Abstract
Background
The new visceral adiposity index (NVAI) was superior to previous obesity indices in predicting cardiovascular diseases among Asians. Nevertheless, the utility of the NVAI for predicting chronic kidney disease is still unclear. The objective of this research was to explore the relationship between the NVAI and subclinical renal damage (SRD) and to investigate whether the NVAI outperforms other common obesity indices in predicting SRD in the Chinese population.
Methods
Participants in this cross-sectional study were from the Hanzhong Adolescent Hypertension Cohort. The NVAI and seven other common obesity indices were calculated, including body mass index, waist circumference, lipid accumulation product, visceral adiposity index, Chinese visceral adiposity index, a body shape index and metabolic score for visceral fat. Logistic regression models revealed the association between NVAI and SRD. The odds ratio (OR) and the 95% confidence interval (CI) were calculated to show the association between the two variables. The predictive power of eight obesity indices for SRD was evaluated through the receiver operating characteristic curve and area under the curve (AUC). In addition, the net reclassification index (NRI) and integrated discrimination improvement (IDI) were also applied to compare the incremental predictive value for SRD of different obesity indices.
Results
The median age of the 2358 subjects was 42.00 years. Across NVAI tertiles, the prevalence of SRD was 7.25%, 11.21%, and 21.60%, respectively. After adjusting for confounders, a high level of NVAI remained a risk factor for SRD. The ORs of the middle and top NVAI tertiles for SRD were 1.920 (95% CI: 1.322, 2.787) and 4.129 (95% CI: 2.750, 6.202), respectively. The AUC of the NVAI was 0.666 (95% CI: 0.647, 0.685), which was significantly larger than the AUC of any of the other obesity indicators. Moreover, the NRI and IDI were significantly improved when NVAI was added to the basic model for predicting SRD. Among eight obesity indices, NVAI had the highest NRI (0.392; 95% CI: 0.280, 0.503), and its IDI (0.021; 95% CI: 0.014, 0.027) was second only to that of the body mass index (0.023; 95% CI: 0.014, 0.032).
Conclusions
NVAI is independently and positively associated with SRD. Among the eight obesity indices, the NVAI shows the strongest predictive power for SRD in the Chinese population. The NVAI may be useful as an effective warning indicator of chronic kidney disease in Chinese adults.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) has become a global health issue [1]. The prevalence and mortality of CKD have increased dramatically since 1990 [2]. In 2017, almost 700 million patients struggled with CKD worldwide, with more than 1 million cases dying as a result [2]. Defined as the continuously abnormal structure or function of the kidney for more than three months [3], CKD consists of different stages based on the level of albuminuria or the glomerular filtration rate [2, 4]. Once in end-stage kidney disease, which means poor renal function with no chance of recovery, the quality of life of subjects is always compromised by a slew of negative symptoms [5, 6]. Therefore, it is necessary to identify and treat subclinical renal damage (SRD) as early as possible.
Obesity greatly contributes to the occurrence and deterioration of CKD [7, 8]. According to previous research, the abundance of visceral fat rather than overall obesity is a crucial factor in the link between obesity and CKD [9, 10]. Body mass index (BMI) is frequently utilized in medical practice to measure obesity, but it is unable to reflect regional fat distribution, especially in CKD patients [11, 12]. However, accurate detection methods such as magnetic resonance imaging or computed tomography are not only costly but may be harmful because of exposure to radiation [13]. Therefore, alternative parameters have been developed, including the visceral adiposity index (VAI) [14], Chinese VAI (CVAI) [15], lipid accumulation product (LAP) [16], a body shape index (ABSI) [17], and metabolic score for visceral fat (METS-VF) [18], which have been shown to be related to CKD [19,20,21,22,23]. Recently, it was documented that a new VAI (NVAI) had a higher predictive value for cardiovascular diseases than other obesity indicators in the Korean population [24, 25]. However, research exploring any association between the NVAI and SRD is still absent.
Therefore, this study aimed to investigate among Chinese adults whether the NVAI is a predictor of SRD and compare its predictive value with other obesity indices.
