Abstract
Background
Materials have been distributed in the European Union to inform physicians on the safe use of intravitreal aflibercept (IVT-AFL) as part of the risk-minimization plan for IVT-AFL.
Objective
We aimed to measure physician knowledge and understanding of key safety information for IVT-AFL.
Methods
The current study was a follow-up cross-sectional survey (‘wave 2’) to an earlier survey (‘wave 1’) examining the effectiveness of the IVT-AFL educational materials by assessing physician knowledge of the key safety information. Based on wave 1 results, the educational materials were revised to focus more on items of key concern (e.g., use in women of childbearing potential, procedural information); physicians in France, Germany, Italy, Spain, and the UK completed a questionnaire to evaluate their knowledge of key safety information in the revised educational materials.
Results
Among 454 physician respondents (of 4715 invited; response rate 9.6%), most reported having received the IVT-AFL Summary of Product Characteristics (SmPC; 89%) and Prescriber Guide (82%). More than half reported receiving the Injection Procedure Video (54%) and Patient Booklet (65%). The highest percentage of correct answers was observed for questions concerning procedural steps, the most important risks, and safe use as emphasized by the educational materials and the SmPC.
Conclusion
Physician knowledge and understanding of safe use of IVT-AFL, including for questions that prompted revisions to the educational materials, suggests the need to reconsider methods for developing educational materials to follow best practices (e.g., focusing on only key messages and pretesting with end users).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We conducted a follow-up cross-sectional survey (‘wave 2’) to an earlier survey (‘wave 1’) examining the effectiveness of educational materials for physicians on the safe use of intravitreal aflibercept. |
The highest proportion of correct answers was observed for content related to procedural steps and to the most important risks and safe use emphasized in the prescriber guide. A lower percentage of correct responses was observed for topics that related to intravitreal injections and for more complex aspects of safe use. |
These results suggest that physicians could benefit from educational materials focusing on the most relevant product-specific content. |
1 Introduction
Aflibercept (Eylea; Bayer AG) is an anti-vascular endothelial growth factor (VEGF) therapy administered via intravitreal injection. It was granted market authorization in 2012 by the European Commission for the treatment of exudative retinal conditions [1]. Intravitreal injections in general are associated with serious ocular complications such as endophthalmitis, increased intraocular pressure, traumatic cataract, and retinal and vitreous detachment. Although less serious, conjunctival hemorrhage, vitreous floaters, and eye pain are other common complications [2, 3]. When prescribing and administering intravitreal treatments, physicians should be well educated about current treatment procedures, safe use, and appropriate monitoring for adverse effects, as well as for product-specific guidelines.
As part of risk-minimization procedures for intravitreal aflibercept (IVT-AFL) in the European Union [4], the manufacturer distributed materials to all physicians who administer IVT-AFL in the European Economic Area, aiming to educate them on the safety information associated with IVT-AFL and on the safe and proper administration of IVT-AFL. The materials, consisting of a Prescriber Guide, the IVT-AFL Summary of Product Characteristics (SmPC), and an Intravitreal Procedure Instructional Video specific to IVT-AFL vials, describe the importance of using the correct sterile injection technique, monitoring procedures following injection, the management of potential injection-related adverse reactions, and proper storage and handling of the medication. These educational materials are intended to raise physicians’ awareness of and minimize the occurrence and consequences of the important risks of treatment, including endophthalmitis, intraocular inflammation, transient intraocular pressure increase, retinal pigment epithelium tears, traumatic cataract, and embryo-fetotoxicity, as well as medication error, misuse, and off-label use.
To evaluate the effectiveness of those educational materials, a survey (‘wave 1’) of physicians and patients was completed in 2017 [5]. Based on the results of wave 1 and as requested by the European Medicines Agency (EMA), the IVT-AFL Prescriber Guide was revised to add more focus on certain aspects of safe use. These included use in women of childbearing potential, procedural information (e.g., such as whether the eye should be dilated prior to giving the injection), the need to evaluate vision immediately after an IVT-AFL injection, the need to monitor patients following an injection for elevation in intraocular pressure, and the restriction against drawing multiple injections from the same vial. The manufacturer then revised the updated IVT-AFL Prescriber Guide for physicians and completed redistribution in May 2019. A second physician survey (‘wave 2’), described here, was conducted between October 2019 and April 2020. The primary objective of the wave 2 survey was to measure physician knowledge and understanding of key safety information contained in the revised materials [6]; exploratory aims were to examine results by ophthalmology subspecialty, by number of IVT-AFL injections administered per month, by country, and by wave.
