Abstract
Problematic gambling has been suggested to be a possible consequence of dopaminergic medications used mainly in neurological conditions, i.e. pramipexole and ropinirole, and possibly by one antipsychotic compound, aripiprazole. Patients with Parkinson’s disease, restless legs syndrome and other conditions potentially treated with dopamine agonists, as well as patients treated for psychotic disorders, are vulnerable patient groups with theoretically increased risk of developing gambling disorder (GD), for example due to higher rates of mental ill-health in these groups. The aim of the present paper is to review the epidemiological, clinical, and neurobiological evidence of the association between dopaminergic medications and GD, and to describe risk groups and treatment options. The neurobiology of GD involves the reward and reinforcement system, based mainly on mesocorticolimbic dopamine projections, with the nucleus accumbens being a crucial area for developing addictions to substances and behaviors. The addictive properties of gambling can perhaps be explained by the reward uncertainty that activates dopamine signaling in a pathological manner. Since reward-related learning is mediated by dopamine, it can be altered by dopaminergic medications, possibly leading to increased gambling behavior and a decreased impulse control. A causal relationship between the medications and GD seems likely, but the molecular mechanisms behind this association have not been fully described yet. More research is needed in order to fully outline the clinical picture of GD developing in patient groups with dopaminergic medications, and data are needed on the differentiation of risk in different compounds. In addition, very few interventional studies are available on the management of GD induced by dopaminergic medications. While GD overall can be treated, there is need for treatment studies testing the effectiveness of tapering of the medication or other gambling-specific treatment modalities in these patient groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The dopamine agonists pramipexole and ropinirole, and the dopamine modulator aripiprazole, are associated with an increased risk of developing problematic gambling. |
The pathological over-activation of the dopaminergic reward and reinforcement system is one possible mechanism for dopaminergic drugs to increase gambling behavior. |
Gambling disorder is a treatable condition, where a number of therapeutic methods, or shorter motivational and normative feedback intervention, can be effective. |
1 Gambling Disorder (GD): The First Non-Substance-Related Addictive Disorder
Among the addictive conditions included in diagnostic manuals, gambling for money is the first to not involve a substance. Gambling disorder (GD), previously referred to as pathological gambling, is a condition defined both in the psychiatric diagnostic manual of the American Psychiatric Association, the DSM-5 [1], and in the World Health Organization’s diagnostic manual, the ICD-11 [2]. Problematic gambling, including both a sub-diagnostic problematic behavior and the more severe clinical picture of a GD, has been reported to occur in up to 6% of the adult population in some settings, whereas the corresponding figures are well below 1% in other settings [3]. Meanwhile, prevalence rates of GD vary around 1% [4]. The concept of problematic gambling is poorly defined, but often used in broader epidemiological studies where face-to-face diagnostic assessments are difficult. In these studies, problematic gambling is typically defined as a score above a defined cut-off on well-established screening measures [3]. Thereby, problematic gambling can be defined as a certain level of harm related to gambling, although not specifically involving a behavior driven by compulsivity or craving. GD is today defined in the DSM-5 as the fulfilment of four out of nine criteria describing several measures of tolerance, withdrawal, “chasing losses” behavior, and different measures of harm [1].
GD typically causes substantial psycho-social problems, and financial problems including over-indebtedness [5,6,7]. In many cases, psychiatric comorbidity is seen, either suspected to cause the gambling problem, or subsequent and secondary to the gambling problem [8, 9]. While comorbidity typically involves co-occurring psychiatric disorders [8, 9], even an increased prevalence of certain physical diseases has been reported [10]. Gambling for money is also the first non-substance-related addictive behavior that has been found to develop as a consequence of dopaminergic medications, likely through specific brain circuits affecting addictive behavior [4, 11, 12].
Typically, debts develop as a part of the GD [7], and the “chasing losses” concept [1, 13] constitutes a key feature and one of the diagnostic criteria of this disorder. The “chasing losses” behavior means that an individual gambles primarily in order to try to win back money lost from recent gambling sessions. Apart from the “chasing losses” behavior, examples of key components of the diagnostic entity are the increased tolerance to gambling, involving higher and higher amounts of money, and repeatedly failed attempts to cut down on gambling. Another key feature of the disorder is the “loss of control,” typically manifested by an intention to gamble to a limited extent, but where the individual repeatedly breaks her/his own pre-set limits after the gambling session starts [1].
