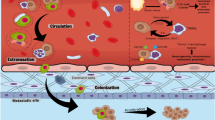
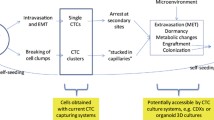
Abstract
Metastasis is the main cause of cancer death. Metastatic foci are derived from tumor cells that detach from the primary tumor and then enter the circulation. Circulating tumor cells (CTCs) are generally associated with a high probability of distant metastasis and a negative prognosis. Most CTCs die in the bloodstream, and only a few cells form metastases. Such metastatic CTCs have a stem-like and hybrid epithelial-mesenchymal phenotype, can avoid immune surveillance, and show increased therapy resistance. Targeting metastatic CTCs and their progenitors in primary tumors and their descendants, particularly disseminated tumor cells, represents an attractive strategy for metastasis prevention. However, current therapeutic strategies mainly target the primary tumor and only indirectly affect metastasis-initiating cells. Here, we consider potential methods for preventing metastasis based on targeting molecular and cellular features of metastatic CTCs, including CTC clusters. Also, we emphasize current knowledge gaps in CTC biology that should be addressed to develop highly effective therapeutics and strategies for metastasis suppression.

Similar content being viewed by others
References
Massagué J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature. 2016;529(7586):298–306. https://doi.org/10.1038/nature17038.
Jin XR, Zhu LY, Qian K, Feng YG, Zhou JH, Wang RW, et al. Circulating tumor cells in early stage lung adenocarcinoma: a case series report and literature review. Oncotarget. 2017;8(14):23130–41. https://doi.org/10.18632/oncotarget.15506.
Jiang X, Wong KHK, Khankhel AH, Zeinali M, Reategui E, Phillips MJ, et al. Microfluidic isolation of platelet-covered circulating tumor cells. Lab Chip. 2017;17(20):3498–503. https://doi.org/10.1039/c7lc00654c.
Sarioglu AF, Aceto N, Kojic N, Donaldson MC, Zeinali M, Hamza B, et al. A microfluidic device for label-free, physical capture of circulating tumor cell clusters. Nat Methods. 2015;12(7):685–91. https://doi.org/10.1038/nmeth.3404.
Nolan J, Nedosekin DA, Galanzha EI, Zharov VP. Detection of apoptotic circulating tumor cells using in vivo fluorescence flow cytometry. Cytometry A. 2019;95(6):664–71. https://doi.org/10.1002/cyto.a.23642.
Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF, et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol. 1998;153(3):865–73. https://doi.org/10.1016/s0002-9440(10)65628-3.
Menyailo ME, Tretyakova MS, Denisov EV. Heterogeneity of circulating tumor cells in breast cancer: identifying metastatic seeds. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21051696.
Rossi T, Gallerani G, Angeli D, Cocchi C, Bandini E, Fici P, et al. Single-cell NGS-based analysis of copy number alterations reveals new insights in circulating tumor cells persistence in early-stage breast cancer. Cancers (Basel). 2020. https://doi.org/10.3390/cancers12092490.
Agnoletto C, Corrà F, Minotti L, Baldassari F, Crudele F, Cook WJJ, et al. Heterogeneity in circulating tumor cells: the relevance of the stem-cell subset. Cancers (Basel). 2019. https://doi.org/10.3390/cancers11040483.
Lowes LE, Allan AL. Circulating tumor cells and implications of the epithelial-to-mesenchymal transition. Adv Clin Chem. 2018;83:121–81. https://doi.org/10.1016/bs.acc.2017.10.004.
Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566(7745):553–7. https://doi.org/10.1038/s41586-019-0915-y.
Ward Y, Lake R, Faraji F, Sperger J, Martin P, Gilliard C, et al. Platelets promote metastasis via binding tumor CD97 leading to bidirectional signaling that coordinates transendothelial migration. Cell Rep. 2018;23(3):808–22. https://doi.org/10.1016/j.celrep.2018.03.092.
Hapach LA, Mosier JA, Wang W, Reinhart-King CA. Engineered models to parse apart the metastatic cascade. Npj Precis Oncol. 2019;3(1):20. https://doi.org/10.1038/s41698-019-0092-3.
Hansen E, Read AF. Cancer therapy: attempt cure or manage drug resistance? Evol Appl. 2020;13(7):1660–72.
Tai W, Mahato R, Cheng K. The role of HER2 in cancer therapy and targeted drug delivery. J Control Release. 2010;146(3):264–75. https://doi.org/10.1016/j.jconrel.2010.04.009.
Bethune G, Bethune D, Ridgway N, Xu Z. Epidermal growth factor receptor (EGFR) in lung cancer: an overview and update. J Thorac Dis. 2010;2(1):48–51.
Aggarwal S. Targeted cancer therapies. Nat Rev Drug Discov. 2010;9(6):427–8. https://doi.org/10.1038/nrd3186.
Cameron D, Casey M, Oliva C, Newstat B, Imwalle B, Geyer CE. Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: final survival analysis of a phase III randomized trial. Oncologist. 2010;15(9):924–34. https://doi.org/10.1634/theoncologist.2009-0181.
Park YH, Park MJ, Ji SH, Yi SY, Lim DH, Nam DH, et al. Trastuzumab treatment improves brain metastasis outcomes through control and durable prolongation of systemic extracranial disease in HER2-overexpressing breast cancer patients. Br J Cancer. 2009;100(6):894–900. https://doi.org/10.1038/sj.bjc.6604941.
Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382(25):2419–30. https://doi.org/10.1056/NEJMoa2004413.
Rades D, Bartscht T, Hunold P, Schmidberger H, König L, Debus J, et al. Radiochemotherapy with or without cetuximab for unresectable esophageal cancer: final results of a randomized phase 2 trial (LEOPARD-2). Strahlenther Onkol. 2020;196(9):795–804. https://doi.org/10.1007/s00066-020-01646-4.
