients with familial adenomatous polyposis. Scand J Gastroenterol 45, 1320–1328 (2010).
Motylewska, E., Stasikowska, O. & Mełeń-Mucha, G. The inhibitory effect of diarylpropionitrile, a selective agonist of estrogen receptor beta, on the growth of MC38 colon cancer line. Cancer Lett. 276, 68–73 (2009).
Rudolph, A. et al. Expression of oestrogen receptor β and prognosis of colorectal cancer. Br J Cancer 107, 831–839 (2012).
Jassam, N., Bell, S. M., Speirs, V. & Quirke, P. Loss of expression of oestrogen receptor beta in colon cancer and its association with Dukes’ staging. Oncol Rep. 14, 17–21 (2005).
Caiazza, F., Ryan, E. J., Doherty, G., Winter, D. C. & Sheahan, K. Estrogen receptors and their implications in colorectal carcinogenesis. Front Oncol. 5, 19. 10.3389/fonc.2015.00019. eCollection 02015 (2015).
Rimando, A. M. et al. Cancer chemopreventive and antioxidant activities of pterostilbene, a naturally occurring analogue of resveratrol. JAgric FoodChem 50, 3453^3457 (2002).
Aggarwal, B. B. & Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol 71, 1397–1421 (2006).
Rimando, A. M. & Suh, N. Biological/chemopreventive activity of stilbenes and their effect on colon cancer. Review. Planta Med. 74, 1635–1643 (2008).
Rimando, A. M., Kalt, W., Magee, J. B., Dewey, J. & Ballington, J. R. Resveratrol, pterostilbene and piceatannol in vaccinium berries. JAgric Food Chem 52, 4713–4719 (2004).
Lin, H. S., Yue, B. D. & Ho, P. C. Determination of pterostilbene in rat plasma by a simple HPLC-UV method and its application in pre-clinical pharmacokinetic study. Biomed Chromatogr. 23, 1308–1315 (2009).
Chiou, Y. S. et al. Pterostilbene is more potent than resveratrol in preventing azoxymethane (AOM)-induced colon tumorigenesis via activation of the NF-E2-related factor 2 (Nrf2)-mediated antioxidant signaling pathway. J Agric Food Chem. 59, 2725–2733 (2011).
Paul, S. et al. Anti-inflammatory action of pterostilbene is mediated through the p38 mitogen-activated protein kinase pathway in colon cancer cells. Cancer Prev Res (Phila). 2, 650–657 (2009).
Wawszczyk, J., Kapral, M., Hollek, A. & Węglarz, L. In vitro evaluation of antiproliferative and cytotoxic properties of pterostilbene against human colon cancer cells. Acta Pol Pharm. 71, 1051–1055 (2014).
Cheng, T. C. et al. Potent anti-cancer effect of 3′-hydroxypterostilbene in human colon xenograft tumors. PLoS One 9, e111814 (2014).
Nutakul, W. et al. Inhibitory effects of resveratrol and pterostilbene on human colon cancer cells: a side-by-side comparison. J Agric Food Chem 59, 10964–10970 (2011).
Robb, E. L. & Stuart, J. A. The stilbenes resveratrol, pterostilbene and piceid affect growth and stress resistance in mammalian cells via a mechanism requiring estrogen receptor beta and the induction of Mn-superoxide dismutase. Phytochemistry. 98, 164–173 (2014).
Arias, A. et al. Regulation of expression and activity of multidrug resistance proteins MRP2 and MDR1 by estrogenic compounds in Caco-2 cells. Role in prevention of xenobiotic-induced cytotoxicity. Toxicology. 5, 46–55 (2014).
Tu, Z. et al. Estrogen receptor β potentiates the antiproliferative effect of raloxifene and affects the cell migration and invasion in HCT-116 colon cancer cells. J Cancer Res Clin Oncol. 138, 1091–1103 (2012).
Tu, Z., Ma, Y., Akers, W., Achilefu, S. & Gu, Y. Therapeutic effect of the treatment for colorectal cancer with adenoviral vectors mediated estrogen receptor β gene therapy combined with thermotherapy. J Cancer Res Clin Oncol. 140, 623–632 (2014).
