Abstract
Twenty-five increment cores and tree discs were sampled for four common trees in Wadi Allaqi, an extremely arid region in South-East Egypt (19 for Acacia tortilis subsp. raddiana (Savi) Brenan and 2 for each of Balanites aegyptiaca (L.) Delile, Acacia ehrenbergiana Hayne, and Tamarix nilotica (Ehrenb.) Bunge). The main aim of the current study is to achieve a longer temporal perspective on growth, longevity, and marginal parenchyma of the wood samples. The growth ring boundaries of the acacias are differentiated by thin parenchyma bands, which run around the entire stem discs. Samples of Acacia tortilis subsp. raddiana were located along this Wadi from its upstream to downstream parts; based on the mean distance between the bands of marginal parenchyma, longevity, based on the marginal parenchyma bands, indicated that Acacia tortilis subsp. raddiana grew slowly and some of its studied individuals reflected 2 age scenarios in the downstream part, while the measurement interval reflected an established date of around 1884 or 1886. Both scenarios grew fast over a long period of time, and so the chiefly recent growth was dated back to 1885. Approximate dates for the midstream part dated back to 1648, while the overall growth for the upstream part dated back to 1482. Samples of Balanites aegyptiaca may be established between 1608 and 1715, while those of Acacia ehrenbergiana may be established between 1945 and 1975. Tamarix nilotica swiftly established itself, and a new ecosystem replaced the severe arid habitat after the dropping of the water level in 1980s.Two scenarios of age are probably true for the downstream part, implying a date of establishment between 1884 and 1886. It was also discovered that the outdated scenario for B. aegyptiaca and A. ehrenbergiana is more in line with asymptotic value and current growth, indicating larger possibility for future expansion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
Growth rings of trees are of ecological significance because the periodicity of their formation can be related to age (Schweingruber 1988). The age structure of a population infers the population pattern, which is crucial for a better understanding of ecosystem dynamics. The study of growth rings in the tropics is much more complex than in the temperate regions due to the greater number of species, habitat diversity, cambial activity rhythm and phenology, anatomical markers for growth rings, and their respective degrees of, in addition to lack of clearly defined seasons (Islam et al. 2018; Silva et al. 2019). Wherever seasonal change in environmental conditions is dramatic, the effect on the cambium is probable to be dramatic, and the resulting growth ring becomes distinct. Indeed, tropical ring counts are not directly predicted age estimates, although many authors continue to assume their annularity (Prins and van der Jeugd 1993). However, such studies have aroused interest in tropical tree rings because of the need for a greater biological understanding of tropical forest dynamics (Nath et al. 2016; Farahat and Gartner 2019).
A broad variety of anatomical characters define the resulting growth ring, including earlywood-latewood transitions, bands of marginal parenchyma, alternating patterns of fiber and parenchyma, and ring-porous structure (Worbes 1989). Such bands are of continuous marginal parenchyma, filled with amorphous substances such as crystals. Contrary, crystals have been identified as a common feature for many leguminous trees (Worbes 1989). However, identifying and quantifying marginal parenchyma bands (growth rings) in dendrochronological research utilizing some species could be difficult. This confusion can be summarized with another wood parenchyma such as paratracheal and apotracheal parenchyma, or difficulties in identifying rings when they are very narrow (Détienne 1989). Other difficulties include identifying rings near the cambium or different numbers on different radii (Mariaux 1981); difficulties in identifying rings near the dark heartwood, pith, and other color variations in the wood (Gourlay 1995). Other confusions include merging, missing, and dividing rings, as well as false and discontinuous rings (Fahn et al. 1968; Martin 1995).
Kyiapi (1994) found that, the average number of rings produced each year was 1.14 rings (0.88 year/ring). Concerning Acacia species, Gammadid (1989) was able to age four samples of Acacia bussei and reported that number of rings was approximately twice the tree’s age, which may be attributed to the bimodal rainfall pattern. Gourlay (1995) counted rings of some Acacia species of known planting date and generally found their number to be slightly ≤ the known age in locations with a single-mode rainfall pattern. Martin and Moss (1997) reported that the relationship between ring count and age was likely to be unique to a particular geographical area, based on its climate. Even within one area, a high degree of variability in the relationship was present, this suggests that some site characteristics such as water-holding capacity of soil and browsing pressure may be important. Lilly (1977); Argent et al. (2004) and February et al. (2006) reported that the transition from saplings to adults may be rare and episodic, depending on contingencies of climate, fire-free intervals, or collapse of a browser population.
Cartwright and Taylor (2008) reported that the woods of 15 Egyptian wooden archery bows from the collections of the British Museum, ranging in date from the Neolithic period to the New Kingdom, have been scientifically identified. Microscopical examination of millimeters-sized samples revealed that all the bows were made from indigenous Egyptian woods (e.g., Ziziphus spina-christi, Acacia, and Tamarix species). The diagnostic anatomical traits of these three taxa indicated that the ring boundary growth is either distinct or indistinct.