GD may develop in the context of many diverse gambling types. Traditionally, gambling involved land-based gambling modalities. Currently, gambling increasingly occurs online and often on more rapid gambling types, with a risk of gambling being performed at high speed and more likely to be addictive. Gambling types with a high addictive potential include online casino games such as “slot” games or online bingo games, rapid online sports betting such as in-play betting during a game, or land-based gambling electronic machine gambling [14].
Gambling, although a behavior typically unrelated to the use of substances, is indeed one of the behaviors most commonly reported to increase in response to the dopamine agonists pramipexole and ropinirole [15, 16]. The risk of increased gambling as an effect of dopaminergic medications has been mostly researched in patients with Parkinson’s disease, which is a striking finding, in the sense that in this specific group of patients, addictive behaviors related to substances are instead typically less common than in the general population [17]. In addition to a problematic gambling pattern, a number of other impulse-related conditions can develop under dopaminergic medications, and may involve urge, craving or increased tolerance (such as in hypersexuality or altered eating behavior), or a more compulsive behavior (such as hobbyism or kleptomania). In either case, these side effects of dopaminergic medication are known to be time-consuming, socially stigmatizing, and often lead to severe financial, emotional, and even legal consequences. Among the conditions described to arise from dopaminergic medications, a problematic gambling pattern is one of the most commonly reported [17].
GD can lead to severe mental health consequences, suicidal ideation [18], and suicide mortality [19], and indebtedness as such has been suggested to be a risk factor of suicide death [20]. In addition, GD has been linked to a more unhealthy life-style and other addictive behaviors such as video gaming or addictive shopping behavior [21]. Psychiatric comorbidity is common in GD. This may include either a concurrent mental health disorder in parallel with the GD, a mental health disorder preceding the onset of GD, or a mental health disorder occurring subsequent to the gambling problem [8, 9]. In a meta-analysis, current comorbidity when seeking GD treatment was very common for mood disorders (around 30% for depression), and different anxiety-related disorders (12–15%) such as, for example, generalized anxiety and social phobia. Alcohol use disorders were common (18% for DSM-IV alcohol abuse, and 15% for alcohol dependence), whereas psychotic disorders were relatively infrequent [8]. Patients with schizophrenia, however, constitute a risk group for problematic gambling [22]. Given the susceptibility of patients with schizophrenia to develop GD, attention has been raised to one of the newer-generation atypical antipsychotic compounds, i.e. the dopamine modulator aripiprazole, which differs from the traditional anti-dopaminergic profile or traditional antipsychotics. Case reports [23, 24] have led to the suspicion that this substance may play a role in gambling in a manner comparable to the case of dopamine agonists.
The order in which comorbid disorders appear may differ in different subgroups of the population [25], and health services are suggested to screen for gambling problems in patients with mental health conditions, and vice versa for mental health problems in patients with known GD. Meanwhile, in many settings, GD is rarely diagnosed and treated, and spontaneous treatment seeking is low [26, 27]. In general, women with gambling problems are more likely than men with gambling problems to suffer from a comorbid mental health disorder [25]. In addition, the clinical course of GD in women may differ from that traditionally seen in men; women are more likely to report a later onset of gambling in life, but a more accelerated course from gambling onset to the development of gambling problems, typically referred to as a “telescoping phenomenon” [28, 29]. Researchers have come to different conclusion regarding the existence of such a “telescoping” phenomenon [28, 30, 31], whereas the higher proportion of mental health comorbidity in female patients with a GD is more clearly demonstrated [25].
With this in mind, there is reason to study and highlight dopaminergic medications as a potential cause, and to increase knowledge and awareness in clinicians working either with the conditions for which these pharmaceuticals are typically used, or with GD and other addictive behaviors. Authors in the area have called for the need for increased attention to this area [4], and the present narrative review attempts to review the literature and to summarize the knowledge base for this potentially underdeveloped area of clinical work.
2 Addictive Reward Mechanisms in GD
The latest versions of the main diagnostic manuals, DSM-5 and ICD-11, classify GD as a behavioral addiction rather than an impulse-control disorder as in previous versions [32]. The change was motivated by findings that GD shares many features with substance use disorders. Both GD and substance use disorders are more common in men, are seen mostly in adolescence and young adult age, and present with similar psychiatric comorbidities [33]. Regarding pharmacotherapy, opioid antagonists, commonly used to treat substance use disorders, can have some beneficial effect in GD [34]. The etiologies of GD and substance use disorders also show substantial similarities. Impulsivity and impaired decision-making are characteristic for both types of addiction, as well as a pathological activation of reward-related learning and changes in striatal activation [35]. Presumably, the key mechanisms of how GD can be induced pharmacologically lie in the neurobiology of reward and reinforcement. Therefore, we summarize the psychological and neurobiological background of GD in this section and the next one, as far as is known to date.