Methods
Study participants
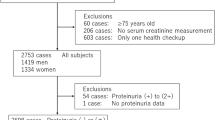
The Hanzhong Adolescent Hypertension Cohort is a currently underway prospective study with the goal of exploring the development of cardiovascular risk factors since childhood. It was established in 1987, and so far, it has been followed up six times for 30 years. Details of the cohort were published previously [26]. The latest follow-up was in 2017, involving 2780 subjects. A total of 417 individuals without relevant data for NVAI or renal function indicators were excluded. After further excluding five patients with severe basic disease, the cross-sectional study eventually included 2358 subjects (Fig. 1).
The research adhered to the guidelines of the Helsinki Declaration. The Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University approved the current study. Informed consent forms were signed by all participants.
Anthropometric measurements
Uniformly trained staff obtained detailed demographic information, history of diseases, smoking habits, and alcohol consumption of the subjects through a standard questionnaire. Participants were measured for waist circumference (WC), height, and weight while wearing only underwear and no shoes. The mean of two measurement values of height was included in the statistical analysis, as was weight. In a quiet environment, a professional used a precalibrated sphygmomanometer to measure the blood pressure of the individuals in a sitting position. Three measurements were conducted at 2-min intervals. The mean was taken as the actual blood pressure level. The value combining 1/3 systolic blood pressure (SBP) with 2/3 diastolic blood pressure (DBP) was determined as the mean arterial pressure (MAP).
Hypertension was defined as SBP ≥ 140 mmHg, DBP ≥ 90 mmHg, taking antihypertensive drugs, or a self-reported history of hypertension. Diabetes was defined as fasting plasma glucose (FPG) ≥ 7.0 mmol/l, taking hypoglycemic medications, or a self-reported history of diabetes.
Biochemical assays
Venous blood samples of individuals who had fasted for at least eight hours were collected by professional staff in the morning. Detection of lipids, renal function, and liver function was subsequently conducted. Relevant biochemical parameters consisted of high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglyceride (TG), total cholesterol (TC), uric acid (UA), creatinine, glutamic pyruvic transaminase (GPT), glutamic oxaloacetic transaminase (GOT), total bile acid (TBA), and FPG. Using spot urine samples, the physicians measured the concentrations of creatinine, albumin, and UA with an automated device.
Obesity indicators
A series of obesity indices were calculated using the following equations [14,15,16,17,18, 24].
For men:
For women:
Subclinical renal damage definition
Using the estimated glomerular filtration rate (eGFR) as the sole index to determine SRD has limitations. Thus, the urinary albumin creatinine ratio (uACR) was used to jointly reflect the actual renal function of the subjects [27]. Based on the modified calculation formula for Chinese individuals, eGFR = 175 × serum creatinine−1.234 × age−0.179 (for men). The value needs to be additionally multiplied by 0.79 for women. In the above equation, the units of serum creatinine concentration, age, and eGFR were mg/dl, years, and ml/min/1.73 m2, respectively [28, 29]. uACR in mg/mmol was computed by dividing albumin by creatinine based on the urine samples. Participants whose eGFR values fluctuated from 30 to 60 were defined as patients suffering from SRD [30]. Men with uACR values ≥ 2.5 and women with uACR values ≥ 3.5 were also included in this category [31].
Statistical analysis
Normally distributed variables were given as the mean ± standard deviation, while other continuous variables were expressed as the median and interquartile range. The number and proportion were used to describe the categorical variables. Based on NVAI tertiles, the overall population was separated into three groups. When comparing parameters among the three groups, the Jonckheere-Terpstra test or the chi-square test was performed where suitable. Spearman correlation analyses revealed the relationships between the obesity indices and the renal function indicators. To examine the connection between NVAI and SRD, binary logistic regression analyses were carried out. Three models were adopted to validate the association, the strength of which was illustrated by the odds ratio (OR). The predictive power of the obesity markers for SRD was proven through receiver operating characteristic (ROC) curves. The area under the curve (AUC), net reclassification index (NRI), and integrated discrimination improvement (IDI) were used to compare the predictive power of the eight adiposity parameters for SRD. All analyses were conducted by SPSS (version 20.0, SPSS Inc., Chicago, IL, USA), Medcalc (version 20.0, Medcalc Ltd., Ostend, Belgium), and R (version 4.2.2, The R Foundation, Vienna, Austria). Two-tailed P values of less than 0.05 were considered statistically significant.
