Abstract
In patients with end-stage renal disease (ESRD) undergoing haemodialysis, hypertension is of common detection and frequently inadequately controlled. Multiple pathophysiological mechanisms are involved in the development and progression of the ESRD-related high blood pressure state, which has been implicated in the increased cardiovascular risk reported in this hypertensive clinical phenotype. Renal sympathetic efferent and afferent nerves play a relevant role in the development and progression of elevated blood pressure values in patients with ESRD, often leading to resistant hypertension. Catheter-based bilateral renal nerves ablation has been shown to exert blood pressure lowering effects in resistant hypertensive patients with normal kidney function. Promising data on the procedure in ESRD patients with resistant hypertension have been reported in small scale pilot studies. Denervation of the native non-functioning kidney’s neural excitatory influences on central sympathetic drive could reduce the elevated cardiovascular morbidity and mortality seen in ESRD patients. The present review article will focus on the promising results obtained with renal denervation in patients with ESRD, its mechanisms of action and future perspectives in these high risk patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
High blood pressure is one of the leading risk factors responsible for the increased incidence of end-stage renal disease (ESRD), contributing to the high rates of cardiovascular complications in chronic haemodialysis patients [1,2,3]. Hypertension is detected in approximately 80–85% of patients with chronic kidney disease (CKD) and in the majority of the ESRD patients [4]. For any given cause of CKD including hypertension itself, the elevation in blood pressure values amplifies the degree at which glomerular filtration rate worsens, making the high blood pressure state an independent risk factor for ESRD [4,5,6]. Hypertension also almost 50–60% of haemodialysis patients and its prevalence varies widely among studies according to its detection before or after dialysis and to the methodologies employed to measure blood pressure (office or ambulatory monitoring) [2, 3].
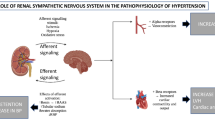
CKD and hypertension are reciprocally connected. In a vicious feedback loop, the presence of hypertension drives CKD severity: uncontrolled resistant hypertension is associated with a marked increase in the risk of developing ESRD over a 5-year period [7]. Multiple crosstalk processes are involved in sustaining the unavoidable high blood pressure state in CKD, and these play an important role in the pathogenesis of the increased cardiovascular risk associated with CKD [2, 3, 8]. One of the main causes of hypertension in haemodialysis patients is represented by the sodium retention and volume expansion [9]. In presence of a volume overload, blood pressure values increase due to an increase in cardiac output and a parallel elevation in systemic vascular resistance [2, 3]. Moreover, previous data indicates that the correction of volume overload by removing excess sodium and reducing target dry weight can improve blood pressure profile in approximately 60% of HD patients [2, 3]. Other factors, such as endothelial dysfunction, activation of the renin-angiotensin-aldosterone axis and overactivity of the sympathetic nervous system represent additional pro-hypertensive pathogenetic mechanisms [9]. The role of sympathetic neural factors is strengthened by recent new data which have also provided new insights on the mechanisms throughout which neuroadrenergic activation may develop [10]. This is the case of the native kidneys, which can send afferent nerve impulses to the central nervous system, leading to the sympathetic overdrive [11]. Furthermore, sympathetic activity increases with CKD progression [12] and afferent renal nerves, in response to intra-renal injury, may play an excitatory influence on central sympathetic neural outflow [13]. Finally, renal sympathetic efferent and afferent nerves play a relevant influence in the development, maintenance and progression of elevated BP values commonly detected in patients with ESRD, often leading to resistant hypertension [10, 14].
Catheter-based renal denervation has been shown to exert some blood pressure lowering effects in resistant hypertensive patients with normal kidney function (see below). Promising data on the procedure have emerged from pilot studies characterized by small sample size, within study populations with ESRD and resistant hypertension. Denervation of the native non-functioning kidney’s neural contribution to central sympathetic drive could reduce the elevated cardiovascular morbidity and mortality reported in ESRD patients. In this review, we will discuss the potential favorable effects of renal nerves ablation in patients with ESRD, mechanisms of action and clinical implications.