Developing an addiction includes the pathological activation of the natural reward and reinforcement system in the brain [36]. This system is responsible for judging external stimuli and giving them either a positive or a negative value, depending on whether they have a beneficial or a detrimental outcome for the individual and the species [37]. Through Pavlovian conditioning, former neutral stimuli are linked to an evaluation regarding their impact on well-being and survival. After being given a positive association, a stimulus will reinforce behavior in the individual that leads to seeking this specific stimulus. It is known that addictive substances or behaviors can over-activate the reward-reinforcement mechanism [38]. Their stimuli cause new positive conditioning every time they are encountered and eventually receive an abnormally high value in the reward-related memory. Therefore, their capability for reinforcement becomes stronger than other stimuli crucial for survival, and negative consequences play a subordinate role in the process of decision-making. Recently, there have been new insights into how gambling has the property to become addictive as a behavior. Reward uncertainty might be the factor that causes the excessive activation of reward-related learning [12]. Every win is evaluated as a reward prediction error, a stimulus that was “better than expected,” which leads to the reward-related memory increasing the value of the behavior every time a win occurs. If this process is activated too extensively, it can impair balanced decision-making and eventually gambling develops into an addiction.
Besides being drawn to reward uncertainty, the pathological form of gambling involves many other neurobehavioral characteristics [35], for instance increased impulsivity. Impulsive behavior has been described as one common feature throughout impulse-control disorders and behavioral addictions, and can be defined as the tendency to act without forethought, including reduced consideration for consequences and an impaired response inhibition [39]. In GD, one typical example for impulsivity is risk-taking behavior, which has been shown to predict gambling severity in pathological gamblers [40,41,42,43]. Another measure for increased impulsivity in addiction is a steeper temporal or delay discounting curve, which reflects the inability to wait for larger rewards received at a later time point and the tendency to instead choose small immediate rewards [44, 45]. Higher temporal discounting rates have been found to correlate with the severity of gambling in GD patients [40, 42].
In addition to certain personality traits, the outer set-up of the game seems to influence the likelihood of gambling to become addictive substantially. Through Pavlovian conditioning, gambling-related cues are linked to supposedly beneficial outcomes and can lead to a severe distraction from other tasks [46, 47]. Notably, this attentional bias has been demonstrated repeatedly in pathological gamblers [41, 48, 49]. Furthermore, near misses in gambling, occasions where the player almost wins, can be perceived as winning with regard to their reinforcement properties [50]. Therefore, games where near misses are a common outcome have been described to have an especially high risk of becoming addictive [35].
3 Neuronal Activity and Dopamine Signaling in GD
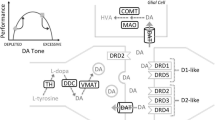
The neurobiology of reward-related learning in GD has only been partly understood. Dopamine plays a key role in the process, but other transmitter systems are involved, especially serotonin and glutamate [51]. The central brain structure of the reward and reinforcement system is the mesocorticolimbic dopamine pathway [52]. It is based on neurons that project from the midbrain to the basal ganglia, more precisely from the ventral tegmental area to the nucleus accumbens in the ventral striatum. Reward prediction errors that occur in gambling are translated into phasic, high peak dopamine signaling in the nucleus accumbens [12]. Therefore, one proposed mechanism for gambling to become addictive is pathologically increased striatal dopamine signaling [53]. In line with this hypothesis, reward uncertainty in GD has been found to be translated into increased dopamine signaling in the nucleus accumbens [54]. While the striatum is described as the central structure for reward-related learning, projections into the cortex and other regions of the limbic system are involved in the process as well [55]. Especially the prefrontal cortex, important in decision-making and response inhibition [56], should be considered to play a role in the pathophysiology of GD.