Results
Characteristics of the NVAI tertiles
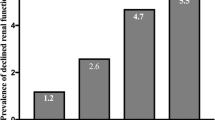
Among 2358 participants, their median age was 42.00 years and 1302 were men (55.22%). The whole population was classified into three groups based on NVAI tertiles (Tertile 1, ≤ 0.56; Tertile 2, 0.56–0.90; Tertile 3, > 0.90). The highest tertile group was the highest for age, men’s proportion, incidence of smoking and alcohol drinking, blood pressure, heart rate, FPG, TC, TG, LDL, TBA, GPT, GOT, serum UA, and values of obesity indices. The proportion of patients who had hypertension or diabetes was also found to be notably higher in the top tertile group than in the other groups. The level of uACR increased significantly as the NVAI tertiles increased, while eGFR decreased gradually. In addition, the higher the NVAI values were, the higher the prevalence of SRD (Table 1).
Association of obesity indicators with markers of renal function
Spearman correlation tests were performed to examine the associations between different obesity indices and markers to evaluate the function of the kidney. The results were shown in Table 2. It was suggested that obesity indices except for ABSI were all negatively linked with eGFR but positively correlated with uACR. In brief, the higher the obesity index score, the worse the kidney function.
Relationship between NVAI and SRD
To investigate the association between NVAI and SRD further, binary logistic regression analyses were conducted. Table 3 demonstrated the relevant results. Generally, the higher the NVAI score was, the greater the risk of SRD in the population. Even after controlling for related confounding variables, including sex, smoking and drinking status, diabetes mellitus, heart rate, TBA, GPT, GOT, TC, LDL, serum UA, and urine UA, a high level of NVAI was still a risk factor for SRD. The OR (95% CI) and variance inflation factor of independent viables in Model 3 were shown in Supplementary Table 1, and no multicollinearity was observed among variables. When choosing the lowest tertile group as a reference, the middle and the top tertile groups of the NVAI demonstrated strong associations with SRD. The ORs of the two groups were 1.920 (95% CI: 1.322, 2.787) and 4.129 (95% CI: 2.750, 6.202), respectively.
Predictive power of the eight obesity indicators for SRD
The predictive ability of the different obesity indices for SRD was evaluated through ROC curve analyses, and P values were obtained by the comparison of the AUCs between the NVAI and the other obesity indicators. As predicted, it was observed that the AUC of the NVAI was 0.666 (95% CI: 0.647, 0.685), which was the largest among all of the obesity indices (Fig. 2, Table 4). Moreover, the incremental predictive values for SRD of different obesity indicators were shown in Table 5. The NRI and IDI of the basic model were significantly enhanced by adding NVAI, CVAI, BMI, WC, LAP, and METS-VF. Among the eight obesity indices, NVAI had the highest NRI (0.392; 95% CI: 0.280, 0.503), and its IDI (0.021; 95% CI: 0.014, 0.027) was second only to that of BMI (0.023; 95% CI: 0.014, 0.032).
Receiver operating characteristic curves of the NVAI and traditional obesity markers for SRD. Legends of Fig. 2: ABSI, a body shape index; BMI, body mass index; CVAI, Chinese visceral adiposity index; LAP, lipid accumulation product; METS-VF, metabolic score for visceral fat; NVAI, new visceral adiposity index; VAI, visceral adiposity index; WC, waist circumference
Discussion
The results of the present study suggested that the higher the NVAI level, the worse the renal function. After correction for various covariates, a high NVAI remained a risk factor for SRD. When compared with other obesity indicators, the NVAI demonstrated greater predictive ability for SRD in Chinese adults.