In order to gain a better knowledge about the age and growth conditions of Acacia tortilis subsp. raddiana in hyper-arid Eastern Desert of Egypt’s, researchers are using pre- and postbomb C14 concentration in wood samples, as well as the existence of narrow marginal parenchymatic bands in the wood (Anderson and Krzywinski 2007). According to age scenarios, the investigated tree ages seem to be 200–650 years old. Radial growth might reach 2.4 mm per year; however, it varies regionally and temporally based on calibrated dates according to post-bomb C14 content of investigated samples. There is a low correlation between the band pattern among trees and within sites. Marginal parenchymatic bands can reveal fine-scale variations in growing environment and historical tree management.
So, the main aim of the present study is to achieve a longer temporal perspective on growth, longevity, and marginal parenchyma of wood samples of 4 species in Wadi Allaqi Biosphere Reserve, South-East Egypt. Presence/absence inventories, tree detection, long-term comparison in between past and present of a period of 47 years (after the dropping of the water level in the 1980s in Wadi Allaqi) of Acacia tortilis subsp. raddiana (Savi) Brenan, Balanites aegyptiaca (L.) Delile, Acacia ehrenbergiana Hayne, and Tamarix nilotica (Ehrenb.) Bunge were assessed.
2 Materials and methods
Study area
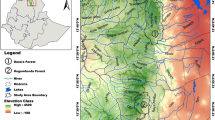
– The study area is located in the South-Eastern Desert of Egypt (i.e., Egyptian Nubian Desert). Wadi Allaqi Biosphere Reserve is situated about 180 km south of Aswan, on the eastern side of Lake Nasser (between latitude 22° and 23° N, and longitude 33° and 35° E), comprises an area of about 22,600 km2. It forms one of the most extensive drainage systems in Egypt’s Eastern Desert. Its upstream tributaries drain some of the mountains that divide the Red Sea coastal plain from the Nile Valley. The wadi extends for about 350 km, in a NW–SE direction. It has an average width of about 1 km being narrower upstream and considerably broader downstream as it approaches the lake (Fig. 1). The southern part of the Eastern Desert is a rocky plateau with rugged topography. This plateau is dissected by numerous wadis, which form a drainage system. These wadis are filled with Quaternary alluvium surrounded by numerous mountainous lands comprising igneous, metamorphic, basement, and Nubian sandstone rocks of essentially Precambrian and Cretaceous ages (Abdel Moneim 2005). Water resources of the Wadi Allaqi are rare and represented mainly by some important wells (e.g., El-Quleib, Ungat, Sidinab, and Umm Ashira) that carry good potable water but in limited quantities. These are all shallow, temporary wells containing water derived from Lake Nasser. Soil in Wadi Allaqi consists of wadi fill deposits and varies in depth, physical, and chemical composition depending on the soil-forming materials, transport processes, and depositions (Springuel 1995).
The climate of the South Eastern Desert of Egypt is extremely arid (Ayyad and Ghabbour 1985), with an aridity index of less than 0.05. The mean temperature ranges from 18.0 to 33.6 °C with total annual mean of 25.6 °C. The long-term monthly mean relative humidity ranged between 14.0 and 38% with a total annual mean of 22.7%, while the annual rainfall rarely exceeds 5 mm and is highly variable in both time and space (Sheded 1992 and Salem 2006). The amount of retained water will depend on the texture and depth of the surface deposits (Kassas 1955). The wind speed in Aswan area ranged between 4 to 8 km h−1 between 1960 and 1980 with an annual mean wind speed of 5.9 km h−1. In contrast, the mean annual wind speed in Wadi Allaqi meteorological station ranged between 7.6 and 10.1 km h−1 from 1996 to 2005 (Springuel et al. 1997).
Methods
– Samples collection and processing. Forty-nine plots were selected to represent 19 locations within Wadi Allaqi Biosphere Reserve (upstream, midstream, and downstream parts, including the different wadi tributaries). These plots were selected to represent the prevailing range of physiographic, environmental variations, and abundance of target species in the study area (Fig. 1). The size of each plot was determined according to the certain parameters such as width of each wadi part, the variation in density and size of the recorded species and the soil variation. The priority was to obtain samples of trees where the felling of tree was probable, and the preferred sample was a whole disc or log from the main stem of the tree. In the cases where trees unable to be felled, samples were collected as increment cores. Within these plots, 25 increment cores and tree discs were sampled during 2008–2010, of those 19 samples for A. tortilis subsp. Raddiana. (Nine represent each of the upstream and midstream parts, while one represents the downstream part.) Two samples were taken to represent each of B. aegyptiaca, A. ehrenbergiana, and T. nilotica. A. tortilis subsp. raddiana, B. aegyptiaca, and T. nilotica have extremely hardwood, so we built a high-torque steel corer that is powered by an electrical drill and can take cores up to 300 mm long and 13 or 20 mm in diameter. In addition, a chain electrical saw was used for dead stem discs’ samples (Gebrekirstos et al. 2008). The samples were cut at breast height (143–150 cm height) wherever possible, or discs were cut between 0.5 and 1.5 m above the ground and dried at room temperature. The stem disk surfaces were sanded gradually using sandpaper of grit size series 80, 100, 120, 150, 210, and 240 μm (Gourlay and Grime 1994). All discs were rough-planed, sanded, and finely polished to ensure that the fine scratches not confused with marginal parenchyma bands.