Fang, Y. J. et al. MMP7 expression regulated by endocrine therapy in ERbeta-positive colon cancer cells. J Exp Clin Cancer Res. 28, 132 (2009).
Francipane, M. G. & Lagasse, E. mTOR pathway in colorectal cancer: an update. Oncotarget. 5, 49–66 (2014).
Liu, X. et al. Estrogen receptor β agonist enhances temozolomide sensitivity of glioma cells by inhibiting PI3K/AKT/mTOR pathway. Mol Med Rep. 11, 1516–1522 (2015).
Bossard, C. et al. Potential role of estrogen receptor beta as a tumor suppressor of epithelial ovarian cancer. PLoS One 7, e44787 (2012).
Lindberg, K., Helguero, L. A., Omoto, Y., Gustafsson, J. Å. & Haldosén, L. A. Estrogen receptor β represses Akt signaling in breast cancer cells via downregulation of HER2/HER3 and upregulation of PTEN: implications for tamoxifen sensitivity. Breast Cancer Res. 13, R43 (2011).
Chen, J. et al. Calycosin suppresses breast cancer cell growth via ERβ-dependent regulation of IGF-1R, p38 MAPK and PI3K/Akt pathways. PLoS One 9, e91245. 91210.91371/journal.pone.0091245. eCollection 0092014 (2014).
Dunn, E. F. et al. Dasatinib sensitizes KRAS mutant colorectal tumors to cetuximab. Oncogene. 30, 561–574 (2011).
Chen, R. J. et al. Chemopreventive effects of pterostilbene on urethane-induced lung carcinogenesis in mice via the inhibition of EGFR-mediated pathways and the induction of apoptosis and autophagy. J Agric Food Chem. 60, 11533–11541 (2012).
Nakajima, Y. et al. Estrogen regulates tumor growth through a nonclassical pathway that includes the transcription factors ERbeta and KLF5. Sci Signal 4, ra22 (2011).
Tolba, M. F. et al. Caffeic acid phenethyl ester synergistically enhances docetaxel and paclitaxel cytotoxicity in prostate cancer cells. . IUBMB life. 65, 716–729 (2013).
Reagan-Shaw, S. & Ahmad, N. The role of Forkhead-box Class O (FoxO) transcription factors in cancer: a target for the management of cancer. Toxicol Appl Pharmacol. 224, 360–368 (2007).
Jia, T. et al. The differential susceptibilities of MCF-7 and MDA-MB-231 cells to the cytotoxic effects of curcumin are associated with the PI3K/Akt-SKP2-Cip/Kips pathway. Cancer Cell Int 14, 126 (2014).
Li, Y. et al. Involvement of post-transcriptional regulation of FOXO1 by HuR in 5-FU-induced apoptosis in breast cancer cells. Oncol Lett. 6, 156–160 (2013).
Reagan-Shaw, S. & Ahmad, N. RNA interference-mediated depletion of phosphoinositide 3-kinase activates forkhead box class O transcription factors and induces cell cycle arrest and apoptosis in breast carcinoma cells. Cancer Res. 66, 1062–1069 (2006).
Vakifahmetoglu, H., Olsson, M. & Zhivotovsky, B. Death through a tragedy: mitotic catastrophe. Cell Death Differ. 7, 1153–1162 (2008).
Ye, L., Tao, K., Yu, Y. & Wang, G. Reduction of G0 phase cells of colon cancer caco-2 cells may enhance 5-fluorouracil efficacy. J Biomed Res. 24, 64–68 (2010).
Chen, R. J., Ho, C. T. & Wang, Y. J. Pterostilbene induces autophagy and apoptosis in sensitive and chemoresistant human bladder cancer cells. Mol Nutr Food Res. 54, 1819–1832 (2010).
Tolomeo, M. et al. Pterostilbene and 3′-hydroxypterostilbene are effective apoptosis-inducing agents in MDR and BCR-ABL-expressing leukemia cells. Int J Biochem Cell Biol 37, 1709–1726 (2005).
Palmqvist, R., Stenling, R., Oberg, A. & Landberg, G. Prognostic significance of p27KipI expression in colorectal cancer: a clinico-pathological characterization. . J Pathol 188, 18–23 (1999).