2 Methods
2.1 Study Design and Sample
Similar to wave 1 [5], this study was an observational, cross-sectional survey of knowledge, understanding, and self-reported behavior among physicians with recent experience in prescribing/administering IVT-AFL. Data were collected from physicians practicing in France, Germany, Italy, Spain, and the UK; these countries were chosen to allow for qualitative comparison of differences in practice patterns and treatment indication and for consistency with the initial wave 1 survey [5]. The study design and implementation followed the best practice guidelines in pharmacoepidemiology and pharmacovigilance [7,8,9,10], under a protocol approved by the EMA [6]. This study was conducted in compliance with the principles of the Declaration of Helsinki and fulfilled all national and local regulatory and ethical requirements. All participants provided informed consent. The study was granted an exemption from review by the Office of Research Protection and Ethics at RTI International.
Eligible physicians were licensed, practicing ophthalmologists who had prescribed or administered IVT-AFL to at least one patient in the past 6 months. The ophthalmologists were primarily recruited from an online physician panel, as described in the methods of the wave 1 study [5]. Physicians’ identities were blinded to the study sponsor. A target size of 60–100 ophthalmologists per country and 300–500 overall was set for the study. Data were collected from 8 October 2019 to 15 April 2020, and collection started in each country at least 3 months after the initial distribution of the revised educational materials to allow time for prescribers to have received the revised Prescriber Guide and used the information in their practice. Physicians received a small compensation for their time in completing the survey.
2.2 Questionnaire
The questionnaire (see Appendix A in the electronic supplementary material [ESM]) contained closed-ended questions on the following topics: physicians’ knowledge of key safety information, including preparation and dosing information, injection procedures (e.g., aseptic techniques to minimize the risk of infection; intravitreal injection techniques) and signs and symptoms of injection-related adverse effects; physicians’ receipt and review of the educational materials; and physicians’ assessment of the usefulness of the educational materials. The wave 2 survey included new and modified items to further address key topics from the wave 1 survey results (e.g., use during pregnancy and breastfeeding and procedural information for anti-VEGF injections) and to collect additional information to describe ophthalmologists’ demographics and experience.
2.3 Statistical Methods
All analyses were performed using SAS 9.4 statistical software (SAS Institute, Inc., Cary, NC, USA). Data analyses were descriptive, and exact 95% confidence intervals around the percentage of correctly answered questions were calculated using the Clopper–Pearson method. The knowledge questions were stratified to examine associations between knowledge levels and the following variables: specialty within ophthalmology, practice setting, years in practice, age, sex, average number of monthly IVT-AFL injections administered, time since last reviewing the Prescriber Guide, and participation (yes/no) in the wave 1 study. No imputation of missing values was performed.
3 Results
3.1 Physician Characteristics
Of the 4715 ophthalmologists invited to participate, 454 completed the survey and were included in the analysis (Fig. 1), making the overall response rate 9.6%. The participants in wave 2 included new participants (n = 347, 76%) as well as participants who had completed the wave 1 survey (n = 107, 24%) to reach the desired sample size. The majority of participants specialized in treatment of the retina (63%) and/or general ophthalmology (57%) (Table 1). Most practiced in hospital (74%) and/or were in office-based (31%) settings and reported having treated patients for 6–10 years (23%), 11–15 years (21%), or 16–20 years (20%). Approximately two-thirds (67%) were male, and more than half (60%) were aged 40–59 years, while 27% were younger than 40 years of age.
Per the screening criteria, all participants had prescribed (94%) and/or administered (78%) IVT-AFL in the past 6 months for indications including neovascular age-related macular degeneration (nAMD; 93%), visual impairment due to diabetic macular edema (81%), macular edema secondary to central (72%) or branch retinal vein occlusion (67%), and myopic choroidal neovascularization (50%) (see Table B1 in Appendix B of the ESM). Approximately half reported administering an average of 5–40 anti-VEGF injections per month (44%) and 5–40 IVT-AFL injections per month (53%); 76% had administered their last IVT-AFL injection < 1 month prior to completing the study survey (Table B1 in the ESM).