Day KC, Lorenzatti Hiles G, Kozminsky M, Dawsey SJ, Paul A, Broses LJ, et al. HER2 and EGFR overexpression support metastatic progression of prostate cancer to bone. Cancer Res. 2017;77(1):74–85. https://doi.org/10.1158/0008-5472.can-16-1656.
Wülfing P, Borchard J, Buerger H, Heidl S, Zänker KS, Kiesel L, et al. HER2-positive circulating tumor cells indicate poor clinical outcome in stage I to III breast cancer patients. Clin Cancer Res. 2006;12(6):1715–20. https://doi.org/10.1158/1078-0432.ccr-05-2087.
Apostolaki S, Perraki M, Pallis A, Bozionelou V, Agelaki S, Kanellou P, et al. Circulating HER2 mRNA-positive cells in the peripheral blood of patients with stage I and II breast cancer after the administration of adjuvant chemotherapy: evaluation of their clinical relevance. Ann Oncol. 2007;18(5):851–8. https://doi.org/10.1093/annonc/mdl502.
Deutsch TM, Riethdorf S, Fremd C, Feisst M, Nees J, Fischer C, et al. HER2-targeted therapy influences CTC status in metastatic breast cancer. Breast Cancer Res Treat. 2020;182(1):127–36. https://doi.org/10.1007/s10549-020-05687-2.
Liu Y, Liu Q, Wang T, Bian L, Zhang S, Hu H, et al. Circulating tumor cells in HER2-positive metastatic breast cancer patients: a valuable prognostic and predictive biomarker. BMC Cancer. 2013;13:202. https://doi.org/10.1186/1471-2407-13-202.
Kuboki Y, Matsusaka S, Minowa S, Shibata H, Suenaga M, Shinozaki E, et al. Circulating tumor cell (CTC) count and epithelial growth factor receptor expression on CTCs as biomarkers for cetuximab efficacy in advanced colorectal cancer. Anticancer Res. 2013;33(9):3905–10.
Saczko J, Michel O, Chwiłkowska A, Sawicka E, Mączyńska J, Kulbacka J. Estrogen receptors in cell membranes: regulation and signaling. Adv Anat Embryol Cell Biol. 2017;227:93–105. https://doi.org/10.1007/978-3-319-56895-9_6.
Lombardi APG, Vicente CM, Porto CS. Estrogen receptors promote migration, invasion and colony formation of the androgen-independent prostate cancer cells PC-3 through β-catenin pathway. Front Endocrinol (Lausanne). 2020;11:184. https://doi.org/10.3389/fendo.2020.00184.
Yan S, Dey P, Ziegler Y, Jiao X, Kim SH, Katzenellenbogen JA, et al. Contrasting activities of estrogen receptor beta isoforms in triple negative breast cancer. Breast Cancer Res Treat. 2021;185(2):281–92. https://doi.org/10.1007/s10549-020-05948-0.
Ahmad I. Tamoxifen a pioneering drug: an update on the therapeutic potential of tamoxifen derivatives. Eur J Med Chem. 2018;143:515–31. https://doi.org/10.1016/j.ejmech.2017.11.056.
Ekholm M, Bendahl PO, Fernö M, Nordenskjöld B, Stål O, Rydén L, et al. Effects of adjuvant tamoxifen over three decades on breast cancer-free and distant recurrence-free interval among premenopausal women with oestrogen receptor-positive breast cancer randomised in the Swedish SBII:2pre trial. Eur J Cancer. 2019;110:53–61. https://doi.org/10.1016/j.ejca.2018.12.034.
Aktas B, Müller V, Tewes M, Zeitz J, Kasimir-Bauer S, Loehberg CR, et al. Comparison of estrogen and progesterone receptor status of circulating tumor cells and the primary tumor in metastatic breast cancer patients. Gynecol Oncol. 2011;122(2):356–60. https://doi.org/10.1016/j.ygyno.2011.04.039.
Babayan A, Hannemann J, Spötter J, Müller V, Pantel K, Joosse SA. Heterogeneity of estrogen receptor expression in circulating tumor cells from metastatic breast cancer patients. PLoS ONE. 2013;8(9): e75038. https://doi.org/10.1371/journal.pone.0075038.
Forsare C, Bendahl PO, Moberg E, Jørgensen CLT, Jansson S, Larsson AM, et al. Evolution of estrogen receptor status from primary tumors to metastasis and serially collected circulating tumor cells. Int J Mol Sci. 2020;21(8):2885. https://doi.org/10.3390/ijms21082885.
Zhang L, Ridgway LD, Wetzel MD, Ngo J, Yin W, Kumar D, et al. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci Transl Med. 2013;5(180):180ra48. https://doi.org/10.1126/scitranslmed.3005109.
Kwan TT, Bardia A, Spring LM, Giobbie-Hurder A, Kalinich M, Dubash T, et al. A digital RNA signature of circulating tumor cells predicting early therapeutic response in localized and metastatic breast cancer. Cancer Discov. 2018;8(10):1286–99. https://doi.org/10.1158/2159-8290.cd-18-0432.
Powell AA, Talasaz AH, Zhang H, Coram MA, Reddy A, Deng G, et al. Single cell profiling of circulating tumor cells: transcriptional heterogeneity and diversity from breast cancer cell lines. PLoS ONE. 2012;7(5): e33788. https://doi.org/10.1371/journal.pone.0033788.
Yu J-j, Xiao W, Dong S-l, Liang H-f, Zhang Z-w, Zhang B-x, et al. Effect of surgical liver resection on circulating tumor cells in patients with hepatocellular carcinoma. BMC Cancer. 2018;18(1):835. https://doi.org/10.1186/s12885-018-4744-4.