Parenchyma bands were identified and marked in two radii for each tree sample under a Wild M3C Wild Heerbrugg Switzerland Light Microscope at 10 magnification lenses. Images captured for each sample were collected using a micro digital camera 30 MP connected to microscope and got a serial code (UAC, MAC, and DAC) refer for Acacia at up-, mid-, and downstream parts of Wadi Allaqi, respectively. Image tool analysis software (CMEIAS v.128) was used to measure band intervals. Mean and standard deviation were calculated for each sample. Nineteen samples of A. tortilis subsp. raddiana collected from these parts of Wadi Allaqi were classified depending on the means of the marginal parenchyma band interval, into 6 size classes as follows: I: 0.25 to < 0.4, II: 0.4 to < 0.6, III: 0.6 to < 1.0, IV: 1.0 to < 2.0, V: 2.0 to < 4.0, and VI: > 4.0 mm. The estimated growth rate (GR in mm/year) was used to create age scenarios. Intervening GR was estimated, based on the time interval between the sample under consideration and the next youngest sample, while the overall GR was estimated based on the time interval between the oldest and sampled dates (2008–2010).
X-ray interaction with wood analysis. Scanning electron microprobe (SEM). The crystals detected in these sections were examined by X-ray scanning electron microscopy (SEM) of the type JEOL JEM 35, with a Link 860 Series energy-dispersive X-ray system and backscattered detector. In the case of discs, two or more radii were examined under the modified light microscope at a magnification of × 10. For core samples, only one radius was sampled, prepared, and examined.
Sample for scanning electron microscope. One and 2 cm3 thick cubes were taken from the discs using a band saw. The samples were air-dried at room temperature to stabilize the moisture content of the samples to be < 15%. Furthermore, the cubic surfaces were sanded gradually using sandpaper of grit size series of 80, 100, 120, 150, 210, and 240 μm. The narrow marginal parenchyma bands were identified, marked, and counted. Parenchyma bands that form a more or less continuous layer of varied width at the borders of a growth ring or are irregularly zonate are referred to as marginal parenchyma (Worbes 1989). This feature can often delimit growth in tropical woods as it may appear more clearly defined than vessel boundaries or other anatomical features used to describe growth rings. Marginal parenchyma is commonly composed of < 5 rows of parenchymatous cells forming continued layer. They were stained with safranin to enable the examination of the crystal chains and their subsequent photography. Identification of peripheral parenchyma zones and crystalliferous chains in samples from these plots allowed annual growth rates to be calculated and compared to climatic variables. Cross-dating follows the technique of Stokes and Smiley (1968). As a result, rings were first measured and then cross-dating was verified and corrected with the CDendro 7.1 and CooReader software.
3 Results
Sample discs and core sampling
– The growth ring boundaries of A. tortilis subsp. raddiana are distinguished by thin parenchyma bands, which go through the whole stem disc (Fig. 2). A thin parenchyma band and aggregation of vessels determine the boundaries of the tree-rings. Distinctness of the growth rings varies between species. In the examined species, heartwood marginal parenchyma bands were less pronounced than sapwood bands.
Ring boundaries and general anatomical characteristics
– Acacia tortilis subsp. raddiana. Nineteen samples of A. tortilis subsp. raddiana were classified into 6 size classes depending on the means of the marginal parenchyma band interval as follows (Table 1, Fig. 3):
-
1.
Class I comprises three samples: UAC 04 at the upstream part (0.27 mm), MAC 09 (0.38 mm), and MAC 16 (0.25 mm) at the midstream part (Table 1). These samples were characterized by narrow marginal parenchyma. Growth rings of samples of UAC 04 and MAC 09 were less distinct to identify and had more prominent streaks of white-banded parenchyma because of prominent-banded confluent parenchyma and numerous rays, while the bands of sample MAC 16 were distinct (Fig. 4).
-
2.
Class II had three samples: UAC 07 at the upstream part (0.42 mm), MAC 10 (0.4 mm), and MAC 19 at the midstream part (0.59 mm). These samples were characterized by thin marginal parenchyma bands. Growth rings of samples MAC 10 and UAC 07 were less distinct and had more prominent streaks of white-banded due to prominent banded confluent parenchyma and numerous rays, while that of MAC 19 was more distinct and had marginal parenchyma bands, rays more clear and a net-like structure appear.
-
3.
Class III comprises three samples: UAC 05 (0.74) and UAC 03 (0.82 mm) at the upstream part, and MAC 18 at the midstream part (0.9 mm). These samples were characterized by thin narrow marginal parenchyma bands. They were less distinct to identify and had prominent streaks of white-banded because of prominent banded confluent parenchyma and numerous rays.
-
4.