3.2 Physician Knowledge
3.2.1 Storage and Preparation
Participants were asked seven true/false questions related to the storage and preparation of IVT-AFL (Fig. 2). Ninety-two percent correctly responded that adequate anesthesia and asepsis must be provided for the patient; 85% correctly indicated that IVT-AFL must be stored in the refrigerator.
3.2.2 Dosing
Three questions were asked about IVT-AFL dosing. Most participants (82%) correctly reported that the vial contains more than the recommended dose and excess volume should be expelled before injecting (Table 2). Likewise, 78% correctly reported that 50 μL (2 mg) is the recommended dose (Table 3). A lower percentage of participants (58%) correctly reported that after removing all drug from the vial with a syringe, the plunger should be depressed until the tip aligns with the line that marks 0.05 mL on the syringe (Table 2).
3.2.3 Safe Use
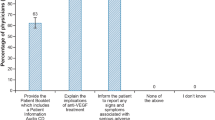
The survey included five questions about safe use. More than three-quarters of participants (78%) correctly identified all three contraindications for IVT-AFL (Fig. 3). Correct responses ranged from 82% for ‘Patients with active severe intraocular inflammation’ as a contraindication, to 92% for ‘Patients with a known hypersensitivity to the active substance aflibercept or to any of the excipients (e.g., nonactive ingredients)’. Overall, 94% of participants correctly indicated that they should ‘Inform the patient to report any signs and symptoms potentially associated with serious adverse events (AEs) and provide information on when to seek medical attention, when preparing a patient to start IVT-AFL treatment’; 90% selected ‘Explain the implications of anti-VEGF treatment’, and 67% selected ‘Provide the Patient Booklet, which includes a patient booklet audio CD and patient information leaflet’ (Fig. B1 in Appendix B of the ESM). Slightly more than half of physicians (53%) selected the correct timeframe for which women of childbearing potential must use effective contraception during treatment and for at least 3 months after the last IVT-AFL injection, while 28% incorrectly indicated that IVT-AFL should never be used in pregnancy. More than three-quarters (76%) correctly indicated that IVT-AFL is not recommended for women who are breastfeeding (Figs. B2 and B3 in Appendix B of the ESM).
3.2.4 Injection Procedure
A series of six questions was asked about proper injection procedure (Fig. B4 in Appendix B of the ESM); 92% of participants correctly responded ‘True’ to a true/false question asking whether disinfectant (e.g., povidone iodine solution) should be applied to the periocular skin, eyelid, and ocular surface, and 89% correctly responded that topical anesthesia should be used prior to injection. When asked to identify steps that should be taken prior to marking the scleral injection site, 84% correctly selected ‘Cover the eye with a sterile drape’, and 76% correctly selected ‘Insert a sterile lid speculum’. Most participants correctly reported that the injection needle should be inserted into the vitreous cavity, avoiding the horizontal meridian, and aiming toward the center of the globe (80%), and that in preparation for the injection, the eye should be marked at a distance of 3.5–4.0 mm posterior to the limbus (77%).
3.2.5 Possible Adverse Effects
Two questions examined post-injection monitoring (Fig. B5 in Appendix B of the ESM). Most participants (83%) correctly responded that increased intraocular pressure has been observed within 60 min of intravitreal injection. When shown a list of potential known undesirable adverse effects, participants correctly selected endophthalmitis (91%), transient increase of intraocular pressure (92%), retinal tear or retinal detachment (70%), and cataract (traumatic, nuclear, subcapsular, cortical) or lenticular opacities (69%) (Fig. B6 in Appendix B of the ESM).
3.3 Receipt and Use of Educational Materials
Rates of self-reported receipt of the materials were relatively high (Table 4): 89% reported receiving the SmPC, 82% received the Eylea Prescriber Guide, 65% received the Indication-Specific Patient Booklet (including a patient information audio CD and the patient information leaflet), and 54% received the Intravitreal Injection Procedure Video. Of those who reported having received the materials, 83% reported having reviewed the SmPC, 82% reviewed the Prescriber Guide, 70% reviewed the patient information and CD, and 72% reviewed the Injection Procedure Video.