Multiple studies have investigated the brain regions and signaling involved in impulsivity and under gambling-related conditions, mostly through neuroimaging [55, 57]. But even preclinical approaches are gaining importance in investigating the etiology of gambling behavior [58, 59]. Since patients with Parkinson’s disease represent the largest group affected by drug-induced GD, many studies focus on a population with Parkinson’s disease or on parkinsonian rodent models. The findings on activity changes in the striatum and the prefrontal cortex in GD and other impulse-control disorders remain controversial. Several studies have found an increase in neuronal activity and functional connectivity in these areas in a gambling context. Larger bet sizes and reward-related learning in patients with impulse-control disorders have been shown to correlate with increased mesocorticolimbic connectivity [60, 61]. Higher impulsivity can be linked to increased activity in the orbitofrontal cortex [62], and reward anticipation in individuals who gamble has been found in connection with elevated activity in the ventral striatum and the orbitofrontal cortex [63]. Furthermore, higher compulsivity has been found to correlate with increased activation of the dorsal striatum and the orbitofrontal cortex in rats treated with the D2 agonist pramipexole [64].
In contrast, patients with impulse-control disorders have shown lower activity in the mesocorticolimbic pathway [65, 66], even when tested under a risk-taking challenge [67]. A reduced connectivity in the reward evaluation and response inhibition network, including the prefrontal cortex, has been associated with gambling behavior [61]. Interestingly, dopamine agonist treatment seems to influence individuals involved in gambling and healthy controls differently with regard to the effect on several brain areas, including the orbitofrontal cortex [68]. While the dopamine agonist increased the activity in controls, it was reduced in people who gamble pathologically. Even though the striatum and the prefrontal cortex have been suggested as the main structures responsible for reward-related learning and response inhibition, other areas also seem to play a role in impulse control, for example the amygdala, the insula, and the cingulate cortex [62, 65, 68, 69].
Another research target has been the dopamine signaling in GD on a cellular level. Even in this field, no consensus has been reached yet, but certain trends are starting to be consistent. Notably, non-replicable neuroimaging results have been pointed out as a limitation in this research area [70]. Many studies suggest an increase in dopamine transmission as part of the pathophysiology in GD, indicated for instance by a higher dopamine synthesis capacity in patients with GD, but also a lower postsynaptic receptor availability and a lower abundance of the presynaptic dopamine transporter [71, 72]. The striatal dopamine synthesis capacity in GD patients has been found to be increased [73], but not without contradictory results [66]. Patients with a GD or another impulse-control disorder have been shown to have a higher dopamine release in the ventral striatum while receiving visual reward cues [74, 75]. Furthermore, certain characteristics of excessive gambling, such as elevated excitement and alertness, or unbalanced decision-making in a reward context, correlate with an increase in striatal dopamine binding [76,77,78]. Higher binding to the inhibitory dopamine receptors D2 and D3, which are expressed predominantly in the nucleus accumbens, has been associated with symptoms characteristic for GD, mainly impulsivity [79]. Several other studies have investigated the dopamine receptor availability in patients with GD. Some findings did not show a difference in comparison to the control group [79,80,81], but there is evidence for a reduced postsynaptic receptor expression as well [53, 82,83,84]. Especially, the receptor availability in the ventral striatum seems to be reduced in patients with GD and, often, the decrease can be linked to impulsive behavior and symptom severity. Even a decreased density of the presynaptic dopamine transporter has been associated with GD and other impulse-control disorders in the ventral striatum, but also in other areas [85,86,87,88]. Overall, there seems to be evidence for a direct connection between GD and dopaminergic signaling alterations in the ventral striatum and some afferent and efferent areas, but as mentioned above, there are also negative findings and the molecular mechanisms remain to be discovered.
4 Drug-Induced Changes in GD
The correlation between dopaminergic drugs and increased rates of GD is beginning to be an established consensus [23]. Especially the dopamine replacement therapy applied in Parkinson’s disease is known to have impaired impulse-control as an adverse effect [89,90,91]. Along with hypersexuality, compulsive shopping and binge eating, GD is the most common manifestation of an impaired impulse control due to dopamine replacement therapy in Parkinson’s disease [16]. Hence, the majority of research on drug-induced GD has been conducted in patients with Parkinson’s disease. Multiple studies with large populations have confirmed an association between GD and levodopa treatment, but even more clearly between GD and the dopamine agonists pramipexole and ropinirole (Table 1). These two drugs are selective for the D3 receptor [92, 93], which could explain their high risk of increasing GD rates compared to other dopamine agonists or levodopa [94, 95]. As mentioned earlier, the D2 and D3 receptor in the striatum could mediate an increase in addictive behavior. Dopaminergic therapy is additionally used as a treatment in restless legs syndrome, fibromyalgia, and pituitary adenomas, and also correlates with an impairment of impulse control in these conditions (Table 1) [96].