It is important to find warning parameters of CKD due to its insidious onset and poor prognosis. Obesity, especially visceral obesity, has been revealed to be strongly linked to CKD [7,8,9,10]. In a population without obvious cardiovascular diseases, the pararenal fat tissue of patients whose renal function deteriorated dramatically was much thicker than that of subjects in the first and second stages of CKD. The accumulation of pararenal fat was a risk factor for renal function decline [32]. Olivero et al. found that the visceral adipose tissue ratio at baseline had the ability to predict the worsening of CKD stages during the 12 months after renal surgery [33].
Previous research has revealed that indicators to evaluate visceral fat are closely related to CKD. A Chinese study suggested that the level of VAI was significantly associated with urine protein excretion among middle-aged and elderly individuals [13]. Another cross-sectional study proposed that LAP was an independent predictor of CKD in hypertensive patients [34]. The CVAI was also reported to be significantly correlated with a greater prevalence of diabetic kidney disease [21]. Similar results were obtained in this study. A range of obesity indicators all correlated with uACR positively and eGFR negatively. Renal function subsequently deteriorated when the NVAI level increased.
The value of the NVAI as a predictor for SRD was significantly higher than that of the other obesity indices. Possible causes are as follows. BMI dose not reflects the fat distribution or even the total adipose tissue level well because it is influenced by muscle mass [12]. It has been documented that all-cause mortality increased when the BMI score was either too low or too high [35]. Many CKD patients in the later stages often suffer from chronic consumption; thus, relying only on BMI to evaluate body fat may lead to the “obesity paradox” [36, 37]. In addition, volume overload accompanying CKD can also interfere with the assessment ability of BMI. WC is a common indicator used to reflect abdominal fat accumulation, but it is unable to distinguish subcutaneous adipose tissue and visceral adipose tissue [38]. ABSI was developed to assess the risk of obesity-related diseases independently of BMI [17], but was reported to be not superior to BMI or WC in reflecting body fat distribution, either total fat or visceral fat [39].
Based on surveys carried out in the United States, researchers developed LAP to reflect lipid overaccumulation. In Caucasians, the buildup of visceral tissue can be accurately reflected by the VAI. However, both indexes may not be suitable for the Asian population because body fat distribution differs among ethnic groups [40]. The CVAI was established to assess visceral fat distribution in Asians and was reported to outperform the VAI in predicting renal injury [15, 41]. As a composite index combining age, sex, height, weight, WC, glucose, and lipids, METS-VF was considered an excellent predictor of CKD [42]. In this study, we were delighted to observe that the NVAI had a stronger predictive power than the CVAI and the METS-VF for SRD in the Chinese population. The NVAI was initially developed and validated in the Korean population and demonstrated high agreement with computed tomography in assessing visceral obesity. A positive relationship was noted between it and atherosclerotic cardiovascular disease risk[24]. Recently, a Korean study also found that the NVAI had the highest predictive value for coronary atherosclerosis and arterial stiffness among five obesity indices [25]. This study introduced a new perspective by extending the research on the correlation between the NVAI and clinical diseases from the cardiovascular field to the renal disease field.
The underlying causes linking obesity to SRD have been uncovered gradually. First, excess adipose tissue located in and around the kidneys mechanically compresses the renal parenchyma and vessels, which results in increased NaCl resorption in the Henle loop and decreased NaCl delivery to the macula densa. Macula densa feedback maintains the sodium balance by increasing glomerular filtration and activating the renin–angiotensin–aldosterone system. Although glomerular hyperfiltration is a compensatory regulation mechanism of the kidney, a continuously elevated glomerular hydrostatic pressure will eventually cause kidney injury [43]. Second, adipose tissue overload leads to ectopic lipid accumulation [44]. Circulating lipids can accumulate in almost all types of renal cells and generate toxic effects [45, 46]. Excessive accumulation of lipids not only accelerates tubular damage and interstitial fibrosis but also contributes to the development of glomerulosclerosis [45]. Third, the impact of adipokines on the decline of renal function cannot be overstated. Adipocytes produce numerous vital adipokines. For example, interleukin 6, leptin and tumour necrosis factor α take part in not only insulin resistance but also inflammation and other physiological processes [47]. All of these pathogenic changes contribute to accelerating the progression of CKD.