Dimitrov-Markov S, Perales-Patón J, Bockorny B, Dopazo A, Muñoz M, Baños N, et al. Discovery of new targets to control metastasis in pancreatic cancer by single-cell transcriptomics analysis of circulating tumor cells. Mol Cancer Ther. 2020;19(8):1751–60. https://doi.org/10.1158/1535-7163.mct-19-1166.
Jayatilleke KM, Hulett MD. Heparanase and the hallmarks of cancer. J Transl Med. 2020;18(1):453. https://doi.org/10.1186/s12967-020-02624-1.
Ferro V, Dredge K, Liu L, Hammond E, Bytheway I, Li C, et al. PI-88 and novel heparan sulfate mimetics inhibit angiogenesis. Semin Thromb Hemost. 2007;33(5):557–68. https://doi.org/10.1055/s-2007-982088.
Dredge K, Hammond E, Handley P, Gonda TJ, Smith MT, Vincent C, et al. PG545, a dual heparanase and angiogenesis inhibitor, induces potent anti-tumour and anti-metastatic efficacy in preclinical models. Br J Cancer. 2011;104(4):635–42. https://doi.org/10.1038/bjc.2011.11.
Ritchie JP, Ramani VC, Ren Y, Naggi A, Torri G, Casu B, et al. SST0001, a chemically modified heparin, inhibits myeloma growth and angiogenesis via disruption of the heparanase/syndecan-1 axis. Clin Cancer Res. 2011;17(6):1382–93. https://doi.org/10.1158/1078-0432.ccr-10-2476.
Zhou H, Roy S, Cochran E, Zouaoui R, Chu CL, Duffner J, et al. M402, a novel heparan sulfate mimetic, targets multiple pathways implicated in tumor progression and metastasis. PLoS ONE. 2011;6(6): e21106. https://doi.org/10.1371/journal.pone.0021106.
Liao BY, Wang Z, Hu J, Liu WF, Shen ZZ, Zhang X, et al. PI-88 inhibits postoperative recurrence of hepatocellular carcinoma via disrupting the surge of heparanase after liver resection. Tumour Biol. 2016;37(3):2987–98. https://doi.org/10.1007/s13277-015-4085-8.
Li XM, Liu WL, Chen X, Wang YW, Shi DB, Zhang H, et al. Overexpression of TMPRSS4 promotes tumor proliferation and aggressiveness in breast cancer. Int J Mol Med. 2017;39(4):927–35. https://doi.org/10.3892/ijmm.2017.2893.
Kim S, Ko D, Lee Y, Jang S, Lee Y, Lee IY, et al. Anti-cancer activity of the novel 2-hydroxydiarylamide derivatives IMD-0354 and KRT1853 through suppression of cancer cell invasion, proliferation, and survival mediated by TMPRSS4. Sci Rep. 2019;9(1):10003. https://doi.org/10.1038/s41598-019-46447-7.
Kerkhoff C, Ghavami S (2011) S100A9 (S100 calcium binding protein A9). Atlas Genet Cytogenet Oncol Haematol. 15(9):746–57. https://doi.org/10.4267/2042/46028
Sherbet GV (2010) S100A4 (S100 Calcium Binding Protein A4). Atlas Genet Cytogenet Oncol Haematol. 14(10):877–86. https://doi.org/10.4267/2042/46036
Fujiwara M, Kashima TG, Kunita A, Kii I, Komura D, Grigoriadis AE, et al. Stable knockdown of S100A4 suppresses cell migration and metastasis of osteosarcoma. Tumour Biol. 2011;32(3):611–22. https://doi.org/10.1007/s13277-011-0160-y.
Kinoshita R, Sato H, Yamauchi A, Takahashi Y, Inoue Y, Sumardika IW, et al. Newly developed anti-S100A8/A9 monoclonal antibody efficiently prevents lung tropic cancer metastasis. Int J Cancer. 2019;145(2):569–75. https://doi.org/10.1002/ijc.31982.
Gires O, Pan M, Schinke H, Canis M, Baeuerle PA. Expression and function of epithelial cell adhesion molecule EpCAM: where are we after 40 years? Cancer Metastasis Rev. 2020;39(3):969–87. https://doi.org/10.1007/s10555-020-09898-3.
Schmidt M, Scheulen ME, Dittrich C, Obrist P, Marschner N, Dirix L, et al. An open-label, randomized phase II study of adecatumumab, a fully human anti-EpCAM antibody, as monotherapy in patients with metastatic breast cancer. Ann Oncol. 2010;21(2):275–82. https://doi.org/10.1093/annonc/mdp314.
Jolly MK, Somarelli JA, Sheth M, Biddle A, Tripathi SC, Armstrong AJ, et al. Hybrid epithelial/mesenchymal phenotypes promote metastasis and therapy resistance across carcinomas. Pharmacol Ther. 2019;194:161–84. https://doi.org/10.1016/j.pharmthera.2018.09.007.
Bocci F, Mandal S, Tejaswi T, Jolly MK. Investigating epithelial-mesenchymal heterogeneity of tumors and circulating tumor cells with transcriptomic analysis and biophysical modeling. Comput Syst Oncol. 2021;1(2): e1015. https://doi.org/10.1002/cso2.1015.
Tashireva LA, Savelieva OE, Grigoryeva ES, Nikitin YV, Denisov EV, Vtorushin SV, et al. Heterogeneous manifestations of epithelial-mesenchymal plasticity of circulating tumor cells in breast cancer patients. Int J Mol Sci. 2021;22(5):2504.
Forte E, Chimenti I, Rosa P, Angelini F, Pagano F, Calogero A, et al. EMT/MET at the crossroad of stemness, regeneration and oncogenesis: the ying-yang equilibrium recapitulated in cell spheroids. Cancers. 2017;9(8):98. https://doi.org/10.3390/cancers9080098.