Class IV has five samples: UAC 06 (1.46 mm), MAC 11 (1.73 mm), MAC 12 (1.0 mm), MAC 13 (1.4 mm), and MAC 17 (1.03 mm). These samples were characterized by thin slightly wider bands, with various distances in between bands. Growth rings of samples of MAC 12 and MAC 13 of marginal bands were highly distinct, while that of samples UAC 06, MAC 11, and MAC 17 were less distinct to identify and had prominent streaks of white-banded because of prominent banded confluent parenchyma and numerous rays.
-
5.
Class V has four samples: UAC 08 (3.29 mm), MAC 14 (3.04 mm), MAC 15 (3.09 mm), and DAC 01 (3.98 mm). The samples of this class were characterized by thin moderately wider marginal bands. Generally, the bands in these samples are distinct, although they had prominent streaks of white-banded that confluent with parenchyma and numerous rays.
-
6.
Class VI has only one sample DAC 02 (4.05 mm), which was characterized by thin highly wider marginal parenchyma bands, which are slightly less distinct to identify and had prominent streaks of white banded, due to prominent banded confluent parenchyma and numerous rays.
Estimation of tree ages: According to the diameter at breast height, the oldest tree recorded in Wadi Allaqi belonged to A. tortilis subsp. raddiana, with radius of 494 mm and a tree girth of 320–340 cm (Fig. 5). Regarding the mean time interval, two age scenarios were probably true for the downstream part, implying a year of foundation between 1884 and 1886 (Table 1), the total recent growth was dated back to 1885. The approximate dates for the midstream part implying the date of establishment between 1848 and 1458 with the exception of sample MAC 19 with suggested date of 1171. The upstream part scenarios implying the date of establishment between 1858 and 1322; the oldest scenario is more in line with recent growth, while the asymptotic value showed there is more room for growth in the future.
Sample correlations: The correlation coefficients between sample DAC 01 and DAC 02 were highly positive (p < 0.01), while DAC 01 correlated negatively with that UAC 03 in upstream part (p < 0.001). UAC 03 correlated positively with UAC 08 (p < 0.05). On the other hand, MAC 13 positively correlated with UAC 03 (p < 0.05), UAC 04 with MAC 11 (p < 0.05), and UAC 05 with UAC 07 (p < 0.001). MAC 12 positively correlated with MAC 16 (p < 0.05), while MAC 14 negatively correlated with MAC 15 (p < 0.001) (Table 2, Fig. 6).
Balanites aegyptiaca. Two samples of B. aegyptiaca in the upstream part had mean distance between bands of 0.78 ± 0.38 mm for sample UBA 01 and 1.06 ± 0.30 mm for UBA 02. Because of large banded confluent parenchyma and numerous rays, the growth ring borders were marked by thin slightly less broadly marginal parenchyma bands with varying distances between them that were less apparent to distinguish and had significant, white-banded streaks. The thin parenchyma band and the aggregation of vessels characterize the tree-rings of this species (Figs. 7, 8). Age scenarios for this species at upstream part are likely, implying an established date of around 1652 or 1715. The two proposed fast growth over a continuous period were dated back to 1608. The upstream scenarios for B. aegyptiaca suggest establishment either around 1715 or 1608. The oldest scenario is, however, in better agreement with recent growth, and the asymptotic value shows greater potential for continued growth. Because of high asymptotic value, the largest tree observed in this part of the upstream part has a radius of 310 mm and hence the oldest scenario is the predicted age.
Acacia ehrenbergiana. Two samples of A. ehrenbergiana within the downstream part had the mean distance in between bands of 1.7 mm (DAE 01) and 0.17 mm (DAE 02) (Table 3). The growth rings were characterized by thin, slightly less spaced marginal parenchyma bands with varying distances between them. The marginal parenchyma bands were less distinct to identify, and had prominent streaks of white-banded because of the prominent banded confluent parenchyma and numerous rays. When the rings are crossed with rays, the rays appear broader (Fig. 9). Age scenarios used to estimate the age of trees as are presented in Table 3 for A. ehrenbergiana at the downstream part. Scenarios are probable suggesting an establishment date around either 1975 or 1658 based on mean measurement interval. The two proposed fast growth over a continuous period, and so as the overall recent growth, which dated back to 1945. The scenarios for this species suggest establishment either around 1975 or 1945. The oldest scenario is, however, in better agreement with the recent growth, and the asymptotic value shows greater potential for continued growth. Because of high asymptotic value, the largest tree observed in this site of the downstream part has a radius of 60 mm, and hence the oldest scenario is the predicted age.
Tamarix nilotica. The two T. nilotica samples were taken in the downstream part: DTN 01 (2.66 mm) and DTN 02 (2.47 mm) (Table 3). Growth rings of these samples were characterized by a thin annual ring in highly polished cross sections and have various spaces between bands. In addition to the multiple rays, the rings had noticeable white streaks banded throughout.
4 Discussion
The findings of the present study indicated that the distinctness of 25 tree sampled rings was rated from low to high, and all of them were utilized to estimate their age scenarios and growth rates. Although the cross dating was not the target of this study, the distinctness of some species rings could encourage us in the future studies of cambial activity of these tree species and then cross-date these species using standard protocols in dendrochronology for accurate dating. Salem (2006) studied the growth rate of several tree species along Wadi Allaqi using tree ring analysis, where he recorded that most tree species are too difficult to core. Acacia species, the species that could be cored, and the tree-ring estimation was less accurate (Shaltout et al. 2010).