The present research investigated the association between NVAI and SRD for the first time. In addition, it compared the predictive value of NVAI and common obesity indices from different perspectives, which could help identify better predictors in the specific population.
However, some limitations of the current study must be acknowledged. First, it was a single-centre study performed in northern China, whose findings need to be further validated in other ethnic groups. Second, because the majority of participants were middle-aged, caution is advised when extending the conclusions to other age groups. Finally, it is difficult to establish the causality between the NVAI and SRD based on this cross-sectional study. This cohort will continue to be followed up in the future to explore the relationship between dynamic changes in the NVAI and CKD progression.
Conclusions
In short, the NVAI was proven to show a positive association with SRD. Among eight obesity indicators (BMI, WC, VAI, CVAI, NVAI, and LAP), the NVAI was the best predictor for SRD in the Chinese population. These findings highlight the significance of greater attention being paid to CKD in the population with visceral obesity. NVAI could serve as a reliable marker to recognize obese individuals with an enhanced risk of SRD and facilitate their early diagnosis and treatment, thus avoiding adverse clinical outcomes.
Availability of data and materials
The relevant data can be reasonably requested from the corresponding author.
Abbreviations
- ABSI:
-
A body shape index
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CKD:
-
Chronic kidney disease
- CVAI:
-
Chinese visceral adiposity index
- DBP:
-
Diastolic blood pressure
- eGFR:
-
Estimated glomerular filtration rate
- FPG:
-
Fasting plasma glucose
- GOT:
-
Glutamic oxaloacetic transaminase
- GPT:
-
Glutamic pyruvic transaminase
- HDL:
-
High-density lipoprotein
- IDI:
-
Integrated discrimination improvement
- LAP:
-
Lipid accumulation product
- LDL:
-
Low-density lipoprotein
- MAP:
-
Mean arterial pressure
- METS-IR:
-
Metabolic score for insulin resistance
- METS-VF:
-
Metabolic score for visceral fat
- NVAI:
-
New visceral adiposity index
- NRI:
-
Net reclassification index
- OR:
-
Odds ratio
- ROC:
-
Receiver operating characteristic curve
- SBP:
-
Systolic blood pressure
- SRD:
-
Subclinical renal damage
- TBA:
-
Total bile acid
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- UA:
-
Uric acid
- uACR:
-
Urinary albumin creatinine ratio
- VAI:
-
Visceral adiposity index
- WC:
-
Waist circumference
- WHtR:
-
Waist to height ratio
References
Murton M, Goff-Leggett D, Bobrowska A, Garcia Sanchez JJ, James G, Wittbrodt E, et al. Burden of chronic kidney disease by KDIGO categories of glomerular filtration rate and albuminuria: a systematic review. Adv Ther. 2021;38(1):180–200.
GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–33.
Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Köttgen A, Levey AS, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382(9887):158–69.
Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85(1):49–61.
Murtagh FE, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. Adv Chronic Kidney Dis. 2007;14(1):82–99.
Davison SN, Jhangri GS. Impact of pain and symptom burden on the health-related quality of life of hemodialysis patients. J Pain Symptom Manage. 2010;39(3):477–85.
Obermayr RP, Temml C, Knechtelsdorfer M, Gutjahr G, Kletzmayr J, Heiss S, et al. Predictors of new-onset decline in kidney function in a general middle-european population. Nephrol Dial Transplant. 2008;23(4):1265–73.
Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144(1):21–8.
Elsayed EF, Sarnak MJ, Tighiouart H, Griffith JL, Kurth T, Salem DN, et al. Waist-to-hip ratio, body mass index, and subsequent kidney disease and death. Am J Kidney Dis. 2008;52(1):29–38.