Simeonov KP, Byrns CN, Clark ML, Norgard RJ, Martin B, Stanger BZ, et al. Single-cell lineage and transcriptome reconstruction of metastatic cancer reveals selection of aggressive hybrid EMT states. Cancer Cell. 2021. https://doi.org/10.1016/j.ccell.2021.05.005.
Savelieva OE, Tashireva LA, Kaigorodova EV, Buzenkova AV, Mukhamedzhanov RK, Grigoryeva ES, et al. Heterogeneity of stemlike circulating tumor cells in invasive breast cancer. Int J Mol Sci. 2020;21(8):2780. https://doi.org/10.3390/ijms21082780.
Sun YF, Guo W, Xu Y, Shi YH, Gong ZJ, Ji Y, et al. Circulating tumor cells from different vascular sites exhibit spatial heterogeneity in epithelial and mesenchymal composition and distinct clinical significance in hepatocellular carcinoma. Clin Cancer Res. 2018;24(3):547–59. https://doi.org/10.1158/1078-0432.CCR-17-1063.
Brechbuhl HM, Vinod-Paul K, Gillen AE, Kopin EG, Gibney K, Elias AD, et al. Analysis of circulating breast cancer cell heterogeneity and interactions with peripheral blood mononuclear cells. Mol Carcinog. 2020;59(10):1129–39.
Polioudaki H, Agelaki S, Chiotaki R, Politaki E, Mavroudis D, Matikas A, et al. Variable expression levels of keratin and vimentin reveal differential EMT status of circulating tumor cells and correlation with clinical characteristics and outcome of patients with metastatic breast cancer. BMC Cancer. 2015;15(1):1–10. https://doi.org/10.1186/s12885-015-1386-7.
Ou H, Huang Y, Xiang L, Chen Z, Fang Y, Lin Y, et al. Circulating tumor cell phenotype indicates poor survival and recurrence after surgery for hepatocellular carcinoma. Dig Dis Sci. 2018;63(9):2373–80. https://doi.org/10.1007/s10620-018-5124-2.
Semaan A, Bernard V, Kim DU, Lee JJ, Huang J, Kamyabi N, et al. Characterisation of circulating tumour cell phenotypes identifies a partial-EMT sub-population for clinical stratification of pancreatic cancer. Br J Cancer. 2021;124(12):1970–7. https://doi.org/10.1038/s41416-021-01350-9.
Nadal R, Ortega FG, Salido M, Lorente JA, Rodríguez-Rivera M, Delgado-Rodríguez M, et al. CD133 expression in circulating tumor cells from breast cancer patients: potential role in resistance to chemotherapy. Int J Cancer. 2013;133(10):2398–407. https://doi.org/10.1002/ijc.28263.
Papadaki MA, Stoupis G, Theodoropoulos PA, Mavroudis D, Georgoulias V, Agelaki S. Circulating tumor cells with stemness and epithelial-to-mesenchymal transition features are chemoresistant and predictive of poor outcome in metastatic breast cancer. Mol Cancer Ther. 2019;18(2):437–47. https://doi.org/10.1158/1535-7163.MCT-18-0584.
Francart ME, Lambert J, Vanwynsberghe AM, Thompson EW, Bourcy M, Polette M, et al. Epithelial–mesenchymal plasticity and circulating tumor cells: travel companions to metastases. Dev Dyn. 2018;247(3):432–50. https://doi.org/10.1002/dvdy.24506.
Mego M, Mani SA, Lee BN, Li C, Evans KW, Cohen EN, et al. Expression of epithelial–mesenchymal transition-inducing transcription factors in primary breast cancer: the effect of neoadjuvant therapy. Int J Cancer. 2012;130(4):808–16. https://doi.org/10.1002/ijc.26037.
Jiménez N, Reig Ò, Montalbo R, Milà-Guasch M, Nadal-Dieste L, Castellano G, et al. Cell plasticity-related phenotypes and taxanes resistance in castration-resistant prostate cancer. Front Oncol. 2020;10: 594023. https://doi.org/10.3389/fonc.2020.594023.
Shi DD, Yang CG, Han S, Wang SY, Xiong B. Dynamic evaluation of mesenchymal circulating tumor cells in patients with colorectal cancer: clinical associations and prognostic value. Oncol Rep. 2020;44(2):757–67. https://doi.org/10.3892/or.2020.7629.72.
Ujiie D, Matsumoto T, Endo E, Okayama H, Fujita S, Kanke Y, et al. Circulating tumor cells after neoadjuvant chemotherapy are related with recurrence in esophageal squamous cell carcinoma. Esophagus. 2021;18(3):566–73. https://doi.org/10.1007/s10388-021-00829-x.73.
Mego M, Karaba M, Minarik G, Benca J, Silvia J, Sedlackova T, et al. Circulating tumor cells with epithelial-to-mesenchymal transition phenotypes associated with inferior outcomes in primary breast cancer. Anticancer Res. 2019;39(4):1829–37. https://doi.org/10.21873/anticanres.13290.
Boral D, Vishnoi M, Liu HN, Yin W, Sprouse ML, Scamardo A, et al. Molecular characterization of breast cancer CTCs associated with brain metastasis. Nat Commun. 2017;8(1):196. https://doi.org/10.1038/s41467-017-00196-1.
Nel I, Gauler TC, Bublitz K, Lazaridis L, Goergens A, Giebel B, et al. Circulating tumor cell composition in renal cell carcinoma. PLoS ONE. 2016;11(4): e0153018. https://doi.org/10.1371/journal.pone.0153018.
Fontebasso Y, Dubinett SM. Drug development for metastasis prevention. Crit Rev Oncog. 2015;20(5–6):449–73. https://doi.org/10.1615/CritRevOncog.v20.i5-6.150.
Denisov EV, Jolly MK, Shubin VP, Tsukanov AS, Cherdyntseva NV. Critical steps in epithelial-mesenchymal transition as target for cancer treatment. In: Approaching complex diseases. Springer; 2020. p. 213–44.
