Abstract
The present study was designed to provide information on the ability of several different anthropometric markers to reflect the renal impairment associated with body weight increase and to predict the development of renal alterations linked to overweight and obesity. In 574 subjects representative of the general population of the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study, with an age range between 57 and 73 years, we investigated the association between different anthropometric markers of body fat, as alternative to body mass index, and renal failure, to obtain information useful for determining their potential predictive value. Renal dysfunction was significantly associated with almost all anthropometric markers of adiposity related to body weight and body shape. After adjustment for confounders, such as age, sex, office blood pressure, serum glucose, antihypertensive drugs and smoking habit, association remained significant only for waist-to-hip ratio (WHR), lipid accumulation product (LAP) and visceral adiposity index (VAI). These 3 markers also displayed at the receiver operating curves (ROC) analysis the best ability to detect subjects with or without kidney dysfunction. The results of the present study provide evidence that WHR, LAP and VAI represent the best markers of renal dysfunction associated with visceral body fat accumulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
It is well established that the obese state not only represents a key variable for defining cardiovascular and metabolic risk but it also contributes to an impaired kidney function [1]. Specifically, excess visceral fat, rather than obesity in general, represents the main link between obesity and kidney function [2]. Obesity may impair renal function directly or indirectly throughout the influence of hormones, development of insulin resistance, low-grade inflammation, adipokines, oxidative stress, protein glycation, sympathetic and endothelial dysfunction, as well as throughout related comorbidities such as type 2 diabetes mellitus, hypertension, dyslipidaemia and accelerated vascular atherosclerosis [3, 4].
Assessment of body mass index (BMI) allows to identify the obese state as a leading cause of increased mortality in various metabolic disorders [5], although BMI does not provide differentiation between fat mass and muscle mass and information on the distribution of fat in specific body areas. These significant limitations cast doubt upon the effectiveness of BMI in practical use, potentially favoring the occurrence of erroneous assessments of adiposity, closely related to individual height. Moreover, the inadequacy in gauging central obesity is of particular concern, given that abdominal fat represents a more accurate indicator of cardiovascular and metabolic risk when compared to overall body fatness [6].
Fat mass and its distribution can be assessed by imaging techniques such as computed tomography and magnetic resonance as well as by ultrasound and dual energy X-ray absorptiometry (DXA). However, the limited availability of expensive devices, high maintenance costs, and requirement of skilled operators may hinder the application of these methods in clinical practice and in research [7]. Given all the above mentioned limitations various body adiposity indices have been proposed throughout the years to assess body shape. Some indicators reflect intra-abdominal fat or “central obesity” such as waist circumference (WC), waist-to-hip ratio (WHR) or weight-adjusted-waist index (WWI). Other markers may better reflect body fat distribution and total fat mass such as ta body shape index (ABSI), waist-to-height ratio (WHtR) and relative fat mass (RFM). Additional others such as the lipid accumulation product (LAP) or the visceral adiposity index (VAI) may be better predictors of visceral fat function associated with cardiovascular risk [8,9,10,11,12,13]. The present study was designed to investigate the association between the above mentioned obesity markers, as alternative to BMI, and renal failure.
2 Methods
2.1 Study Design and Population
Details related to the study population have been previously reported [14]. The PAMELA (Pressioni Arteriose Monitorate E Loro Associazioni) study was performed in 3200 subjects of the population of Monza (a town near Milan, Italy) stratified according to gender and age decades. Demographic and clinical characteristics of participants and non-participants were similar, as assessed by phone interviews. After obtaining an informed consent, participants were invited to attend the outpatient clinic of S. Gerardo Hospital of Monza in the morning of a working day (Monday to Friday), after an overnight fast and abstinence from alcohol and smoking since the previous day. From the original sample of 2051 subjects who underwent the initial evaluation in the nineties, for the present cross-sectional study a sample of 574 survived subjects who attended the third survey in 2016-2018 was examined. All variables reported in the present paper were collected during this third survey. The study protocol complied with the Declaration of Helsinki, and it was approved by the Ethics Committee of the Institution involved. All participants provided written consent after being informed of the study nature and purpose.
2.2 Measurements
Data collection included medical history, a thorough physical examination, body weight and height, standard blood tests and three sphygmomanometer systolic (S) and diastolic (D) BP measurements in the sitting position (office BP). Body weight was recorded to the nearest 0.1 kg using a calibrated electronic scale with patients wearing indoor clothing without shoes and body mass index (BMI) was then calculated. Height was recorded to the nearest 0.5 cm using a standardized wall-mounted height board. Waist circumference (WC) was assessed halfway between the lower ribs and the iliac crest. Hip circumference (HC) was measured from the widest part of pelvis. Additionally, several other obesity indices were calculated using the following equations showed in Table 1 [8,9,10,11,12,13].