Evans PD, McIntyre NJ, Fluck RJ, McIntyre CW, Taal MW. Anthropomorphic measurements that include central fat distribution are more closely related with key risk factors than BMI in CKD stage 3. PLoS ONE. 2012;7(4):e34699.
Agarwal R, Bills JE, Light RP. Diagnosing obesity by body mass index in chronic kidney disease: an explanation for the “obesity paradox?” Hypertension. 2010;56(5):893–900.
Zeng Q, Wang L, Dong S, Zha X, Ran L, Li Y, et al. CT-derived abdominal adiposity: Distributions and better predictive ability than BMI in a nationwide study of 59,429 adults in China. Metabolism. 2021;115:154456.
Sun K, Lin D, Li F, Qi Y, Feng W, Ren M, et al. Visceral adiposity index is associated with increased urinary albumin excretion: a population-based study. Clin Nutr. 2019;38(3):1332–8.
Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, et al. Visceral adiposity index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33(4):920–2.
Xia MF, Chen Y, Lin HD, Ma H, Li XM, Aleteng Q, et al. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Sci Rep. 2016;6:38214.
Kahn HS. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: a population-based comparison. BMC Cardiovasc Disord. 2005;5:26.
Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS ONE. 2012;7(7):e39504.
Bello-Chavolla OY, Antonio-Villa NE, Vargas-Vázquez A, Viveros-Ruiz TL, Almeda-Valdes P, Gomez-Velasco D, et al. Metabolic Score for Visceral Fat (METS-VF), a novel estimator of intra-abdominal fat content and cardio-metabolic health. Clin Nutr. 2020;39(5):1613–21.
Yan P, Xu Y, Miao Y, Tang Q, Wu Y, Bai X, et al. Association of lipid accumulation product with chronic kidney disease in Chinese community adults: a report from the REACTION study. Lipids Health Dis. 2021;20(1):131.
Chen IJ, Hsu LT, Lu MC, Chen YJ, Tsou MT, Chen JY. Gender differences in the association between obesity indices and chronic kidney disease among middle-aged and elderly Taiwanese population: a community-based cross-sectional study. Front Endocrinol (Lausanne). 2021;12:737586.
Wan H, Wang Y, Xiang Q, Fang S, Chen Y, Chen C, et al. Associations between abdominal obesity indices and diabetic complications: Chinese visceral adiposity index and neck circumference. Cardiovasc Diabetol. 2020;19(1):118.
Kim B, Kim G, Kim E, Park J, Isobe T, Sakae T, et al. The a body shape index might be a stronger predictor of chronic kidney disease than BMI in a senior population. Int J Environ Res Public Health. 2021;18(24):12874.
Yu P, Meng X, Kan R, Wang Z, Yu X. Association between metabolic scores for visceral fat and chronic kidney disease: a cross-sectional study. Front Endocrinol (Lausanne). 2022;13:1052736.
Oh SK, Cho AR, Kwon YJ, Lee HS, Lee JW. Derivation and validation of a new visceral adiposity index for predicting visceral obesity and cardiometabolic risk in a Korean population. PLoS ONE. 2018;13(9):e0203787.
Son DH, Ha HS, Lee HS, Han D, Choi SY, Chun EJ, et al. Association of the new visceral adiposity index with coronary artery calcification and arterial stiffness in Korean population. Nutr Metab Cardiovasc Dis. 2021;31(6):1774–81.
Zheng W, Mu J, Chu C, Hu J, Yan Y, Ma Q, et al. Association of blood pressure trajectories in early life with subclinical renal damage in middle age. J Am Soc Nephrol. 2018;29(12):2835–46.
Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165–80.
Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–22.
Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–44.
Mulè G, Calcaterra I, Costanzo M, Geraci G, Guarino L, Foraci AC, et al. Relationship between short-term blood pressure variability and subclinical renal damage in essential hypertensive patients. J Clin Hypertens (Greenwich). 2015;17(6):473–80.
Leoncini G, Viazzi F, Conti N, Baratto E, Tomolillo C, Bezante GP, et al. Renal and cardiac abnormalities in primary hypertension. J Hypertens. 2009;27(5):1064–73.