2 Resistant Hypertension in End-Stage Renal Disease
In the most recent European Society of Hypertension guidelines on high blood pressure, a hypertensive state is defined as resistant to treatment when appropriate lifestyle measures and pharmacological interventions with optimal or best tolerated doses of three or more drugs fail to achieve office blood pressure below 140/90 mmHg [15]. The inadequate blood pressure control should be confirmed by out-of-office measurement demonstrating uncontrolled 24-h blood pressure values. Evidence of full adherence to therapy and exclusion of secondary causes of hypertension are required to define true resistant hypertension, otherwise this condition is only apparent and termed as “pseudoresistant” hypertension [15]. As mentioned in the previous section, in CKD patients hypertension development and maintenance depend on several factors, whose therapeutical correction not necessarily leads to a blood pressure reduction.
Observational studies report an extremely variable prevalence of resistant hypertension, ranging between 2 and 30%, depending partly on how this clinical hypertensive phenotype is defined [15]. It should be mentioned that for a proper diagnosis careful anamnestic data collection and evaluation of ambulatory blood pressure, in order to avoid the so-called office blood pressure related “alarm reaction”, are mandatory [15]. Not infrequently presumed resistant hypertensive states, once properly evaluated, appear to be pseudo-resistant hypertension. Direct assessment of sympathetic nerve traffic via the microneurographic technique allowed to demonstrate that true resistant hypertension is characterized by a much consistent sympathetic overdrive as compared to the pseudo-resistant hypertensive state (Fig. 1) [16]. Resistant hypertension is significantly more common in patients with CKD and in patients with cardiovascular disease [17, 18] and it is also associated with an increased risk of cardiovascular events, compared with patients with treated hypertension [15]. A subanalysis of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) has shown that progression to ESRD is almost 2-fold higher in patients with resistant hypertension as compared with normotensive patients [19]. Although fluid overload is a central feature of resistant hypertension in haemodialysis patients [20], it explains only 1/3 of the hypertensive states.

From data of Ref. [16]
Muscle sympathetic nerve traffic (MSNA) expressed as bursts incidence over time (left panel) and as bursts incidence corrected for heart rate (right panel) in true resistant hypertension (RHT) and in apparent resistant hypertension (ARHT). Data are shown as means ± SEM. Asterisks (P < 0.01) refer to the statistical significance between groups.
3 Sympathetic Activation in Chronic Kidney Disease
There is overwhelming evidence that CKD is frequently characterized by the presence of sympathetic hyperactivity. Importantly, data are accumulating that this neurogenic alteration may affect cardiovascular and renal prognosis [10, 21]. Sympathetic overactivity exerts pro-hypertensive effects by increasing cardiac output and total peripheral resistance. This may result from direct actions on cardiac and vascular receptors, or by the renin-angiotensin system’s influence on the release of renin and sodium retention by the kidney [21]. Using the above mentioned microneurographic technique, Converse first documented evidence of sympathetic hyperactivity in haemodialysis patients [11]. Subsequent studies have shown a progressive increase in sympathetic activity during the various stages of chronic renal failure, providing in addition evidence that adrenergic activation in patients with HD is greater for magnitude than that characterizing uncomplicated essential hypertension [12]. Some indirect evidence demonstrated that the sympathetic overactivity in patients with ESRD is caused by neurogenic signals originating in the damaged kidneys [22]. In patients with ESRD on haemodialysis, there is a more pronounced increase in nerve endings in the internal area of the adventitia compared with patients with a less severe degree of CKD or a normal renal function. The pathological activation of the sympathetic nervous system is associated with a higher incidence of sudden cardiac death in CKD and ESRD [23, 24].
4 Blood Pressure and Sympathetic Lowering Effects of Renal Denervation
Since the publication of the results of the first non randomized, proof-of-concept study (Simplicity HTN1) a number of clinical trials based on different catheter-based methodologies to achieve renal nerves ablation have been performed. Documents issued by different scientific national and international societies have highlighted the main features of the procedure. In particular in the document issued by the Italian Society of Hypertension (SIIA) a particular focus has been made on a crucial point, namely the criteria to be followed for the patients selection to the procedure [25].