Padthaisong S, Thanee M, Techasen A, Namwat N, Yongvanit P, Liwatthakun A, et al. Nimotuzumab inhibits cholangiocarcinoma cell metastasis via suppression of the epithelial-mesenchymal transition process. Anticancer Res. 2017;37(7):3591–7. https://doi.org/10.21873/anticanres.11729.
Tian L, Shen D, Li X, Shan X, Wang X, Yan Q, et al. Ginsenoside Rg3 inhibits epithelial-mesenchymal transition (EMT) and invasion of lung cancer by down-regulating FUT4. Oncotarget. 2016;7(2):1619–32. https://doi.org/10.18632/oncotarget.6451.
Chang JH, Lai SL, Chen WS, Hung WY, Chow JM, Hsiao M, et al. Quercetin suppresses the metastatic ability of lung cancer through inhibiting Snail-dependent Akt activation and Snail-independent ADAM9 expression pathways. Biochim Biophys Acta Mol Cell Res. 2017;1864(10):1746–58. https://doi.org/10.1016/j.bbamcr.2017.06.017.
Zhou C, Li J, Lin L, Shu R, Dong B, Cao D, et al. A targeted transforming growth factor-beta (TGF-β) blocker, TTB, inhibits tumor growth and metastasis. Oncotarget. 2018;9(33):23102–13. https://doi.org/10.18632/oncotarget.24562.
Thaiparambil JT, Bender L, Ganesh T, Kline E, Patel P, Liu Y, et al. Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int J Cancer. 2011;129(11):2744–55. https://doi.org/10.1002/ijc.25938.
Rhodes LV, Tate CR, Segar HC, Burks HE, Phamduy TB, Hoang V, et al. Suppression of triple-negative breast cancer metastasis by pan-DAC inhibitor panobinostat via inhibition of ZEB family of EMT master regulators. Breast Cancer Res Treat. 2014;145(3):593–604. https://doi.org/10.1007/s10549-014-2979-6.
Ferrari-Amorotti G, Chiodoni C, Shen F, Cattelani S, Soliera AR, Manzotti G, et al. Suppression of invasion and metastasis of triple-negative breast cancer lines by pharmacological or genetic inhibition of slug activity. Neoplasia. 2014;16(12):1047–58. https://doi.org/10.1016/j.neo.2014.10.006.
Roccaro AM, Mishima Y, Sacco A, Moschetta M, Tai YT, Shi J, et al. CXCR4 regulates extra-medullary myeloma through epithelial-mesenchymal-transition-like transcriptional activation. Cell Rep. 2015;12(4):622–35. https://doi.org/10.1016/j.celrep.2015.06.059.
Wu CX, Xu A, Zhang CC, Olson P, Chen L, Lee TK, et al. Notch Inhibitor PF-03084014 inhibits hepatocellular carcinoma growth and metastasis via suppression of cancer stemness due to reduced activation of Notch1-Stat3. Mol Cancer Ther. 2017;16(8):1531–43. https://doi.org/10.1158/1535-7163.mct-17-0001.
Ferrarotto R, Mitani Y, Diao L, Guijarro I, Wang J, Zweidler-McKay P, et al. Activating NOTCH1 mutations define a distinct subgroup of patients with adenoid cystic carcinoma who have poor prognosis, propensity to bone and liver metastasis, and potential responsiveness to notch1 inhibitors. J Clin Oncol. 2017;35(3):352–60. https://doi.org/10.1200/jco.2016.67.5264.
Qu H, Ma B, Yuan H-F, Wang Z-Y, Guo S-J, Zhang J. Effect of salinomycin on metastasis and invasion of bladder cancer cell line T24. Asian Pac J Trop Med. 2015;8(7):578–82. https://doi.org/10.1016/j.apjtm.2015.06.004.
Dinicola S, Fabrizi G, Masiello MG, Proietti S, Palombo A, Minini M, et al. Inositol induces mesenchymal-epithelial reversion in breast cancer cells through cytoskeleton rearrangement. Exp Cell Res. 2016;345(1):37–50. https://doi.org/10.1016/j.yexcr.2016.05.007.
Zhou P, Wang C, Hu Z, Chen W, Qi W, Li A. Genistein induces apoptosis of colon cancer cells by reversal of epithelial-to-mesenchymal via a Notch1/NF-κB/slug/E-cadherin pathway. BMC Cancer. 2017;17(1):813. https://doi.org/10.1186/s12885-017-3829-9.
Sadhu SS, Wang S, Dachineni R, Averineni RK, Seefeldt T, Xie J, et al. In vitro and in vivo antimetastatic effect of glutathione disulfide liposomes. Cancer Growth Metastasis. 2017;10:1179064417695255. https://doi.org/10.1177/1179064417695255.
Ishay-Ronen D, Diepenbruck M, Kalathur RKR, Sugiyama N, Tiede S, Ivanek R, et al. Gain fat-lose metastasis: converting invasive breast cancer cells into adipocytes inhibits cancer metastasis. Cancer Cell. 2019;35(1):17-32.e6. https://doi.org/10.1016/j.ccell.2018.12.002.
Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5(1):8. https://doi.org/10.1038/s41392-020-0110-5.
Kakar SS, Ratajczak MZ, Powell KS, Moghadamfalahi M, Miller DM, Batra SK, et al. Withaferin a alone and in combination with cisplatin suppresses growth and metastasis of ovarian cancer by targeting putative cancer stem cells. PLoS ONE. 2014;9(9): e107596. https://doi.org/10.1371/journal.pone.0107596.
Marangoni E, Lecomte N, Durand L, de Pinieux G, Decaudin D, Chomienne C, et al. CD44 targeting reduces tumour growth and prevents post-chemotherapy relapse of human breast cancers xenografts. Br J Cancer. 2009;100(6):918–22. https://doi.org/10.1038/sj.bjc.6604953.