Diabetes mellitus was defined as fasting plasma glucose (FPG) ≥ 126 mg/dl or use of hypoglycaemic medications. Renal failure defined as estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2, estimated by the Modification of Diet in Renal Disease (MDRD) equation [15]. Laboratory analyses included serum glucose, total serum cholesterol, high density lipoprotein (HDL) cholesterol, serum triglycerides, and creatinine and low-density lipoprotein (LDL) cholesterol estimated according to the Friedewald equation [16].
2.3 Statistical Analysis
Data were expressed as median (lower quartile-upper quartile) or percent values. Comparisons between subjects with and without renal dysfunction were performed by Mann–Whitney test or prevalence test (chi-square test). Logistic regression model was used to investigate the association between anthropometric indicators of adiposity and renal dysfunction. Models were unadjusted (model 1) and adjusted as follows: age and sex (model 2); age, sex, office SBP, serum glucose, antihypertensive drug and smoking habit (model 3). Receiver operating curve (ROC) and their area under the curve (AUC) were used to compare discrimination in detecting subjects with or without renal dysfunction. The optimal cut point was chosen optimizing sensibility and specificity by Youden index. A P value < 0.05 was considered statistically significant. All statistical analyses were conducted using SAS statistical software version 9.4 (SAS Institute, Cary, North Carolina, USA).
3 Results
3.1 Population Characteristics
As shown in Table 2, among 574 participants, median age was 66 (57–73) years, male prevalence 49.4% and 10.6% smokers. Median systolic and diastolic clinic BP amounted to 136.2 (124.3–148) and 83.3 (76.7–89) mmHg respectively, 49.5% of the total population being under antihypertensive drug treatment and 17.6% under statins. About 9.0% of the total population was diabetic. Table 2 also shows baseline office BP, office heart rate, biochemical values and obesity indices of the participants.
3.2 Subgroups Without and with Renal Dysfunction
Table 3 shows data collected in the study population, when classified according to the presence or absence of renal impairment. Prevalence of renal dysfunction amounted to 11.1% and patients with renal impairment showed statically significant differences in all the evaluated variables, with the exception of office heart rate and total cholesterol. They were older, more frequently males without habit of smoking and with higher SBP values more frequently treated with antihypertensive drugs. Lipid profile was similar in the two groups, patients with renal impairment showing lower LDL and HDL levels and higher TG levels.
As far as anthropometric values are concerned, with the only exception of RFM, all other indices evaluated in the present study were significantly greater in the subjects with as compared to those without renal dysfunction. Finally, in terms of adiposity indices both LAP and VAI were significantly higher in subjects with renal dysfunction.
3.3 Association Between Anthropometric Markers and Renal Dysfunction
Figure 1 shows the association between anthropometric indicators of adiposity related to body weight and body shape and renal dysfunction. Renal dysfunction was significantly associated with all anthropometric markers of adiposity related to body weight and body shape, with the exception of RFM and VAI. Association remained significant for WC, WHR, LAP and VAI after adjustment for age, sex, office SBP, serum glucose, antihypertensive drugs, and smoking habit.
Unadjusted and adjusted odds ratios (OR) for association between kidney function alterations and anthropometric indices in the study population. CI confidence interval, WC waist circumference, BMI body mass index, WHR waist-to-hip ratio, ABSI a body shape index, BRI body roundness index, WHtR waist-to-height ratio, RFM relative fat mass, WWI weight-adjusted-waist index, LAP lipid accumulation product, VAI visceral adiposity index. Model 1: unadjusted variables, Model 2: adjusted variables for age and sex, Model 3: adjusted variables for age, sex, office systolic blood pressure (SBP), serum glucose levels, antihypertensive drug treatment and cigarette smoking
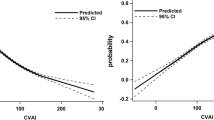
Figure 2 shows the ROC curves for WHR, LAP and VAI. These three markers displayed the largest area under the curves values among the various obesity markers evaluated in the present study (Table 4), confirming their ability in detecting subjects with or without renal dysfunction.
4 Discussion
The results of the present study provide evidence that among different anthropometric markers of adiposity WHR, LAP and VAI represent those more closely associated with renal dysfunction. After correction for various covariates, these variables confirmed their nature as risk factors for renal impairment, showing a greater predictive ability for kidney dysfunction than other anthropometric indices. Interestingly, one of these markers, WHR (and its closely related covariable WHtR) has been shown to represent the best predictor of cardiovascular disease and hypertension development in Asian populations [11, 13]. The present findings expand this information providing evidence that the predictive value of WHR also applies to renal dysfunction as well and that this association can be detected also in a general European population.