Bragina A, Osadchiy KK, Rodionova JN, Bayutina D, Cherepanov AG, Podzolkov VI. Pararenal fat and renal dysfunction in patients without significant cardiovascular disease. Am J Nephrol. 2022;53(5):416–22.
Olivero A, Basso L, Barabino E, Milintenda P, Testino N, Chierigo F, et al. The impact of visceral adipose tissue on postoperative renal function after radical nephrectomy for renal cell carcinoma. Minerva Urol Nephrol. 2021;73(6):789–95.
Shi Y, Hu L, Li M, Zhou W, Wang T, Zhu L, et al. Association between the surrogate markers of insulin resistance and chronic kidney disease in Chinese hypertensive patients. Front Med (Lausanne). 2022;9:831648.
Mei X, Hu S, Mi L, Zhou Y, Chen T. Body mass index and all-cause mortality in patients with percutaneous coronary intervention: a dose-response meta-analysis of obesity paradox. Obes Rev. 2021;22(2):e13107.
Kalantar-Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81(3):543–54.
Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Paradoxical association between body mass index and mortality in men with CKD not yet on dialysis. Am J Kidney Dis. 2007;49(5):581–91.
Pou KM, Massaro JM, Hoffmann U, Lieb K, Vasan RS, O’Donnell CJ, et al. Patterns of abdominal fat distribution: the Framingham Heart Study. Diabetes Care. 2009;32(3):481–5.
Parente EB, Mutter S, Harjutsalo V, Ahola AJ, Forsblom C, Groop PH. Waist-height ratio and waist are the best estimators of visceral fat in type 1 diabetes. Sci Rep. 2020;10:18575.
Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord. 1998;22(12):1164–71.
Lin M, Li N, Heizhati M, Gan L, Zhu Q, Yao L, et al. Chinese visceral adiposity index is associated with incident renal damage in patients with hypertension and abnormal glucose metabolism: a longitudinal study. Front Endocrinol (Lausanne). 2022;13:910329.
Feng L, Chen T, Wang X, Xiong C, Chen J, Wu S, et al. Metabolism Score for Visceral Fat (METS-VF): A New Predictive Surrogate for CKD Risk. Diabetes Metab Syndr Obes. 2022;15:2249–58.
Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat Rev Nephrol. 2019;15(6):367–85.
de Vries AP, Ruggenenti P, Ruan XZ, Praga M, Cruzado JM, Bajema IM, et al. Fatty kidney: emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2014;2(5):417–26.
Gai Z, Wang T, Visentin M, Kullak-Ublick GA, Fu X, Wang Z. Lipid accumulation and chronic kidney disease. Nutrients. 2019;11(4):722.
Moorhead JF, Chan MK, El-Nahas M, Varghese Z. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet. 1982;2(8311):1309–11.
Stasi A, Cosola C, Caggiano G, Cimmarusti MT, Palieri R, Acquaviva PM, et al. Obesity-related chronic kidney disease: principal mechanisms and new approaches in nutritional management. Front Nutr. 2022;9:925619.
Acknowledgements
We especially thank the Cardiovascular Research Institute of Hanzhong People’s Hospital for their contribution to data collection.
Funding
This work was supported by the National Natural Science Foundation of China No. 82070437 (J.-J.M.) and the Key Project of the Ministry of Science and Technology of China (2017YFC0211703).
Author information
Authors and Affiliations
Contributions
YS and JJM came up with the idea and refined the whole study. CC, TSG, QM and YW collected and collated the data. YS, YY, YYL, DW and HJ analyzed the data. YS and JJM wrote and checked the manuscript. The final version was approved by all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The research adhered to the guidelines of the Helsinki Declaration and was approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University. All participants signed the informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sun, Y., Yan, Y., Liao, Y. et al. The new visceral adiposity index outperforms traditional obesity indices as a predictor of subclinical renal damage in Chinese individuals: a cross-sectional study. BMC Endocr Disord 23, 78 (2023). https://doi.org/10.1186/s12902-023-01330-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-023-01330-5