Five sham-controlled randomized trials showed safety and efficacy of second generation radiofrequency or ultrasound systems in patients with or without concomitant medical therapy [26,27,28,29,30]. The reduction in office systolic blood pressure ranged from − 9.0 to − 10.8 mmHg and in the diastolic blood pressure from − 5.0 to − 5.5 mmHg, the corresponding ambulatory blood pressure decreases ranged from − 4.7 to − 9.0 mmHg and − 3.7 to − 6.0 mmHg, respectively [31]. Recently, the final analysis of the Symplicity HTN-3 trial showed that patients in the renal denervation group had significantly larger reductions from baseline to 36-month follow-up in both office and 24-h ambulatory systolic blood pressure compared with the sham control group [32]. Taken together all these positive results represent the background for the favorable evaluation of the renal denervation procedure in the recent European Society of Hypertension guidelines [15].
Along with the blood pressure reduction, bilateral renal nerves ablation has been shown to decrease sympathetic nerve traffic with a considerable reduction in systemic and in renal norepinephrine spillover [33,34,35]. A recent meta-analysis of available studies, although showing significant blood pressure reduction following the procedure (Fig. 2), reported a limited relationship between the renal denervation-dependent reduction in sympathetic nerve traffic as measured by microneurography and the blood pressure reduction [36]. It is thus possible that mechanisms beside the neuroadrenergic inhibition may be involved in determining the blood pressure lowering effects of the procedure.

From data of Ref. [36]
Blood pressure changes induced by renal denervation in the meta-analysis assessing the effects of the procedure on sympathetic nerve traffic in resistant or uncontrolled hypertension. Data (3 or 6 months post-renal denervation) are shown in systolic blood pressure (SBP, upper panel) and diastolic blood pressure (DBP, lower panel).
5 Renal Denervation in End-Stage Renal Disease
Pioneering studies performed more than 70 years ago reported optimistic effects on hypertension control after extensive surgical sympathectomy. Despite improved mortality rates in these patients, the procedures were associated with significant comorbidity and adverse events. Nephrectomy of native kidney in patients undergoing haemodialysis has been reported to reduce blood pressure and sympathetic nerve traffic [37]. Spinal rhizotomy of rats with hypertension following partial nephrectomy has been shown to simultaneously reduce blood pressure and hypothalamic norepinephrine content, confirming that the kidneys are neurologically active and contribute to the neurogenic hypertension [38]. Thus, blocking overactivity of the renal sympathetic nerve in CKD may be a rationale treatment option for lowering blood pressure and delaying the decline of kidney function. Severe resistant hypertension detected in patients undergoing haemodialysis has been an indication for bilateral nephrectomy. However, it is infrequently carried out since the clinical benefits in improving blood pressure values usually do not balance the high peri-operative morbidity risk. Nonetheless, bilateral nephrectomy may be considered in rare cases of non-compliant patients with life-threatening hypertension that cannot be controlled by any other intervention [39]. In recent years, observations on bilateral native nephrectomy as antihypertensive treatment have provided the rationale for catheter-based renal denervation in CKD patients with true resistant hypertension. Surgical ablation ameliorates sympathetic overactivity and prevents both hypertension and the progression of renal disease in experimental models.
Renal nerves ablation is of special interest to nephrologists as it may provide additional benefits to hypertensive patients with CKD, growing body of clinical evidence supporting the safety and efficacy of the procedure in this high risk population. In addition, preclinical and clinical research investigations indicate potential nephroprotective effects in CKD patients. The potential safety and efficacy of therapeutic denervation of native non-functioning kidney in patients with ESRD are however limited. Over the past decade, encouraging data on renal denervation have emerged from pilot studies characterized by the small sample size, within study populations with ESRD and resistant hypertension. Five case studies have reported the procedure as feasible and effective in ESRD, despite the presence of smaller renal artery luminal diameter and atrophic kidneys [40,41,42,43,44]. Schlaich and colleagues described the largest cohort of nine successful denervations in ESRD. Post renal denervation office systolic blood pressure and sympathetic nerve traffic were reduced, but ambulatory blood pressure values remained unaffected [44]. Beyond the impact on blood pressure, reductions in left ventricular mass index were highlighted. Impressively, these effects became evident as early as 3 months following the procedure and persisted for up to 12 months.