The measured tree-ring widths depending on the distance between the marginal parenchyma bands indicated that A. tortilis subsp. raddiana grows slowly. For individuals of some studied trees in the present study, age scenarios were used to estimate the age of trees at the downstream part. Two scenarios are probable, implying the date of establishment of approximately 1884 or 1886, with a recent growth dated back to 1885. The approximate dates for the midstream part dated back to 1648, while for the upstream part dated back to 1482. The suggested establishment for B. aegyptiaca was around 1715 or 1608, while for A. ehrenbergiana was either around 1975 or 1945. T. nilotica swiftly established itself, and a new ecosystem replaced the severe arid habitat after the water level in Wadi Allaqi dropped in the 1980s. The long-term trend in tree growth was well captured by these age scenarios (Zeide 1993). The growth rate is depicted by bell-shaped curve, which means that it increases at first and declines after that in the tree’s life. On the other hand, trees can live for > 1000 years in some species (Higgins et al. 2000; Abdoun et al. 2005).
According to a recent research using C14 dating by February et al. (2006), some Acacia nigrescens and Acacia nilotica individuals are < 100 years old. Longevity estimates of > 100 years are depending on mortality rates in the Mojave Desert (> 100 years; Cody 2000) and repeat photography in Arizona (> 100 years; Bowers et al. 1995) and are expressed as general assertions (150 years; Pellew 1983). As a result, this figure should not be used for all acacias. In the Negev Desert, Ward and Rohner (1997) estimated the age of Acacia raddiana depending on a Serengeti size-age connection in which large trees are ≥ 200 years old (Wiegand et al. 1999). This assumption suggests that long-term environmental influences are stable, resulting in the water-limited circumstances that characterize desert habitats. Short-term growth fluctuation is predictable and does not contradict growth curve model’s long-term tendencies. Comparing with the previous results, and considering the hyper-aridity of Wadi Allaqi (rainfall < 30 mm yr−1), our results of most samples were 0.2 to 2.2 mm yr−1, and lifespan is approximately 200 years, which lie within the previously cited ranges as well as the range of Anderson and Krzywinski (2006).
In the present study, the distances between bands varied among samples, and the width of rings varied considerably between years and among the species (in average maximum ring widths). At midstream part, there is a short distance between bands (ranged between 0.14 and 4.72 mm), compared with the upstream (0.11 to 4.29 mm) and downstream (1.85 to 5.63 mm) parts. Some relatively long distances also appear especially near mountains. Narrow, parenchymatic bands like those present in all A. tortilis subsp. raddiana wood cores have been successfully used as time markers for dendrochronological dating and in climatological studies elsewhere (Gourlay 1995; Eshete and Stahl 1999). Such methods seem to be applicable to A. tortilis subsp. raddiana wood from the extremely arid area of Wadi Allaqi. Marginal parenchyma was formed on slightly regular basis, and the band patterns of trees correlated with each other, either between, or within sites.
Low significant and negative correlations between the sampled plots indicate that the local factors are changeable and influence growth condition, and thus the establishment of marginal parenchyma bands. Within the three-stream parts of Wadi Allaqi, these factors affect the band formation. Annual rainfall in Wadi Allaqi rarely exceeds 5 mm and is highly variable in both time and space. Precipitation comes in discontinuous cloudbursts, varying from one to 15 days in the year, and rainless in many years (Abdou et al. 2016). Since the rainfall is very local and there are so few measuring stations, rain occurrences were unable to be correctly recognized. The water amount received by the soil based on the catchment areas of the receiving basin, are controlled by land topography, and retained based on the texture and depth of surface deposits (Kassas 1955). During the previous decades, rainfall was recorded in south Aswan in autumn 1982, October 1987, December 1990, October 1992, May 1993, October 1994, and November 1996, which caused strong torrents in the surrounding wadis (Salem 2006). Autumn rain events indicate that the S–E Desert is a transitional zone where the precipitation pattern gradually changes from predominantly winter rains in the north (i.e., Mediterranean climate) to predominantly summer rains in the south (i.e., Tropical climate) (IDRC 1997). Generally, the overall amount of rainfall from 1960 to 2013 ranged from 0 to 0.8 mm month−1 (Carlquist 2001; Yacoub 2018). However, the negative correlations could be due to the contrasting conditions in downstream (DAC), midstream (MAC), and upstream (UAC) parts. To clarify these points, further studies need to be carried out.
The growth conditions varied between plots and were shown by the loss of between-plot band correlations. Within plots, however, regional variables are probable to influence all trees by the same way, unless local landscape variability has a large impact. According to Gourlay and Grime (1994), phenology and temperature are associated with band formation and cambial activity. Seasonal temperature changes exist in Wadi Allaqi (Ayyad and Ghabbour 1985); therefore, one might predict ordinary band formation, especially at the high altitudes among cold winter temperatures (e.g., Eiqat core area in the upstream part). The absence of ordinary band formation shows that the temperature in Wadi Allaqi does not create major regular variations or is insufficiently cold to establish a spell of consistently cold temperatures in the winter (Fahn et al. 1968).