Participants were asked to rate the materials they reviewed on a scale from 1 (not at all helpful) to 4 (extremely helpful). Overall, 73–85% of participants rated all educational materials 3 or 4. When asked how many patients under their care who were receiving IVT-AFL injections were provided the Eylea Patient Booklet, 37% reported that they provided the booklet to all of their patients, and 24% reported providing it to most patients (Table B2 of Appendix B in the ESM). The most common reasons given not to provide the booklet were 'I provide the same information from the Eylea Patient Booklet to patients verbally and give them the chance to ask questions' (46%), 'I did not receive enough copies of the Eylea Patient Booklet to distribute to all my Eylea patients' (38%), and 'I provide alternate materials (e.g., treatment consent form)' (35%).
3.4 Other Analyses
3.4.1 Stratified Analyses
Knowledge questions were stratified by subspecialty and average number of monthly IVT-AFL injections. Self-identified retina specialists had the highest proportion of correct responses on all knowledge questions. Ophthalmologists who performed more IVT-AFL injections per month consistently provided more correct responses than those who performed fewer injections. Both sets of stratified knowledge questions can be found in Appendix C of the ESM.
3.4.2 Comparison of Results Across Waves
While ophthalmologists participating in waves 1 and 2 had broadly similar demographic and clinical characteristics and experience with prescribing IVT-AFL, a notably lower proportion of ophthalmologists selected retina as their specialty in wave 2 (63%) than in wave 1 (74%). Reported rates of having received the educational materials were similar among wave 1 participants (range, 50% for the Intravitreal Injection Procedure Video to 87% for the SmPC) and wave 2 participants (range, 54% for the Intravitreal Injection Procedure Video to 89% for the SmPC) (Table 4), as were reported rates of having reviewed the materials among those who had received them (ranges, 67% for the Intravitreal Injection Procedure Video to 89% for the SmPC for wave 1; 70% for the Patient Booklet to 83% for the SmPC for wave 2) (Table 4). On average, results on knowledge questions were similar across waves, except the percentages of correct responses for some questions were slightly higher in wave 1 than in wave 2, with + 5% to 6% differences.
Knowledge about the use of IVT-AFL in women of childbearing potential was similar across waves: 48% of participants in wave 1 and 53% in wave 2 knew that effective contraception must be used during and up to 3 months after treatment. Similar proportions (27% in wave 1 and 28% in wave 2) indicated that IVT-AFL should not be used during pregnancy. In wave 2, 76% of participants responded correctly that IVT-AFL is not recommended for use in women who are breastfeeding; the wave 1 physician survey did not specifically address use of IVT-AFL in breastfeeding women.
Regarding procedural steps, 28% of participants in wave 1 and 22% in wave 2 responded incorrectly that eye dilation is necessary prior to injection. The most common reasons for this response in wave 2 were ‘Eye dilation is done for regular assessment of the underlying disease or other assessments (e.g., fundus examination)’ (58%), ‘Requirements/recommendations by national or local guidelines for intravitreal injections, local protocols, or other recommendations’ (54%), and ‘Personal preference’ (41%). Most participants from both waves (82% in wave 1 and 83% in wave 2) knew that a vial of aflibercept should not be used for more than one patient, as 84% from wave 2 indicated that they knew that drawing multiple injections from the same vial could lead to contamination and subsequent infection.
Knowledge of evaluation of adverse effects was similar across waves. A majority of participants knew that evaluating vision after the injection was done ‘By hand movements or counting fingers’ (wave 1, 60%; wave 2, 54%) and that they needed to ‘Ensure that sterile equipment is available to perform paracentesis if necessary’ if there was increased intraocular pressure following an injection (wave 1, 65%; wave 2, 56%). Finally, on almost all of the questions, physicians in the wave 2 survey who had participated in the wave 1 survey had higher correct response proportions than those who were participating in the survey for the first time; however, the differences between the two groups were 10% or less for two-thirds of the questions.
4 Discussion
The current study is a follow-up wave 2 survey that assessed whether ophthalmologists received and reviewed the revised IVT-AFL educational materials and evaluated their knowledge of key safety information within the revised materials. The survey included new and/or revised questions to cover information deemed to be of interest following the wave 1 results, including knowledge about the need for effective contraception during treatment with IVT-AFL in women of childbearing potential, the restriction on using the same vial for multiple injections, the need to evaluate visual function immediately after an IVT-AFL injection, the need to monitor patients following an injection for elevation in intraocular pressure, and the fact that pupil dilation is not required prior to injection.