The effect of renal denervation was evaluated by our group in a small non-randomized study on haemodialysis patients who showed resistant hypertension despite maximal medical therapy with confirmed adherence [45]. As illustrated in Fig. 3, a reduction in ambulatory systolic blood pressure was observed early in the course of the follow-up post procedure, remaining evident during the 12 months observation after the procedure. In the sham-treated group no blood pressure change was observed. The blood pressure lowering effects were statistically significant during both the daytime and the nighttime periods, suggesting the beneficial role of renal denervation also in haemodialysis patients [44]. Collectively, to date, a unique meta-analysis has been conducted, focusing on impact of renal denervation in 238 resistant hypertensive patients with CKD/ESRD. This analysis encompassed not only patients undergoing haemodialysis but also those with CKD stages 1–5, drawing data from 11 single-center studies, non-randomized, uncontrolled [46]. The results revealed that RDN exhibited effectiveness in reducing both office and 24-h ambulatory blood pressure over a time span of at least 24 months [46].

From data of Ref. [45]
Effects of renal denervation on systolic (SBP) and diastolic (DBP) ambulatory blood pressure in resistant hypertensive patients with chronic renal failure during 12 months follow-up. Data are shown in the group undergoing renal denervation (black columns) and in the sham group (grey columns). Data are shown as means ± SDM. Asterisks (P < 0.01) refer to the statistical significance between groups.
Special attention in these patients should be devoted to the patients follow-up, which, according to recent indications [15, 25, 31], should include during the first year following the procedure periodic assessment of renal function (every month), monthly check of clinic blood pressure and performance of ambulatory blood pressure monitoring every 6 months.
6 Conclusions
Data discussed in the present paper suggest that in patients with ESRD under long-term haemodialysis and with a marked blood pressure elevation despite multiple drug treatment renal nerves ablation may exert blood pressure and sympathetic lowering effects.
The blood pressure reduction occurs early after the denervation procedure, is maintained over a long follow-up and includes both office and ambulatory daytime and nighttime values. This scores in favour of the adoption of this procedure in these patients to improve the chance of achieving blood pressure control in the frequent cases of unresponsiveness to drug treatment. Although renal denervation has been evaluated in selected patients with ESRD, multicenter trials on the effects of this interventional treatment in large cohorts of patients with ESRD have not yet been performed. It is predictable that the preliminary experiences available will promote the design of large scale clinical studies in a near future.
References
Agarwal R, Nissenson AR, Batlle D, Coyne DW, Trout JR, Warnock DG. Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med. 2003;115:291–7.
Agarwal R. Epidemiology of interdialytic ambulatory hypertension and the role of volume excess. Am J Nephrol. 2011;34:381–90.
Leoncini G, Viazzi F, Agabiti-Rosei E, Ambrosioni E, Costa FV, Leonetti G, Pessina A, Trimarco B, Volpe M, Deferrari G, Pontremoli R. Chronic kidney disease in the hypertensive patient. High Blood Press Cardiovasc Prev. 2011;18:31–6.
Johansen KL, Chertow GM, Gilbetrtson DT, Ishani A, Israni A, Ku E, Li S, Liu J, Obrador GT, Schulman I, Chan K, Abbot KC, O’Hare AM, Powe NR, Roetker NS, Scherer JS, Peter WS, Snyder J, Winkelmayer WC, Wong SPY, Wetmore JB. US renal data system 2022 annual data report. Am J Kid Dis. 2023;81(Suppl 1):A8–11.