The present results revealed that the outdated scenario for B. aegyptiaca and A. ehrenbergiana is more in line with the new growth, while asymptotic value indicates that there is more room for expansion in the future because of the high asymptotic value, the greatest tree in the upstream part with a radius of 310 mm for B. aegyptiaca, and 60 mm for A. ehrenbergiana, hence the outdated scenario for them is the predicted age. In addition, Tamarix has a great resilience to sand erosion and wind. Meanwhile, the Tamarix’s internal directional growth (eccentricity) is influenced by wind–sand erosion. Understanding the relationship between eccentric growth, surface cracks, and growth stress is crucial to comprehending the Tamarix’s active defense mechanism. This can interpret a function mechanism of the self-adaptive process for Tamarix wind–sand erosion stress. Under external environmental stress, the cambium cell division rate increases in Wadi Allaqi (Han et al. 2013). The structure and appearance of Tamarix trunks were altered by environmental stimulation. Tamarix that was stimulated throughout its growth produced broad rings in rainy years and very thin narrower rings in dry years. Atypical growth of Tamarix was linked to environmental encouragement (its pith was deviated toward the windward aspect). These findings are consistent with Farahat et al. (2022) who studied the Moringa peregrina tree in the Egyptian hyper arid desert (see also Farahat and Gartner 2019).
Dendrochronology can and must serve as a key bridge toward the formation of a fuller understanding of Egypt and the ancient world around it, even if it is not a panacea for all of the chronological and environmental concerns. This development will not occur quickly or easily; a project of this magnitude will be protracted, hard, and fraught with difficulties (including scientific and logistical). Furthermore, regardless of its importance, dendrochronology must be used in conjunction with other tools, such as historical records (e.g., king lists), archaeological interpretations, C14 dating, Sothic cycles, and synchronisms with other cultures, on which the problematic chronologies currently in use are based. Only by combining dendrochronology with other types of research, we can fulfill dendrochronology’s potential for Egypt and nearby regions, and so provide specific dates for other historians to discuss (Creasman 2014; Kuniholm et al. 2014).
5 Conclusions
The current study detected the presence/absence inventories, tree detection, long-term comparison in between past and present through 47 years of Acacia tortilis subsp. raddiana, Balanites aegyptiaca, Acacia ehrenbergiana, and Tamarix nilotica in Wadi Allaqi Biosphere Reserve. Nineteen samples of Acacia tortilis subsp. raddiana collected from the up-, mid-, and down-stream parts of Wadi Allaqi were classified into 6 size classes depending on means of the marginal parenchyma band interval. Our results indicated that the growth ring boundaries of A. tortilis subsp. raddiana are differentiated by thin parenchyma bands around the whole stem. Regarding the mean time interval, two scenarios of age are probably true for the downstream part, implying a date of establishment between 1884 and 1886. B. aegyptiaca was thought to have been established around 1715 or 1608, whereas A. ehrenbergiana was thought to have been established around 1975 or 1945. T. nilotica swiftly established itself, and a new ecosystem replaced the severe arid habitat after the dropping of water level in Wadi Allaqi. It was also discovered that the outdated scenario for B. aegyptiaca and A. ehrenbergiana is more in line with asymptotic value and current growth, indicating larger possibility for future expansion.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- UAC:
-
Samples of Acacia tortilis subsp. raddiana in upstream part
- MAC:
-
Samples of Acacia tortilis subsp. raddiana in midstream stream
- DAC:
-
Samples of Acacia tortilis subsp. raddiana in downstream
- UBA:
-
Samples of Balanites aegyptiaca in the upstream part
- DAE:
-
Samples of Acacia ehrenbergiana in downstream part
- DTN:
-
Samples of Tamarix nilotica in downstream part
References
Abdel Moneim AA (2005) Overview of the geomorphological and hydrogeological characteristics of the Eastern Desert of Egypt. Hydrogeol J 13:416–425. https://doi.org/10.1007/s10040-004-0364-y
Abdou HH, Yacoub HA, Gerkema MP, Swart AA (2016) Vanishing knowledge of plant species in the Wadi Allaqi Desert Area of Egypt. Hum Ecol 44:493–504. https://doi.org/10.1007/s10745-016-9826-9
Abdoun F, Jull A, Guibal F, Thinon M (2005) Radial growth of the Sahara’s oldest trees: Cupressus duprezana A. Camus Trees Struct Funct 19:661–670. https://doi.org/10.1007/s00468-005-0430-7
Andersen G, Krzywinski K (2006) Longevity and growth of Acacia tortilis; Insights from C14 Content and Anatomy of Wood, Bergen, Norway