A moderate to high percentage of physicians reported having received the educational materials (54% for the Injection Procedure Video, 65% for the Patient Booklet, 82% for the Prescriber Guide, and 89% for the SmPC) and reported them to be helpful or extremely helpful (73–85%). Although demographic characteristics, years in practice, and frequency of prescribing or administering IVT-AFL injections were similar across waves 1 and 2, the percentage of correct knowledge responses for approximately two-thirds of the questions was slightly higher in wave 1 compared with wave 2. One explanation for this finding could be that wave 1 included a higher proportion of retina specialists: 74% in wave 1 compared with 63% in wave 2.
There has been substantial research on effective communication practices [11] as well as guidance on best practices [8, 12]. The distribution process for educational materials has evolved since implementation of the General Data Protection Regulation in 2018 in the European Union, influencing how sponsors obtain distribution lists for physicians. Physicians may also engage with educational materials differently depending on the issuing organization, tending to prioritize communications from national competent authorities and to give less priority to communications from pharmaceutical companies, potentially considering them to be promotional by default [13]. The results of this survey showing that high percentages (73–85%) of physicians reported the educational materials to be helpful is encouraging; however, the lack of improvement across waves for some of the knowledge questions suggests the need to reconsider methods for developing educational materials for healthcare professionals (e.g., engaging their input on the development of the materials). Important factors in developing effective communications include consideration of the target audience, language/word choices, layout of the information, messaging structure and organization, and the most relevant and appropriate content [11, 12]. A key feature particularly relevant here is the need to keep the content simple, informative, and actionable, as well as to minimize the number of messages provided and avoid repetition. In this case, we might suggest that the most critical information for the IVT-AFL communication would be those clinical items specific to safety and contraindications, for which knowledge would be most problematic in terms of risks for complications. Information that applies to detailed procedures for intraocular injections in general, but which may vary between the countries, may lead to discrepant results among participating regions. For example, results from the question about pupil dilation prior to injection indicated that some physicians performed this step because it was part of the specific procedural guidelines in their country (54%). Moreover, knowledge patterns for issues that are infrequently encountered in clinical practice, such as the use of IVT-AFL during pregnancy or breastfeeding, are somewhat uncommon in the target population; this might suggest that physicians are more likely to consult the SmPC or practice guidelines for guidance on safe use [14] than to rely on recall. Taken together, our findings suggest that educational materials that focus on product-specific content may be most relevant to physicians, who may consult other resources such as practice guidelines for more general information about administration procedures.
The current results are consistent with other studies examining the receipt and use of educational materials. A previous study evaluating Canadian physicians’ knowledge of IVT-AFL demonstrated that the physicians’ knowledge was high, as indicated by high rates of correct responses for questions regarding proper IVT-AFL dosing (75–91%), injection procedure (91–99%), and contraindications (89%) [5]. Another multinational study was conducted to evaluate the proportion of physicians who received educational materials for metabolic monitoring with quetiapine. Although receipt of materials varied across countries, monitoring rates were generally higher among physicians who recalled receiving the materials than among those who did not recall receiving or were unsure they had received them [15]. Artime and colleagues conducted a study to examine the effectiveness of updated risk-minimization measures in a safety information packet (SIP) for alglucosidase alfa across two survey waves. The study found relatively high rates of receipt of the SIP across waves (77.7% in wave 1, 74.5% in wave 2), a high proportion of clinicians who read the SIP (88.6% in wave 1, 89.5% in wave 2), and high usage rates of the SIP among providers (88.2% in wave 1, 89.5% in wave 2). Finally, the rates of knowledge among providers were similar across the waves, with an overall median percentage correct answers of 61.9% in wave 1 and 66.7% in wave 2 [16].
The present study had limitations. Although efforts were made to recruit a diverse and representative sample, the study relied on a physician panel for recruitment. Hence, the panel may not have been representative of all ophthalmologists who have prescribed/administered IVT-AFL, potentially limiting generalizability. Owing to the relatively small sample size, the results are also subject to potential response and volunteer biases, the magnitude and direction of which is not known. Despite efforts to increase participation, the response rate for the online surveys was low (9.6%), a recognized challenge in conducting physician surveys to evaluate the effectiveness of risk-minimization measures [17]. The response rate was slightly higher than the response rate for the wave 1 survey (5.1%) and is broadly consistent with response rates for physician knowledge surveys [5, 18, 19]. Finally, the data collection period (8 October 2019–15 April 2020) coincided in part with the coronavirus disease 2019 (COVID-19) pandemic, which may have interfered with physicians’ ability to participate in the survey at that time.