Ku E, Lee BJ, Wei J, Weir MR. Hypertension in CKD: core curriculum 2019. Am J Kidney Dis. 2019;74:120–31.
Tozawa M, Iseki K, Iseki C, Kinjo K, Ikemiya Y, Takishita S. Blood pressure predicts risk of developing end-stage renal disease in men and women. Hypertension. 2003;41:1341–5.
Sim JJ, Bhandari SK, Shi J, Reynolds K, Calhoun DA, Kalantar-Zadeh K, Jacobsen SJ. Comparative risk of renal, cardiovascular, and mortality outcomes in controlled, uncontrolled resistant, and non-resistant hypertension. Kidney Int. 2015;88:622–32.
Muntner P, He J, Astor BC, Folsom AR, Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities’ study. J Am Soc Nephrol. 2005;16:529–38.
Sarafidis PA, Persu A, Agarwal R, Burnier M, de Leeuw P, Ferro CJ, Halimi JM, Heine GH, Jadoul M, Jarraya N. Hypertension in dialysis patients: a consensus document by the European Renal and Cardiovascular Medicine (EURECA-m) working group of the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) and the hypertension and the kidney working group of the European Society of Hypertension (ESH). Nephrol Dial Transplant. 2017;32:620–40.
Quarti-Trevano F, Seravalle G, Dell’Oro R, Mancia G, Grassi G. Autonomic cardiovascular alterations in chronic kidney disease; effects of dialysis, kidney transplantation and renal denervation. Curr Hypertens Rep. 2021;23:10.
Converse RL Jr, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, Victor RG. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912–8.
Grassi G, Quarti-Trevano F, Seravalle G, Arenare F, Volpe M, Furiani S, Dell’Oro R, Mancia G. Early sympathetic activation in the initial clinical stages of chronic renal failure. Hypertension. 2011;57:846–51.
Schlaich MP, Socratous F, Hennebry S, Eikelis N, Lambert EA, Straznicky N, Esler MD, Lambert GW. Sympathetic activation in chronic renal failure. J Am Soc Nephrol. 2009;20:933–9.
Mazza A, Dell’Avvocata F, Torin G, Bulighin F, Battaglia Y, Fiorini F. Does renal denervation a reasonable treatment option in hemodialysis-dependent patients with resistant hypertension? A narrative review. Curr Hypertens Rep. 2023;25:353–63.
Mancia G, Kreutz R, Brunström M, Burnier M, Grassi G, Januszewicz A, Muiesan ML, Tsioufis K, Agabiti-Rosei E, Algharably EAE, Azizi M, Benetos A, Borghi C, Hitij JB, Cifkova R, Coca A, Cornelissen V, Cruickshank JK, Cunha PG, Danser AHJ, Pinho RM, Delles C, Dominiczak AF, Dorobantu M, Doumas M, Fernández-Alfonso MS, Halimi JM, Járai Z, Jelaković B, Jordan J, Kuznetsova T, Laurent S, Lovic D, Lurbe E, Mahfoud F, Manolis A, Miglinas M, Narkiewicz K, Niiranen T, Palatini P, Parati G, Pathak A, Persu A, Polonia J, Redon J, Sarafidis P, Schmieder R, Spronck B, Stabouli S, Stergiou G, Taddei S, Thomopoulos C, Tomaszewski M, Van de Borne P, Wanner C, Weber T, Williams B, Zhang ZY, Kjeldsen SE. 2023 ESH guidelines for the management of arterial hypertension. J Hypertens. 2023;41:1874–2071.
Dell’Oro R, Quarti-Trevano F, Seravalle G, Bertoli S, Airoldi F, Mancia G, Grassi G. Sympathetic nerve traffic and arterial baroreflex function in apparent drug-resistant hypertension. Hypertension. 2019;74:903–9.
