Abstract
Background
Renal hypouricemia (RHUC), a rare inherited disorder characterized by impaired uric acid reabsorption and subsequent profound hypouricemia, occurs mainly due to variants in SLC22A12 or SLC2A9. Only anecdotal cases and one small-scale RHUC screening study have been reported in the Chinese population.
Methods
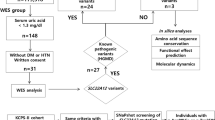
A total of 19 patients with RHUC from 17 unrelated families were recruited from our center. The medical history, clinical manifestations, biochemical exam, and clinical outcomes were collected. Next-generation sequencing-based targeted gene sequencing or whole exon sequencing was performed.
Results
A total of 22 variants in SLC22A12 or SLC2A9 were found in 19 patients. The variant c.944G>A (p.W315X) in SLC2A9 was identified in three patients. Three variants c.165C>A (p.D55E), c.1549_1555delGAGACCC (p.E517Rfs*17), and c.1483T>C (p.W495R) in SLC22A12 and three variants c.1215+1G>A (splicing variant), c.643A>C (p.T215P), and c.227C>A (p.S76X) in SLC2A9 were novel. A proportion of 10 out of 19 patients presented with exercise-induced acute kidney injury (EIAKI). The renal outcome was favorable. Five patients had nephrolithiasis, in whom three had hypercalciuria.
Conclusion
The current study reported six novel variants in SLC22A12 and SLC2A9 genes of Chinese patients with RHUC. The variant c.944G>A (p.W315X) in SLC2A9 may be common in Chinese patients. EIAKI is the main clinical phenotype associated with RHUC in our cohort, with a favorable outcome. Hypercalciuria presented in some RHUC patients is a new finding.



Similar content being viewed by others
References
Nakayama A, Matsuo H, Ohtahara A, et al. Clinical practice guideline for renal hypouricemia (1st edition). Hum Cell. 2019;32(2):83–7.
Dinour D, Gray NK, Ganon L, et al. Two novel homozygous SLC2A9 mutations cause renal hypouricemia type 2. Nephrol Dial Transplant. 2012;27(3):1035–41.
Cha DH, Gee HY, Cachau R, et al. Contribution of SLC22A12 on hypouricemia and its clinical significance for screening purposes. Sci Rep. 2019;9(1):14360.
Kawasoe S, Ide K, Usui T, et al. Distribution and characteristics of hypouricemia within the Japanese general population: a cross-sectional study. Medicina (Kaunas). 2019;55(3):61.
Ichida K, Hosoyamada M, Kamatani N, et al. Age and origin of the G774A mutation in SLC22A12 causing renal hypouricemia in Japanese. Clin Genet. 2008;74(3):243–51.
Mou LJ, Jiang LP. Hu Y A novel homozygous GLUT9 mutation cause recurrent exercise-induced acute renal failure and posterior reversible encephalopathy syndrome. J Nephrol. 2015;28(3):387–92.
Li Z, Ding H, Chen C, Chen Y, Wang DW, Lv Y. Novel URAT1 mutations caused acute renal failure after exercise in two Chinese families with renal hypouricemia. Gene. 2013;512(1):97–101.
Zhou Z, Wang K, Zhou J, et al. Amplicon targeted resequencing for SLC2A9 and SLC22A12 identified novel mutations in hypouricemia subjects. Mol Genet Genomic Med. 2019;7(7): e00722.
Yan MT, Cheng CJ, Chen JS, Lin SH. The case: a young man with acute kidney injury after exercise. The diagnosis: exercise induced acute kidney injury in hereditary renal hypouricemia. Kidney Int. 2010;77(10):935–6.
Shen H, Feng C, Jin X, et al. Recurrent exercise-induced acute kidney injury by idiopathic renal hypouricemia with a novel mutation in the SLC2A9 gene and literature review. BMC Pediatr. 2014;14:73.
Wang C, Wang J, Liu S, et al. Idiopathic renal hypouricemia: a case report and literature review. Mol Med Rep. 2019;20(6):5118–24.
Teng L, Zhang Y, Ye L, et al. Donor-derived hypouricemia in irrelevant recipients caused by kidney transplantation. Ann Transl Med. 2020;8(6):330.
Sun W, Yang J, Zhang Y, et al. Identification of two novel heterozygous SLC2A9 mutations in a Chinese woman and review of literature. Clin Chim Acta. 2021;523:58–64.
Mou L, Wu F. Simultaneous homozygous mutations in SLC12A3 and CLCNKB in an inbred Chinese pedigree. Genes (Basel). 2021;12(3):369.
Iwai N, Mino Y, Hosoyamada M, Tago N, Kokubo Y. Endou H A high prevalence of renal hypouricemia caused by inactive SLC22A12 in Japanese. Kidney Int. 2004;66(3):935–44.
Enomoto A, Kimura H, Chairoungdua A, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417(6887):447–52.
Ichida K, Hosoyamada M, Hisatome I, et al. Clinical and molecular analysis of patients with renal hypouricemia in Japan-influence of URAT1 gene on urinary urate excretion. J Am Soc Nephrol. 2004;15(1):164–73.
Stiburkova B, Sebesta I, Ichida K, et al. Novel allelic variants and evidence for a prevalent mutation in URAT1 causing renal hypouricemia: biochemical, genetics and functional analysis. Eur J Hum Genet. 2013;21(10):1067–73.
Sugihara S, Hisatome I, Kuwabara M, et al. Depletion of uric acid due to SLC22A12 (URAT1) loss-of-function mutation causes endothelial dysfunction in hypouricemia. Circ J. 2015;79(5):1125–32.
Backman JD, Li AH, Marcketta A, et al. Exome sequencing and analysis of 454,787 UK Biobank participants. Nature. 2021;599(7886):628–34.