The present study was not aimed at investigating the pathophysiological mechanism responsible for the adverse impact of the obese state, particularly of visceral type, on renal function. Several pathways have been identified by previous studies, however [3, 4, 17] Indeed obesity impairs kidney function via the direct effects that adiposity exerts on the kidney, and indirectly due the systemic complications of obesity including diabetes mellitus, vascular atherosclerosis and hypertension [18]. Excess adipose tissue in and around the kidneys may increase the volume of renal sinus fat and peri-renal fat and might result in compression of the thin loop of Henle and vasa recta of the renal medulla, determining alterations in tubular function. Furthermore, the reduction in sodium chloride concentration triggers a macula densa feedback which increases glomerular filtration and maintains sodium balance by activating the renin-angiotensin-aldosterone system. Two additional mechanisms involved in the association between obesity and renal dysfunction are worthy to be mentioned. First, the sympathetic overactivity characterizing the obese state which may represent a factor favoring the progression of the renal disease [19]. An additional factor is represented the adipocytes synthesis of several important adipokines which are implicated not only in insulin resistance but also in inflammation and other physiological processes. All these pathogenetic changes may promote progression of renal dysfunction [20, 21].
There is a consensus that adipose tissue anatomical distribution, and particularly central obesity, is important for determining the impact of obesity on renal dysfunction [22]. BMI does not reflect the fat distribution or even the total adipose tissue level because it is affected by muscle mass [8]. Indeed, many patients with chronic kidney disease in the advanced clinical stages often suffer from chronic caloric consumption, thus, relying only on BMI to evaluate body fat may lead to the so called “obesity paradox” [23, 24]. Likewise, volume overload often detected in kidney disease may also interfere with the ability to assess BMI. WC represents a marker commonly used to reflect abdominal fat accumulation. However it is unable to distinguish subcutaneous adipose and visceral adipose tissue [25]. Evans and coworkers found significant correlations between WHR and estimated GFR decline, increase in urinary albumin‐creatinine ratio and uric acid levels [2]. In another study, Lin and colleagues found in non-diabetic patients with chronic kidney disease that visceral obesity and large proportion of body fat mass was associated with an adverse clinical outcome when compared to normal weight patients and patients with overweight and obesity alone [26].
The present study has some limitations. First, the present study was cross sectional not allowing to determine the ability of the various anthropometric variables to predict 10 year renal disease risk. Second, due the observational nature of this study, the possibility of unmeasured confounding factors, despite adjustment for known prognostic variables, still remains. In addition, because the majority of participants was middle-aged, caution should be made in extrapolating the conclusions of the present study to other age groups. Finally, it is difficult to establish the causality between the WHR, VAI, LAP and renal dysfunction based on this cross-sectional study. Future studies are thus needed to explore the relationships between dynamic chances in these anthropometric markers throughout years and renal disease progression.
5 Conclusions
In conclusion, WHR, LAP and VAI documented the best positive association with renal dysfunction among other obesity indicators. Anthromorphometric measures that include assessment of central fat deposition such WHR, LAP and VAI are more related to kidney disease than BMI. Relying on BMI alone may underestimate the risk for kidney disease and those anthropomorphic measurements focused on central fat deposition may be more important risk factors for chronic kidney disease than BMI. Future studies are required to investigate in longitudinal studies the ability of these indices in predicting outcomes and identifying high risk subjects for possible intervention.
References
Obermayr RP, Temml C, Knechtelsdorfer M, Gutjahr G, Kletzmayr J, Heiss S, Ponholzer A, Madersbacher S, Oberbauer R, Klauser-Braun R. Predictors of new-onset decline in kidney function in a general middle-european population. Nephrol Dial Transplant. 2008;23:1265–73.
Evans PD, McIntyre NJ, Fluck RJ, McIntyre CW, Taal MW. Anthropomorphic measurements that include central fat distribution are more closely related with key risk factors than BMI in CKD stage 3. PLoS ONE. 2012;7: e34699.
Antonini L, Grosso A, Francalanci C, Mattei R. Cardiovascular pathophysiology of obesity. High Blood Press Cardiovasc Prev. 2007;14:207–12.
Wickman C, Kramer H. Obesity and kidney disease: potential mechanisms. Semin Nephrol. 2013;33:14–22.
Cameron AJ, Magliano DJ, Shaw JE, Zimmet PZ, Carstensen B, Alberti KGMM, et al. The influence of hip circumference on the relationship between abdominal obesity and mortality. Int J Epidemiol. 2012;41:484–94.