Andersen G, Krzywinski K (2007) Mortality, recruitment and change of desert tree populations in a hyper-arid environment. PLoS ONE 2:208–218. https://doi.org/10.1371/journal.pone.0000208
Argent RM, Mcmahon JM, Bowler JM, Finlayson BL (2004) The dendrochronological potential of Eucalyptus camaldulensis Dehnhardt (River red gum) from the Barmah Forest, Victoria, Australia. Aus Geogr Stud 42:89–102. https://doi.org/10.1111/j.1467-8470.2004.00245.x
Ayyad MA, Ghabbour SI (1985) Hot deserts of Egypt and Sudan. In: Noy-Meir I, Goodall DW (eds) Evenari M. Hot Desert and Arid Shrublands, Elsevier, pp 149–202
Bowers JE, Webb RH, Rondeau RJ (1995) Longevity, recruitment and mortality of desert plants in Grand-Canyon, Arizona. USA J Veg Sci 6:551–564. https://doi.org/10.2307/3236354
Carlquist S (2001) Comparative wood anatomy systematic: ecological, and evolutionary aspects of dicotyledon wood. Springer, Berlin
Cartwright C, Taylor J (2008) Wooden Egyptian Archery bows in the collections of the British Museum. British Museum Tech Res Bull 2:77–83
Cody ML (2000) Slow-motion population dynamics in Mojave Desert perennial plants. J Veg Sci 11:351–358. https://doi.org/10.2307/3236627
Creasman PP (2014) The rings and the chronology of ancient Egypt. Radiocarbon 56:85–92. https://doi.org/10.2458/azu_rc.56.18324
Détienne P (1989) Appearance and periodicity of growth rings in some tropical woods. IAWA Bull 10:123–132. https://doi.org/10.1163/22941932-90000480
Eshete G, Stahl G (1999) Tree rings as indicators of growth periodicity of acacias in the Rift Valley of Ethiopia. For Ecol Manag 116:107–117. https://doi.org/10.1016/S0378-1127(98)00442-3
Fahn A, Waisel Y, Benjamin L (1968) Cambial activity in Acacia raddiana Savi. Ann Bot 32:677–682. https://doi.org/10.1093/oxfordjournals.aob.a084240
Farahat E, Cherubini P, Saurer M, Gartner H (2022) Wood anatomy and tree-ring stable isotopes indicate a recent decline in water-use efficiency in the desert tree Moringa peregrina. Int J Biometeorol 66:127–137. https://doi.org/10.1007/s00484-021-02198-7
Farahat E, Gartner H (2019) Anatomy and dendrochronological potential of Moringa peregrina from the hyper-arid desert in Egypt. Dendrochronologia 56:125606. https://doi.org/10.1016/j.dendro.2019.125606
February EC, Mader AD, Bond WJ (2006) Age determination of two South African Acacia species using ring counts and radiocarbon dating. Afr J Ecol 44:417–419. https://doi.org/10.1111/j.1365-2028.2006.00651.x
Gammadid ID (1989) Growth rates of Acacia species in the bay region of somalia with implications for management. M.Sc. Thesis, University of Oxford
Gebrekirstos A, Mitlohoner S, Hner R (2008) Climate–growth relationships of the dominant tree species from semi-arid savanna woodland in Ethiopia. Trees 22:631–641. https://doi.org/10.1007/s00468-008-0221-z
Gourlay ID (1995) Growth ring characteristics of some African Acacia species. J Trop Ecol 11:121–140. https://doi.org/10.1017/S0266467400008488
Gourlay ID, Grime GW (1994) Calcium-oxalate crystals in African Acacia species and their analysis by scanning proton microprobe (SPM). IAWA Bull J 15:137–148. https://doi.org/10.1163/22941932-90001353
Han Z, Yin W, Zhang J, Niu S, Ren L (2013) Active anti-erosion protection strategy in Tamarisk (Tamarix aphylla). Sci Rep 3:1–10. https://doi.org/10.1038/srep03429
Higgins S, Bond W, Trollope W (2000) Fire, re-sprouting and variability: a recipe for grass-tree coexistence in savanna. J Ecol 88:213–229. https://doi.org/10.1046/j.1365-2745.2000.00435.x
IDRC (1997) Environmental Valuation and Management of Plants in Wadi Allaqi. International Development Research Center, Ottawa, Canada
Islam M, Rahman M, Bräuning A (2018) Growth–ring boundary anatomy and dendrochronological potential in a moist tropical forest in Northeastern Bangladesh. Tree-Ring Res 74:76–93. https://doi.org/10.3959/1536-1098-74.1.76
Kassas M (1955) Rainfall and vegetation in arid Northeast Africa. Plant Ecology Proc. Montpellier Symp., UNESCO, pp 49–57
Kyiapi JL (1994) Structure and characteristics of Acacia tortilis woodland on the Njemps flats. In: Bryan BR (ed) Soil erosion, land degradation and social transition. Geoecological analysis of a semi-arid tropical region, Kenya. Catena Verlag: Advances in geoecology, vol 27, pp 47–69
Kuniholm P, Newton M, Shebiny H, Bassir H (2014) Dendochronological dating in Egypt: work accomplished and future prospects. Radiocarbon 56:93–102. https://doi.org/10.2458/azu_rc.56.18344
Lilly MA (1977) An assessment of the dendrochronological potential of indigenous tree species in south Africa. Department of Geography and Environmental Studies. University of the Witwatersrand, Johannesburg, South Africa. Occasional Paper 18
Mariaux A (1981) Past efforts in measuring age and annual growth in tropical trees. In: Bormann, F.H., Berlyn, G. (Eds.), Age and growth rate of tropical trees: new directions for Research Yale University. Bull.