Thomas G, Xie D, Chen HY, Anderson AH, Appel LJ, Bodana S, Brecklin CS, Drawz P, Flack JM, Miller ER III. Prevalence and prognostic significance of apparent treatment resistant hypertension in chronic kidney disease: report from the chronic renal insufficiency cohort study. Hypertension. 2016;67:387–96.
de Beus E, van der Sande NGC, Bots ML, Spiering W, Voskuil M, Visseren FLJ, Blankestijn PJ. Prevalence and clinical characteristics of apparent therapy-resistant hypertension in patients with cardiovascular disease: a cross-sectional cohort study in secondary care. BMJ Open. 2017;7: e016692.
Muntner P, Davis BR, Cushman WC, Bangalore S, Calhoun DA, Pressel SL, Black HR, Kostis JB, Probstfield JL, Whelton PK, ALLHAT Collaborative Research Group. Treatment-resistant hypertension and the incidence of cardiovascular disease and end-stage renal disease: results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension. 2014;64:1012–21.
Zoccali C, Moissl U, Chazot C, Mallamaci F, Tripepi G, Arkossy O, Wabel P, Stuard S. Chronic fluid overload and mortality in ESRD. J Am Soc Nephrol. 2017;28:2491–7.
Seravalle G, Mancia G, Grassi G. Role of the sympathetic nervous system in hypertension and hypertension-related cardiovascular disease. High Blood Press Cardiovasc Prev. 2014;21:89–105.
Rubinger D, Backenroth R, Sapoznikov D. Sympathetic nervous system function and dysfunction in chronic hemodialysis patients. Semin Dial. 2013;26:333–43.
Saravanan P, Davidson NC. Risk assessment for sudden cardiac death in dialysis patients. Circ Arrhythm Electrophysiol. 2010;3:553–9.
Zoccali C, Mallamaci F, Parlongo S, Cutrupi S, Benedetto FA, Tripepi G, Bonanno G, Rapisarda F, Fatuzzo P, Seminara G, Cataliotti A, Stancanelli B, Malatino LS, Cateliotti A. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation. 2002;105:1354–9.
Bruno RM, Taddei S, Borghi C, Colivicchi F, Desideri G, Grassi G, Mazza A, Muiesan ML, Parati G, Pontremoli R, Trimarco B, Volpe M, Ferri C. Italian Society of Hypertension (SIIA) position paper on the role of renal denervation in the management of the difficult-to-treat hypertensive patient. High Blood Press Cardiovasc Prev. 2020;27:109–17.
Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, SPYRAL HTN-OFF MED Trial Investigators. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017;390:2160–70.
Kandzari DE, Böhm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, SPYRAL HTN-ON MED Trial Investigators. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346–55.
Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, RADIANCE-HTN Investigators. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391:2335–45.
Böhm M, Kario K, Kandzari DE, Mahfoud F, Weber MA, Schmieder RE, SPYRAL HTN-OFF MED Pivotal Investigators. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395:1444–51.
Azizi M, Sanghvi K, Saxena M, Gosse P, Reilly JP, Levy T, Rump LC, Persu A, basile J, Bloch MJ, Daemen J, Lobo MD, Mafhoud F, Schmieer R, Sharp ASP, Weber MA Sapoval M, Fong P, RADIANCE-HTN Investigators, Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet. 2021;397:2476–86.
Schmieder RE, Mahfoud F, Mancia G, Azizi M, Böhm M, Dimitriadis K, Kario K, Kroon AA, Lobo MD, Ott C, Patack A, Persu A, Scalise F, Schlaoch M, Kreutz R, Tsioufis C. European Society of Hypertension position paper on renal denervation 2021. J Hypertens. 2021;39:1733–41.
Bhatt DL, Vaduganathan M, Kandzari DE, Leon MB, Rocha-Singh K, Townsend RR, Katzen BT, Oparil S, Brar S, DeBruin V, SYMPLICITY HTN-3 Steering Committee Investigators. Long-term outcomes after catheter-based renal artery denervation for resistant hypertension: final follow-up of the randomised SYMPLICITY HTN-3 Trial. Lancet. 2022;400:1405–16.