Galizia, G. et al. Prognostic value of p27, p53 and vascular endothelial growth factor in Dukes A and B colon cancer patients undergoing potentially curative surgery. Dis Colon Rectum. 47, 1904–1914 (2004).
Medema, R. H., Kops, G. J., Bos, J. L. & Burgering, B. M. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 404, 782–787 (2000).
Raisova, M. et al. The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J Invest Dermatol. 117, 333–340 (2001).
Mackey, T. J., Borkowski, A., Amin, P., Jacobs, S. C. & Kyprianou, N. bcl-2/bax ratio as a predictive marker for therapeutic response to radiotherapy in patients with prostate cancer. Urology 52, 1085–1090 (1998).
Shakibaei, M. et al. Curcumin enhances the effect of chemotherapy against colorectal cancer cells by inhibition of NF-κB and Src protein kinase signaling pathways. PLoS One. 8, e57218 (2013).
Ferrer, P. et al. Association between pterostilbene and quercetin inhibits metastatic activity of B16 melanoma. Neoplasia. 7, 37–47 (2005).
Mannal, P., McDonald, D. & McFadden, D. Pterostilbene and tamoxifen show an additive effect against breast cancer in vitro. Am J Surg. 200, 577–580 (2010).
Pan, M. H., Chang, Y. H., Badmaev, V., Nagabhushanam, K. & Ho, C. T. Pterostilbene induces apoptosis and cell cycle arrest in human gastric carcinoma cells. J Agric Food Chem. 55, 7777–7785 (2007).
Barone, M. et al. ERβ expression in normal, adenomatous and carcinomatous tissues of patients with familial adenomatous polyposis. Scand J Gastroenterol. 45, 1320–1328 (2010).
Cheng, J., Lee, E. J., Madison, L. D. & Lazennec, G. Expression of estrogen receptor beta in prostate carcinoma cells inhibits invasion and proliferation and triggers apoptosis. FEBS Lett 566, 169–172 (2004).
Choi, K. C., Kang, S. K., Tai, C. J., Auersperg, N. & Leung, P. C. Estradiol up-regulates antiapoptotic Bcl-2 messenger ribonucleic acid and protein in tumorigenic ovarian surface epithelium cells. Endocrinology 142, 2351–2360 (2001).
Datta, S. R. et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91, 231–241 (1997).
Oliver, F. J. et al. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. J Biol Chem. 273, 33533–33539 (1998).
Skehan, P. et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82, 1107–1112 (1990).
Chou, T.-C. & Talalay, P. Analysis of combined drug effects: A new look at a very old problem. . Trends Pharmacol. Sci. 4, 450–454 (1983).
Chou, T. C. & Talalay, P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22, 27–55 (1984).
Acknowledgements
The authors thank Mohamed Fares, Pharmaceutical Chemistry Department, Faculty of Pharmacy, Egyptian Russian University for his help in molecular docking.
Author information
Authors and Affiliations
Contributions
M.T. and S.R. conceived the work idea. M.T. designed and performed the experiments. M.T. and S.R. analyzed the data and wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Tolba, M., Abdel-Rahman, S. Pterostilbine, an active component of blueberries, sensitizes colon cancer cells to 5-fluorouracil cytotoxicity. Sci Rep 5, 15239 (2015). https://doi.org/10.1038/srep15239
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep15239
- Springer Nature Limited
This article is cited by
-
Hyaluronated nanoparticles deliver raloxifene to CD44-expressed colon cancer cells and regulate lncRNAs/miRNAs epigenetic cascade
Cancer Nanotechnology (2023)
-
Pterostilbene promotes mitochondrial apoptosis and inhibits proliferation in glioma cells
Scientific Reports (2021)
-
FOXO transcription factor family in cancer and metastasis
Cancer and Metastasis Reviews (2020)
-
The role of GLI1 for 5-Fu resistance in colorectal cancer
Cell & Bioscience (2017)
-
Pterostilbene exerts anticancer activity on non-small-cell lung cancer via activating endoplasmic reticulum stress
Scientific Reports (2017)