Millar SR, Perry IJ, Phillips CM. Assessing cardiometabolic risk in middle-aged adults using body mass index and waist-height ratio: are two indices better than one? A cross-sectional study. Diabetol Metab Syndr. 2015;7:73.
Vilalta A, Gutierrez JA, Chves S, Hernandez M, Urbina S, Hompesch M. Adipose tissue measurement in clinical research for obesity, type 2 diabetes and NAFLD/NASH. Endocrinol Diab Metab. 2022;5: e00335.
Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS ONE. 2012;7: e39504.
Thomas DM, Bredlau C, Bosy-Westphal A, Mueller M, Shen W, Gallagher D, et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity. 2013;21:2264–71.
Ashwell M, Gibson S. Waist-to-height ratio as an indicator of ‘early health risk’: simpler and more predictive than using a ‘matrix’ based on BMI and waist circumference. BMJ Open. 2016;6: e010159.
Janghorbani M, Aminorroaya A, Amini H. Comparison of different obesity indices for predicting incident hypertension. High Blood Press Cardiovasc Prev. 2017;24:157–66.
Park Y, Kim NH, Kwon TY, Kim SG. A novel adiposity index as an integrated predictor of cardiometabolic disease morbidity and mortality. Sci Rep. 2018;8:16753.
Wu Q, Zhang F, Li R, Li W, Gou D, Wang L. Identification of the best anthropometric index for predicting the 10-year cardiovascualr disease in southwest China: a large single center, cross-sectional study. High Blood Press Cardiovasc Prev. 2022;29:417–28.
Grassi G, Bombelli M, Sega R, Quarti-Trevano F, Madotto F, Facchetti R, Panzeri MF, Mancia G. The PAMELA (Pressioni Arteriose Monitorate E Loro associazioni) Study. High Blood Press Cardiovasc Prev. 2007;14:83–8.
Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kuseek JW, Van Lette F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
Elsayed EF, Sarnak MJ, Tighiouart H, Griffith JL, Kurth T, Salem DN, et al. Waist-to-hip ratio, body mass index, and subsequent kidney disease and death. Am J Kidney Dis. 2008;52:29–38.
Nawaz S, Chinnadurai R, Al-Chalabi S, Evans P, Kalra PA, Syed AA, Sinha S. Obesity and chronic kidney disease: a current review. Obes Sci Pract. 2023;9:61–74.
Servalle G, Grassi G. Sympathetic nervous system, hypertension, obesity and metabolic syndrome. High Blood Press Cardiovasc Prev. 2016;23:175–9.
Stasi A, Cosola C, Caggiano G, Cimmarusti MT, Palieri R, Acquaviva PM, Rana G, Gesualdo L. Obesity-related chronic kidney disease: principal mechanisms and new approaches in nutritional management. Front Nutr. 2022;9: 925619.
de Vries APJ, Ruggenenti P, Ruan XZ, Praga M, Cruzado JM, Bajema IM, et al. Fatty kidney: emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2014;2:417–26.
Fitzpatrick J, Sozio SM, Jaar BG, McAdams-DeMarco MA, Estrella MM, Tereshchenko LG, Monroy-Trujillo JM, Parehk RS. Association of abdominal adiposity with cardiovascular mortality in incident hemodialysis. Am J Nephrol. 2018;48:406–14.
Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Paradoxical association between body mass index and mortality in men with CKD not yet on dialysis. Am J Kidney Dis. 2007;49:581–91.
Soohoo M, Streja E, Hsiung JT, Kovesdy CP, Kalantar-Zadeh K, Arah OA. Cohort study and bias analysis of the obesity paradox across stages of chronic kidney disease. J Renal Nutr. 2022;32:529–36.
Pou KM, Massaro JM, Hoffmann U, Lieb K, Vasan RS, O’Donnell CJ, Fox CS. Patterns of abdominal fat distribution: the Framingham Heart Study. Diabetes Care. 2009;32:481–5.
Lin TY, Lim PS, Hung SC. Normal-weight obesity and clinical outcomes in nondiabetic chronic kidney disease patients: a cohort study. Am J Clin Nutr. 2018;107:664–72.
Funding
Open access funding provided by Università degli Studi di Milano - Bicocca within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared by the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Vela-Bernal, S., Facchetti, R., Dell’Oro, R. et al. Anthropometric Measures of Adiposity as Markers of Kidney Dysfunction: A Cross-Sectional Study. High Blood Press Cardiovasc Prev 30, 467–474 (2023). https://doi.org/10.1007/s40292-023-00600-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40292-023-00600-6