Sperling O. Hereditary renal hypouricemia. Mol Genet Metab. 2006;89(1–2):14–8.
So A, Thorens B. Uric acid transport and disease. J Clin Invest. 2010;120(6):1791–9.
Waring WS, McKnight JA, Webb DJ, Maxwell SR. Uric acid restores endothelial function in patients with type 1 diabetes and regular smokers. Diabetes. 2006;55(11):3127–32.
Ohta T, Sakano T, Igarashi T, Itami N, Ogawa T, Group ARFAwRHR. Exercise-induced acute renal failure associated with renal hypouricaemia: results of a questionnaire-based survey in Japan. Nephrol Dial Transplant. 2004;19(6):1447–53.
Hosoyamada M, Tsurumi Y, Hirano H, et al. Urat1-Uox double knockout mice are experimental animal models of renal hypouricemia and exercise-induced acute kidney injury. Nucleosides Nucleotides Nucleic Acids. 2016;35(10–12):543–9.
Miyamoto D, Sato N, Nagata K, et al. Analysis of purine metabolism to elucidate the pathogenesis of acute kidney injury in renal hypouricemia. Biomedicines. 2022;10(7):1584.
Kaneko K, Taniguchi N, Tanabe Y, Nakano T, Hasui M, Nozu K. Oxidative imbalance in idiopathic renal hypouricemia. Pediatr Nephrol. 2009;24(4):869–71.
Uribarri J, Oh MS. Renal hypouricemia and absorptive hypercalciuria: a real syndrome. Nephron. 1993;63(2):172–5.
Chen W, Roncal-Jimenez C, Lanaspa M, et al. Uric acid suppresses 1 alpha hydroxylase in vitro and in vivo. Metabolism. 2014;63(1):150–60.
Cheong HI, Kang JH, Lee JH, et al. Mutational analysis of idiopathic renal hypouricemia in Korea. Pediatr Nephrol. 2005;20(7):886–90.
Stiburkova B, Gabrikova D, Cepek P, et al. Prevalence of URAT1 allelic variants in the Roma population. Nucleosides Nucleotides Nucleic Acids. 2016;35(10–12):529–35.
Stiburkova B, Bohata J, Pavelcova K, et al. Renal hypouricemia 1: rare disorder as common disease in eastern Slovakia Roma population. Biomedicines. 2021;9(11):1607.
Tasic V, Hynes AM, Kitamura K, et al. Clinical and functional characterization of URAT1 variants. PLoS One. 2011;6(12): e28641.
Tsuji K, Kitamura M, Muta K, et al. Transplantation of a kidney with a heterozygous mutation in the SLC22A12 (URAT1) gene causing renal hypouricemia: a case report. BMC Nephrol. 2020;21(1):282.
Matsuo H, Chiba T, Nagamori S, et al. Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am J Hum Genet. 2008;83(6):744–51.
Yohe S, Thyagarajan B. Review of clinical next-generation sequencing. Arch Pathol Lab Med. 2017;141(11):1544–57.
Vargas-Poussou R, Dahan K, Kahila D, et al. Spectrum of mutations in Gitelman syndrome. J Am Soc Nephrol. 2011;22(4):693–703.
Mou L, Tang M, Zhu L, Lin W, Gu Y. Spectrum of variants in a large Chinese Gitelman syndrome cohort. Clin Genet. 2023;104(6):674–8.
Tseng MH, Yang SS, Hsu YJ, et al. Genotype, phenotype, and follow-up in Taiwanese patients with salt-losing tubulopathy associated with SLC12A3 mutation. J Clin Endocrinol Metab. 2012;97(8):E1478–82.
Members C-N. Partners database resources of the national genomics data center, China National Center for bioinformation in 2022. Nucleic Acids Res. 2022;50(D1):D27–38.
Chen T, Chen X, Zhang S, et al. The genome sequence archive family: toward explosive data growth and diverse data types. Genomics Proteomics Bioinformatics. 2021;19(4):578–83.
Acknowledgement
Special thanks are given to Dr. Yong Du for his guidance with writing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by Zhejiang Provincial Natural Science Foundation of China [LY23H050002].
Conflicts of Interest
The authors (Lijun Mou, Lina Zhu, Xujiao Chen, Ying Hu, Hong Zhu, Ying Xu) report that there are no competing interests to declare.
Availability Of Data And Material
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (Genomics, Proteomics and Bioinformatics 2021) in the National Genomics Data Center (Nucleic Acids Res 2021), and China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: HRA004533) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa. [40, 41]. The data will be shared on reasonable request to the corresponding author.
Ethical Approval of Studies
Study approval statement: This study protocol was reviewed and approved by the Ethics Committee of Zhejiang University School of Medicine Second Affiliated Hospital. The approval number was 2021-0427. Consent to participate statement: Written informed consents were obtained from all participants to participate in the study.
Consent to Participate
Informed consents were obtained from all subjects involved in the study.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Author Contributions
Lijun Mou and Lina Zhu conceived the idea, and designed and planned the study; Xujiao Chen, Ying Hu, Hong Zhu, and Ying Xu gave important suggestions on study design and improved the study protocol; all authors participated in the collection of samples and the clinical data of patients; Ying Hu and Ying Xu analyzed the data; Lijun Mou and Lina Zhu drafted the manuscript and designed the figures; all authors discussed and interpreted the results, commented on the manuscript, provided critical feedback, and helped shape the research; and all authors read and approved the final manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mou, L., Zhu, L., Chen, X. et al. Genotype and Phenotype of Renal Hypouricemia: A Single-Center Study from China. Mol Diagn Ther 28, 87–99 (2024). https://doi.org/10.1007/s40291-023-00683-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-023-00683-w