Li M, Zhang B, Zhang Z, Liu X, Qi X, Zhao J, et al. Stem cell-like circulating tumor cells indicate poor prognosis in gastric cancer. Biomed Res Int. 2014;2014: 981261. https://doi.org/10.1155/2014/981261.
Baccelli I, Schneeweiss A, Riethdorf S, Stenzinger A, Schillert A, Vogel V, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013;31(6):539–44. https://doi.org/10.1038/nbt.2576.
Fang C, Fan C, Wang C, Huang Q, Meng W, Yu Y, et al. CD133+CD54+CD44+ circulating tumor cells as a biomarker of treatment selection and liver metastasis in patients with colorectal cancer. Oncotarget. 2016;7(47):77389–403. https://doi.org/10.18632/oncotarget.12675.
Armstrong AJ, Marengo MS, Oltean S, Kemeny G, Bitting RL, Turnbull JD, et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res. 2011;9(8):997–1007. https://doi.org/10.1158/1541-7786.mcr-10-0490.
Kasimir-Bauer S, Hoffmann O, Wallwiener D, Kimmig R, Fehm T. Expression of stem cell and epithelial-mesenchymal transition markers in primary breast cancer patients with circulating tumor cells. Breast Cancer Res. 2012;14(1):R15. https://doi.org/10.1186/bcr3099.
Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339(6119):580–4. https://doi.org/10.1126/science.1228522.
Stott SL, Hsu CH, Tsukrov DI, Yu M, Miyamoto DT, Waltman BA, et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci USA. 2010;107(43):18392–7. https://doi.org/10.1073/pnas.1012539107.
Cho EH, Wendel M, Luttgen M, Yoshioka C, Marrinucci D, Lazar D, et al. Characterization of circulating tumor cell aggregates identified in patients with epithelial tumors. Phys Biol. 2012;9(1): 016001. https://doi.org/10.1088/1478-3975/9/1/016001.
Wendel M, Bazhenova L, Boshuizen R, Kolatkar A, Honnatti M, Cho EH, et al. Fluid biopsy for circulating tumor cell identification in patients with early-and late-stage non-small cell lung cancer: a glimpse into lung cancer biology. Phys Biol. 2012;9(1): 016005. https://doi.org/10.1088/1478-3967/9/1/016005.
Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158(5):1110–22. https://doi.org/10.1016/j.cell.2014.07.013.106.
Cheung KJ, Padmanaban V, Silvestri V, Schipper K, Cohen JD, Fairchild AN, et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc Natl Acad Sci USA. 2016;113(7):E854–63. https://doi.org/10.1073/pnas.1508541113.
Reduzzi C, Di Cosimo S, Gerratana L, Motta R, Martinetti A, Vingiani A, et al. Circulating tumor cell clusters are frequently detected in women with early-stage breast cancer. Cancers. 2021;13(10):2356. https://doi.org/10.3390/cancers13102356.
Mao S, Zhang Q, Li H, Zhang W, Huang Q, Khan M, et al. Adhesion analysis of single circulating tumor cells on a base layer of endothelial cells using open microfluidics. Chem Sci. 2018;9(39):7694–9. https://doi.org/10.1039/c8sc03027h.
Gkountela S, Castro-Giner F, Szczerba BM, Vetter M, Landin J, Scherrer R, et al. Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell. 2019;176(1):98-112.e14. https://doi.org/10.1016/j.cell.2018.11.046.
Liu X, Adorno-Cruz V, Chang YF, Jia Y, Kawaguchi M, Dashzeveg NK, et al. EGFR inhibition blocks cancer stem cell clustering and lung metastasis of triple negative breast cancer. Theranostics. 2021;11(13):6632–43. https://doi.org/10.7150/thno.57706.
Sionov RV, Fridlender ZG, Granot Z. The multifaceted roles neutrophils play in the tumor microenvironment. Cancer Microenviron. 2015;8(3):125–58. https://doi.org/10.1007/s12307-014-0147-5.
Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183–94. https://doi.org/10.1016/j.ccr.2009.06.017.
Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau C-S, et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522(7556):345–8. https://doi.org/10.1038/nature14282.
Spiegel A, Brooks MW, Houshyar S, Reinhardt F, Ardolino M, Fessler E, et al. Neutrophils suppress intraluminal nk cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. 2016;6(6):630–49. https://doi.org/10.1158/2159-8290.cd-15-1157.
Spicer JD, McDonald B, Cools-Lartigue JJ, Chow SC, Giannias B, Kubes P, et al. Neutrophils promote liver metastasis via Mac-1–mediated interactions with circulating tumor cells. Cancer Res. 2012;72(16):3919–27. https://doi.org/10.1158/0008-5472.can-11-2393.
Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Loughran P, Mowen K, et al. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res. 2016;76(6):1367–80. https://doi.org/10.1158/0008-5472.can-15-1591.
Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123(8):3446–58. https://doi.org/10.1172/jci67484.
Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med. 2016;8(361):361ra138. https://doi.org/10.1126/scitranslmed.aag1711.
Salado C, Olaso E, Gallot N, Valcarcel M, Egilegor E, Mendoza L, et al. Resveratrol prevents inflammation-dependent hepatic melanoma metastasis by inhibiting the secretion and effects of interleukin-18. J Transl Med. 2011;9:59. https://doi.org/10.1186/1479-5876-9-59.
Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–90. https://doi.org/10.1016/j.ccr.2011.09.009.
Olsson AK, Cedervall J. The pro-inflammatory role of platelets in cancer. Platelets. 2018;29(6):569–73. https://doi.org/10.1080/09537104.2018.1453059.
Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol. 2018;11(1):125. https://doi.org/10.1186/s13045-018-0669-2.