5 Conclusions
The observed knowledge patterns were similar across waves, and a higher percentage of correct responses was observed among ophthalmologists who specialized in the retina and who performed more monthly IVT-AFL injections. The highest knowledge was seen in content related to procedural steps and with the most important risks and safe use emphasized in the Prescriber Guide. A lower percentage of correct responses was observed in topics that related to intravitreal injections (e.g., dilating the pupil before injection, evaluating vision after injection) and for more complex aspects of safe use. Moderate to high percentages of physicians reported having received the educational materials, with highest receipt reported for the SmPC and Eylea Prescriber Guide; physicians reported lower rates of having received the Patient Booklet and having provided it to their patients. Overall, a majority of physicians reported the materials to be helpful or extremely helpful. These results suggest that physicians could benefit from prescriber guides and other educational materials that focus only on the most relevant product-specific content, using a language style that is simple to understand, informative and actionable. Furthermore, the involvement of the end users, in this case ophthalmologists who perform intravitreal injections, in the creation and content development of educational materials may be beneficial to ensure that their contents are relevant and effectively convey essential information to promote the safe use of a drug.
References
Eylea summary of product characteristics. European Medicines Agency. 2023. https://www.ema.europa.eu/en/documents/product-information/eylea-epar-product-information_en.pdf. Accessed 31 May 2023.
Csaky K, Do DV. Safety implications of vascular endothelial growth factor blockade for subjects receiving intravitreal anti-vascular endothelial growth factor therapies. Am J Ophthalmol. 2009;148(5):647–56. https://doi.org/10.1016/j.ajo.2009.06.014.
Jeganathan VS, Verma N. Safety and efficacy of intravitreal anti-VEGF injections for age-related macular degeneration. Curr Opin Ophthalmol. 2009;20(3):223–5. https://doi.org/10.1097/ICU.0b013e328329b656.
European Medicines Agency. Assessment report: Eylea (aflibercept). European Medicines Agency; 2013.
Zografos LJ, Andrews E, Wolin DL, Calingaert B, Davenport EK, Hollis KA, et al. Physician and patient knowledge of safety and safe use information for aflibercept in Europe: evaluation of risk-minimization measures. Pharmaceut Med. 2019;33(3):219–33. https://doi.org/10.1007/s40290-019-00279-y.
ENCePP. Postauthorization safety study: evaluation of physician knowledge of safety and safe use information for aflibercept administered by intravitreal injection in europe: a follow-up physician survey. 2018. https://www.encepp.eu/encepp/openAttachment/fullProtocol/31273;jsessionid=GvdqfdROVXWcDRp2VPwP5t7Ro6iCeW15ZCFALdUwDabDi8CAwmfY!456884888. Accessed 7 Feb 2022.
International Society for Pharmacoepidemiology (ISPE). Guidelines for good pharmacoepidemiology practices (GPP). 2015. https://www.pharmacoepi.org/resources/policies/guidelines-08027/. Accessed 5 May 2020.
EMA. European Medicines Agency. Guideline on good pharmacovigilance practices (GVP). Module VIII–post-authorisation safety studies (Rev 3). 2017. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129137.pdf. Accessed 26 Jun 2020.
ENCePP. European Network of Centres for Pharmacoepidemiology and Pharmacovigilance. Guide on methodological standards in pharmacoepidemiology (revision 7). EMA/95098/2010. 2017. http://www.encepp.eu/standards_and_guidances/methodologicalGuide.shtml. Accessed 26 Jun 2020.
European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP). The European Union Electronic Register of Post-Authorisation Studies (EU PAS Register). 26 March 2020. http://www.encepp.eu/encepp_studies/indexRegister.shtml. Accessed 26 June 2020.
US FDA. Communicating Risks and Benefits: An Evidence-Based User's Guide. 2022.
Centers for Disease Control and Prevention (CDC). Simply Put. 2009. https://www.cdc.gov/healthliteracy/pdf/simply_put.pdf. Accessed 11 Mar 2022.