Martin DM (1995) Age Determination of Acacia tortilis from Marsabit District. Thesis, University of Oxford, Kenya. M.Sc
Martin DM, Moss JM (1997) Age determination of Acacia tortilis (Forsk.) Hayne from northern Kenya. Afr J Ecol 35:266–277. https://doi.org/10.1111/j.1365-2028.1997.067-89067.x
Nath C, Munoz F, Pélissier R, Burslem D, Muthusankar G (2016) Growth rings in tropical trees: role of functional traits, environment, and phylogeny. Trees 30:2153–2175. https://doi.org/10.1007/s00468-016-1442-1
Pellew RA (1983) The impacts of elephant, giraffe and fire upon the Acacia tortilis woodlands of the Serengeti. Afr J Ecol 21:41–74. https://doi.org/10.1111/j.1365-2028.1983.tb00311.x
Prins HH, van der Jeugd HP (1993) Herbivore population crashes and woodland structure in east Africa. J Ecol 81:305–314. https://doi.org/10.2307/2261500
Salem AH (2006) Demographic study on the woody vegetation in Wadi Allaqi, South Eastern Egypt. M.Sc. Thesis, Faculty of Science at Aswan. South Valley University
Schweingruber FH (1988) Tree rings: basics and applications of dendrochronology 3rd ed. Reidel, Dordrecht. J Trop For
Shaltout KH, Sheded MG, Salem AH (2010) Vegetation spatial heterogeneity in a hyper and biosphere reserve area in north Africa. Acta Bot Croat 69:31–46
Sheded MG (1992) Environment and vegetation in the South Eastern Desert, Egypt. Ph D. Thesis, Faculty of Science at Aswan. Assiut University.
Silva MS, Funch LS, da Silva LP (2019) The growth ring concept: seeking a broader and unambiguous approach covering tropical species. Biol Rev 94:1161–1178. https://doi.org/10.1111/brv.12495
Springuel IV (1995) Environment and producers in Wadi Allaqi ecosystems. Faculty of Science at Aswan, Assiut University. Allaqi project, working paper No. (28).
Springuel I, Sheded MG, Murphy KJ (1997) The plant biodiversity of the Wadi Allaqi Biosphere Reserve (Egypt): impact of Lake Nasser on a desert wadi ecosystem. Biodivers Conserv 6:1259–1275
Stokes MA, Smiley TL (1968) An introduction of tree ring dating. Chicago University Press
Ward D, Rohner C (1997) Anthropogenic causes of high mortality and low recruitment in three Acacia tree taxa in the Negev desert, Israel. Biodivers Conserv 6:877–893. https://doi.org/10.1023/B:BIOC.0000010408.90955.48
Wiegand K, Jeltsch F, Ward D (1999) Analysis of the population dynamics of Acacia trees in the Negev desert, Israel with a spatially-explicit computer a simulation model. Ecol Modell 117:203–224. https://doi.org/10.1016/S0304-3800(98)00199-9
Worbes M (1989) Growth rings, increment and age of trees in inundation forests, savannas and a mountain forest in the Neotropics. IAWA Bull 10:109–122. https://doi.org/10.1163/22941932-90000479
Yacoub H (2018) Knowledge and community resilience in rangelands recovery: the case of Wadi Allaqi Biosphere Reserve, South Eastern Desert, Egypt. Restor Ecol 26:S37–S43. https://doi.org/10.1111/rec.12667
Zeide B (1993) Analysis of growth equations. For Sci 39:594–616. https://doi.org/10.1093/FORESTSCIENCE/39.3.594
Acknowledgements
The Authors acknowledged the help of Prof. Emad A. Farahat, Prof. of Plant Ecology, Botany and Microbiology Department, Faculty of Science, Helwan University, for reading the first draft of this manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This work was funded by the Springer nature-Egypt agreement.
Author information
Authors and Affiliations
Contributions
Conceptualization was performed by K.H.S.; methodology was carried out by A.H.S. and M.G.S.; statistical analysis was done by A.H.S., M.G.S., Y.M.A.-S., and K.H.S.; writing—original draft preparation was written by A.H.S., M.G.S., Y.M.A.-S., and K.H.S.; writing—review and editing was provided by Y.M.A.-S. and K.H.S..; funding acquisition was given by Y.M.A.-S., M.G.S., and K.H.S. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salem, A., Shedded, M., Al-Sodany, Y. et al. Tree-ring dating of the common trees in Wadi Allaqi Biosphere Reserve, South-East Egypt. Braz. J. Bot 46, 983–996 (2023). https://doi.org/10.1007/s40415-023-00918-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-023-00918-4