Razak NBA, Jones G, Bhandari M, Berndt MC, Metharom P. Cancer-associated thrombosis: an overview of mechanisms, risk factors, and treatment. Cancers (Basel). 2018. https://doi.org/10.3390/cancers10100380.
Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci USA. 2001;98(6):3352–7. https://doi.org/10.1073/pnas.061615598.
Schwarz S, Gockel LM, Naggi A, Barash U, Gobec M, Bendas G, et al. Glycosaminoglycans as tools to decipher the platelet tumor cell interaction: a focus on P-selectin. Molecules. 2020. https://doi.org/10.3390/molecules25051039.
Thiery JP, Lim CT. Tumor dissemination: an EMT affair. Cancer Cell. 2013;23(3):272–3. https://doi.org/10.1016/j.ccr.2013.03.004.
Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376(9754):1741–50. https://doi.org/10.1016/s0140-6736(10)61543-7.
Nakchbandi W, Müller H, Singer MV, Löhr M, Nakchbandi IA. Effects of low-dose warfarin and regional chemotherapy on survival in patients with pancreatic carcinoma. Scand J Gastroenterol. 2006;41(9):1095–104. https://doi.org/10.1080/00365520600575720.129.
Bruno A, Dovizio M, Tacconelli S, Contursi A, Ballerini P, Patrignani P. Antithrombotic agents and cancer. Cancers (Basel). 2018. https://doi.org/10.3390/cancers10080253.
Li J, King MR. Adhesion receptors as therapeutic targets for circulating tumor cells. Front Oncol. 2012;2:79. https://doi.org/10.3389/fonc.2012.00079.
Kim YJ, Borsig L, Varki NM, Varki A. P-selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci USA. 1998;95(16):9325–30. https://doi.org/10.1073/pnas.95.16.9325.
Köhler S, Ullrich S, Richter U, Schumacher U. E-/P-selectins and colon carcinoma metastasis: first in vivo evidence for their crucial role in a clinically relevant model of spontaneous metastasis formation in the lung. Br J Cancer. 2010;102(3):602–9. https://doi.org/10.1038/sj.bjc.6605492.
Husted S, Van Giezen J. Ticagrelor: the first reversibly binding oral P2Y12 receptor antagonist. Cardiovasc Ther. 2009;27(4):259–74.
Wright JR, Chauhan M, Shah C, Ring A, Thomas AL, Goodall AH, et al. The TICONC (Ticagrelor-Oncology) Study: implications of P2Y12 inhibition for metastasis and cancer-associated thrombosis. JACC CardioOncol. 2020;2(2):236–50. https://doi.org/10.1016/j.jaccao.2020.04.009.
Yu L, Zhao ZJ. Engineered platelets with antithrombotic and antimetastatic properties. Stem Cell Investig. 2019;6:27. https://doi.org/10.2137/sci.2019.08.02.
Papa AL, Jiang A, Korin N, Chen MB, Langan ET, Waterhouse A, et al. Platelet decoys inhibit thrombosis and prevent metastatic tumor formation in preclinical models. Sci Transl Med. 2019. https://doi.org/10.1126/scitranslmed.aau5898.
Xu Y, Liu J, Liu Z, Ren H, Yong J, Li W, et al. Blockade of platelets using tumor-specific NO-releasing nanoparticles prevents tumor metastasis and reverses tumor immunosuppression. ACS Nano. 2020;14(8):9780–95. https://doi.org/10.1021/acsnano.0c01687.
Nieswandt B, Hafner M, Echtenacher B, Männel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59(6):1295–300.
Placke T, Örgel M, Schaller M, Jung G, Rammensee HG, Kopp HG, et al. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res. 2012;72(2):440–8. https://doi.org/10.1158/0008-5472.can-11-1872.
Cluxton CD, Spillane C, O’Toole SA, Sheils O, Gardiner CM, O’Leary JJ. Suppression of natural killer cell NKG2D and CD226 anti-tumour cascades by platelet cloaked cancer cells: implications for the metastatic cascade. PLoS ONE. 2019;14(3): e0211538. https://doi.org/10.1371/journal.pone.0211538.
Zhao YK, Jia CM, Yuan GJ, Liu W, Qiu Y, Zhu QG. Expression and clinical value of the soluble major histocompatibility complex class I-related chain A molecule in the serum of patients with renal tumors. Genet Mol Res. 2015;14(2):7233–40. https://doi.org/10.4238/2015.June.29.16.
Holdenrieder S, Stieber P, Peterfi A, Nagel D, Steinle A, Salih HR. Soluble MICB in malignant diseases: analysis of diagnostic significance and correlation with soluble MICA. Cancer Immunol Immunother. 2006;55(12):1584–9. https://doi.org/10.1007/s00262-006-0167-1.
de Andrade LF, Tay RE, Pan D, Luoma AM, Ito Y, Badrinath S, et al. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell-driven tumor immunity. Science. 2018;359(6383):1537–42. https://doi.org/10.1126/science.aao0505.
Qin Z, Chen J, Zeng J, Niu L, Xie S, Wang X, et al. Effect of NK cell immunotherapy on immune function in patients with hepatic carcinoma: a preliminary clinical study. Cancer Biol Ther. 2017;18(5):323–30. https://doi.org/10.1080/15384047.2017.1310346.
Matzner P, Sorski L, Shaashua L, Elbaz E, Lavon H, Melamed R, et al. Perioperative treatment with the new synthetic TLR-4 agonist GLA-SE reduces cancer metastasis without adverse effects. Int J Cancer. 2016;138(7):1754–64. https://doi.org/10.1002/ijc.29885.
Tai LH, de Souza CT, Bélanger S, Ly L, Alkayyal AA, Zhang J, et al. Preventing postoperative metastatic disease by inhibiting surgery-induced dysfunction in natural killer cells. Cancer Res. 2013;73(1):97–107. https://doi.org/10.1158/0008-5472.can-12-1993.