Lem J, Younus M, Aram JA, Moosavi S, Freivogel K, Lewis A, et al. Evaluation of the effectiveness of additional risk minimization measures for voriconazole in the EU: findings and lessons learned from a healthcare professional survey. Pharmaceut Med. 2019;33(2):121–33. https://doi.org/10.1007/s40290-019-00273-4.
Grzybowski A, Told R, Sacu S, Bandello F, Moisseiev E, Loewenstein A, et al. 2018 update on intravitreal injections: euretina expert consensus recommendations. Ophthalmologica. 2018;239(4):181–93. https://doi.org/10.1159/000486145.
Brody RS, Liss CL, Wray H, Iovin R, Michaylira C, Muthutantri A, et al. Effectiveness of a risk-minimization activity involving physician education on metabolic monitoring of patients receiving quetiapine: results from two postauthorization safety studies. Int Clin Psychopharmacol. 2016;31(1):34–41. https://doi.org/10.1097/yic.0000000000000102.
Artime E, Vora P, Asiimwe A, Soriano-Gabarro M, Qizilbash N. Variability in reporting participation data in survey studies evaluating the effectiveness of risk minimisation measures in the European Union. Pharmacoepidemiol Drug Saf. 2017;26(S2):3–636.
Madison T, Arias A, DiSantostefano R, Gilsenan A, Matus D, Primatesta P, et al. Evaluating the effectiveness of additional risk minimization measures via surveys in Europe: challenges and recommendations. Endorsed by ISPE Board of Directors. 2016. https://pharmacoepi.org/pub/f46953df-de69-31e7-8f74-725bd7fa685f. Accessed 11 Sep 2023.
Madison T, Huang K, Huot-Marchand P, Wilner KD, Mo J. Effectiveness of the crizotinib therapeutic management guide in communicating risks, and recommended actions to minimize risks, among physicians prescribing crizotinib in Europe. Pharmaceut Med. 2018;32:343–52. https://doi.org/10.1007/s40290-018-0248-4.
Davis KH, Asiimwe A, Zografos LJ, McSorley DJ, Andrews EB. Evaluation of risk-minimization activities for cyproterone acetate 2 mg/ethinylestradiol 35 µg: a cross-sectional physician survey. Pharmaceut Med. 2017;31(5):339–51. https://doi.org/10.1007/s40290-017-0203-9.
Acknowledgments
The authors thank Melissa Mehalick, PhD, and Kate Lothman of RTI Health Solutions for medical writing assistance. Bayer provided funding for publication support in the form of manuscript writing, styling, and submission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was performed under a research contract between RTI Health Solutions and Bayer AG and was funded by Bayer AG. The contract between RTI Health Solutions and the sponsor includes independent publication rights. Preparation of this article, including medical writing assistance, was funded by Bayer AG.
Conflicts of interest/competing interests
LZ, DW, BC, and ED are employees of RTI Health Solutions, which received funding from Bayer AG to conduct this study; EA was an employee of RTI Health Solutions when the study was conducted. The contract between RTI Health Solutions and the sponsor includes independent publication rights. RTI International conducts work for government, public, and private organizations, including pharmaceutical companies. AM, ML, UMSO, NL, LB, KJ, and KSW are employees of Bayer AG, the sponsor of this study.
Availability of data and materials
The data generated for the analyses presented are included in the published article or its supplementary appendices.
Code availability
Not applicable.
Authors’ contributions
All authors declare that they have made significant contributions to this work in the conception, study design, execution, acquisition of data, and/or analysis and interpretation of data, and have drafted, written, substantially revised, or critically reviewed the article; have agreed on the journal to which the manuscript was submitted; have reviewed and agreed on all versions of the manuscript before submission and during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage; and agree to take responsibility and be accountable for the contents of this article.
Ethics approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the applicable institutional review board and all applicable country-level Ethics Committees and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zografos, L.J., Andrews, E., Wolin, D.L. et al. Evaluation of Physician Knowledge of Safety and Safe Use Information for Intravitreal Aflibercept Injection in Europe: A Second Survey of Physicians Following Dissemination of Updated Risk-Minimization Materials. Pharm Med 38, 63–73 (2024). https://doi.org/10.1007/s40290-023-00506-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40290-023-00506-7