Schlaich MP, Sobotka PA, Krum H, Whitbourn R, Walton A, Esler MD. Renal denervation as a therapeutic approach for hypertension: novel implications for an old concept. Hypertension. 2009;54:1195–201.
Grassi G, Seravalle G, Brambilla G, Pini C, Trabattoni D, Cuspidi C, Corso R, Pieruzzi F, Genovesi S, Stella A, Facchetti R, Spaziani D, Bartorelli A, Mancia G. Blood pressure responses to renal denervation precede and are independent of the sympathetic and baroreflex effects. Hypertension. 2015;65:1209–16.
Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, Esler MD, Schlaich MP. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension. 2013;61:457–64.
Biffi A, Dell’Oro R, Quarti-Trevano F, Cuspidi C, Corrao G, Mancia G, Grassi G. Effects of renal denervation on sympathetic nerve traffic and correlates in drug-resistant and uncontrolled hypertension: a systematic review and meta-analysis. Hypertension. 2023;80:659–67.
Sata Y, Schlaich MP. The potential role of catheter-based renal sympathetic denervation in chronic and end-stage kidney disease. J Cardiovasc Pharmacol Ther. 2016;21:344–52.
Hausberg M, Kosch M, Harmelink P, Barenbrock M, Hohage H, Kisters K, Dietl KH, Rahn KH. Sympathetic nerve activity in end-stage renal disease. Circulation. 2002;106:1974–9.
Aldiabat M, Alabdallah K, Kofahi A, Aziz S. Bilateral nephrectomy, the forgotten measure in the treatment of refractory hypertension in patients with end-stage renal disease: a case report and literature review. Cureus. 2020;12: e9031.
Lambert EA, Krum H, Sobotka PA, Schmieder RE, Ika-Sari C, Eikelis N, Straznicky N, Lambert GW, Esler MD. Feasibility of catheter-based renal nerve ablation and effects on sympathetic nerve activity and blood pressure in patients with end-stage renal disease. Int J Cardiol. 2013;168:2214–20.
Di Daniele N, De Francesco M, Violo L, Spinelli A, Simonetti G. Renal sympathetic nerve ablation for the treatment of difficult-to-control or refractory hypertension in a haemodialysis patient. Nephrol Dial Transplant. 2012;27:1689–90.
Prochnau D, Lauten A, Busch M, Kuehnert H, Figulla HR, Surber R. Catheter-based radiofrequency ablation therapy of the renal sympathetic-nerve system for drug resistant hypertension in a patient with end-stage renal disease. Int J Cardiol. 2012;154:e29–30.
Ott C, Schmid A, Ditting T, Sobotka PA, Veelken R, Uder M. Renal denervation in a hypertensive patient with end-stage renal disease and small arteries: a direction for future research. J Clin Hypertens. 2012;14:799–801.
Schlaich MP, Bart B, Hering D, Walton A, Marusic P, Mahfoud F. Feasibility of catheter- based renal nerve ablation and effects on sympathetic nerve activity and blood pressure in patients with end-stage renal disease. Int J Cardiol. 2013;168:2214–20.
Scalise F, Sole A, Singh G, Sorropago A, Sorropago Balla-Beni C, Maccario M, Vettoretti S, Grassi G, Mancia G. Renal denervation in patients with end-stage renal disease and resistant hypertension on long-term haemodialysis. J Hypertens. 2020;38:936–42.
Xia M, Liu T, Chen D, Huang Y. Efficacy and safety of renal denervation for hypertension in patients with chronic kidney disease: a meta-analysis. Int J Hyperth. 2021;38:732–42.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors report no conflict of interest.
Funding
Open access funding provided by Università degli Studi di Milano - Bicocca within the CRUI-CARE Agreement.
Data Availability Statement
Non applicable, the paper is a review.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Scalise, F., Quarti-Trevano, F., Toscano, E. et al. Renal Denervation in End-Stage Renal Disease: Current Evidence and Perspectives. High Blood Press Cardiovasc Prev 31, 7–13 (2024). https://doi.org/10.1007/s40292-023-00621-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40292-023-00621-1