Zhang J, Tai LH, Ilkow CS, Alkayyal AA, Ananth AA, de Souza CT, et al. Maraba MG1 virus enhances natural killer cell function via conventional dendritic cells to reduce postoperative metastatic disease. Mol Ther. 2014;22(7):1320–32. https://doi.org/10.1038/mt.2014.60.
Blake SJ, Stannard K, Liu J, Allen S, Yong MC, Mittal D, et al. Suppression of metastases using a new lymphocyte checkpoint target for cancer immunotherapy. Cancer Discov. 2016;6(4):446–59. https://doi.org/10.1158/2159-8290.cd-15-0944.
Zhang Z, King MR. Nanomaterials for the capture and therapeutic targeting of circulating tumor cells. Cell Mol Bioeng. 2017;10(4):275–94. https://doi.org/10.1007/s12195-017-0497-4.
Gribko A, Künzel J, Wünsch D, Lu Q, Nagel SM, Knauer SK, et al. Is small smarter? Nanomaterial-based detection and elimination of circulating tumor cells: current knowledge and perspectives. Int J Nanomed. 2019;14:4187–209. https://doi.org/10.2147/IJN.S198319.
Wang D, Ge C, Liang W, Yang Q, Liu Q, Ma W, et al. In vivo enrichment and elimination of circulating tumor cells by using a black phosphorus and antibody functionalized intravenous catheter. Adv Sci. 2020;7(17):2000940. https://doi.org/10.1002/advs.202000940.
Raschpichler M, Preis E, Pinnapireddy SR, Baghdan E, Pourasghar M, Schneider M, et al. Photodynamic inactivation of circulating tumor cells: an innovative approach against metastatic cancer. Eur J Pharm Biopharm. 2020;157:38–46. https://doi.org/10.1016/j.ejpb.2020.10.003.
Ghaderinia M, Khayamian MA, Abadijoo H, Shalileh S, Faramarzpour M, Zandi A, et al. Capture-free deactivation of CTCs in the bloodstream; a metastasis suppression method by electrostatic stimulation of the peripheral blood. Biosens Bioelectron. 2021;183: 113194. https://doi.org/10.1016/j.bios.2021.113194.
Ye H, Wang K, Lu Q, Zhao J, Wang M, Kan Q, et al. Nanosponges of circulating tumor-derived exosomes for breast cancer metastasis inhibition. Biomaterials. 2020;242: 119932. https://doi.org/10.1016/j.biomaterials.2020.119932.
Restivo A, Cocco IM, Casula G, Scintu F, Cabras F, Scartozzi M, et al. Aspirin as a neoadjuvant agent during preoperative chemoradiation for rectal cancer. Br J Cancer. 2015;113(8):1133–9. https://doi.org/10.1038/bjc.2015.336.
Khoo BL, Grenci G, Lim JSY, Lim YP, Fong J, Yeap WH, et al. Low-dose anti-inflammatory combinatorial therapy reduced cancer stem cell formation in patient-derived preclinical models for tumour relapse prevention. Br J Cancer. 2019;120(4):407–23. https://doi.org/10.1038/s41416-018-0301-9.
Uluçkan O, Eagleton MC, Floyd DH, Morgan EA, Hirbe AC, Kramer M, et al. APT102, a novel adpase, cooperates with aspirin to disrupt bone metastasis in mice. J Cell Biochem. 2008;104(4):1311–23. https://doi.org/10.1002/jcb.21709.
D’Avola D, Villacorta-Martin C, Martins-Filho SN, Craig A, Labgaa I, von Felden J, et al. High-density single cell mRNA sequencing to characterize circulating tumor cells in hepatocellular carcinoma. Sci Rep. 2018;8(1):11570. https://doi.org/10.1038/s41598-018-30047-y.
Cheng Y-H, Chen Y-C, Lin E, Brien R, Jung S, Chen Y-T, et al. Hydro-Seq enables contamination-free high-throughput single-cell RNA-sequencing for circulating tumor cells. Nat Commun. 2019;10(1):2163. https://doi.org/10.1038/s41467-019-10122-2.
Klotz R, Thomas A, Teng T, Han SM, Iriondo O, Li L, et al. Circulating tumor cells exhibit metastatic tropism and reveal brain metastasis drivers. Cancer Discov. 2020;10(1):86–103. https://doi.org/10.1158/2159-8290.cd-19-0384.
Specht H, Emmott E, Petelski AA, Huffman RG, Perlman DH, Serra M, et al. Single-cell proteomic and transcriptomic analysis of macrophage heterogeneity using SCoPE2. Genome Biol. 2021;22(1):50. https://doi.org/10.1186/s13059-021-02267-5.
Cong Y, Liang Y, Motamedchaboki K, Huguet R, Truong T, Zhao R, et al. Improved single-cell proteome coverage using narrow-bore packed nanoLC columns and ultrasensitive mass spectrometry. Anal Chem. 2020;92(3):2665–71. https://doi.org/10.1021/acs.analchem.9b04631.
Cong Y, Motamedchaboki K, Misal SA, Liang Y, Guise AJ, Truong T, et al. Ultrasensitive single-cell proteomics workflow identifies > 1000 protein groups per mammalian cell. Chem Sci. 2021;12(3):1001–6.
Acknowledgements
We thank Ms. Ekaterina Khitrinskaya for the preparation of the figure.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by the Russian Science Foundation (grant #19-75-30016).
Conflict of Interest
The authors declare no conflicts of interest.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Author contributions
All authors drafted and critically revised the manuscript.
Rights and permissions
About this article
Cite this article
Menyailo, M.E., Bokova, U.A., Ivanyuk, E.E. et al. Metastasis Prevention: Focus on Metastatic Circulating Tumor Cells. Mol Diagn Ther 25, 549–562 (2021). https://doi.org/10.1007/s40291-021-00543-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-021-00543-5