Another drug associated with an increase in GD rates is the atypical antipsychotic aripiprazole [23, 97]. It targets dopamine and serotonin receptors, and is mainly prescribed in schizophrenia and bipolar disorder [98]. Even for this drug, an association with GD has been established in large-scale studies (Table 1). The proposed mechanism of action for aripiprazole is its stabilizing effect on dopamine levels, acting as a partial agonist. Similar to pramipexole and ropinirole, this drug has high affinity for the D2 and D3 receptor, and these receptors playing a role in the increase of addictive behavior seems likely. While most of the evidence of a role of aripiprazole in GD has relied on more anecdotal case presentations [23, 24, 99, 100], recent large-scale clinical and register studies have demonstrated a statistical association between aripiprazole medication and GD [101], even specifically within the sample of patients with psychotic disorders [102]. As mentioned previously, even serotonin could be involved in the pathophysiology of GD, and aripiprazole might additionally have an effect on impulse control through the serotonergic transmitter system [98]. This could also apply to the atypical antidepressant agomelatine, which acts as a melatonin agonist and a serotonin antagonist. Agomelatine has been described to increase GD, although it has also been proposed as a potential treatment for this disorder [103, 104], so more knowledge is needed regarding its role in relation to gambling.
Furthermore, other psychotropic drugs have been reported to have an effect on impulse control and GD. A common mechanism could be increasing dopamine levels through unselective monoamine reuptake inhibition. A study in rats showed increased impulsivity under treatment with a nonselective serotonin/noradrenaline reuptake inhibitor, which also inhibits dopamine reuptake, but not under the treatment with a serotonin-selective reuptake inhibitor [105]. Interestingly, the anti-attention deficit hyperactivity disorder (ADHD) drug atomoxetine has shown some beneficial effects in treating impulsivity and executive dysfunctions in patients with Parkinson’s disease [106,107,108]. This reuptake inhibitor is known to increase the levels of dopamine, noradrenaline, and possibly serotonin [109], and could be promising in treating impulse-control disorders like GD in patients with Parkinson’s disease in the future. Even the antidepressant venlafaxine, a nonselective monoamine reuptake inhibitor, has been associated with higher impulsivity in humans [110]. Paradoxically, venlafaxine, like agomelatine, also can be an effective treatment against impulse-control disorders [111]. Another example of a drug influencing GD is the stimulant modafinil, which is selective for dopamine reuptake inhibition and has been shown to increase reward seeking in patients with GD, but not in a control group [112]. Notably, amphetamines as dopamine reuptake inhibitors can prime gambling motivation and increase the striatal dopamine release in patients with GD more than in healthy controls [113, 114]. However, these results should be interpreted taking into considering that amphetamines are addictive substances themselves.
Another mechanism of action that could influence impulse control is D2-like receptor antagonism, including D2 and D3. The typical antipsychotic haloperidol, a D2 antagonist, enhanced reward effects and priming to gambling only in patients with GD and significantly changed the perception of slot-machine gambling in these patients in comparison with controls [115, 116]. How a dopamine antagonist could increase gambling behavior, rather than reduce it, remains unclear, given that reduced impulsivity and gambling behavior through dopamine antagonism have been proposed as a mechanism to find effective pharmacotherapy in GD. One of the few substances, the D2-like and serotonin receptor antagonist olanzapine, used as an atypical antipsychotic, has been tested as a treatment in patients with GD. One randomized, double-blind clinical trial involving 42 individuals with GD did not find a change in gambling behavior under olanzapine treatment compared to a placebo condition [117]. Neither the scores in the Pathological Gambling Adaptation of the Yale-Brown Obsessive Compulsive Scale, nor the amount of gambling episodes or hours gambled per week differed between the drug and placebo treatment. A high discontinuation rate occurred during the study, especially within the patient group treated with olanzapine (52%). Another randomized, double-blind clinical trial explored a similar study design in 21 individuals with problematic gambling involving video poker gambling [118]. Here, gambling did not change differently in patients treated with olanzapine compared with those taking a placebo either.
5 Patient Populations Exposed to Dopaminergic Medications: Patients at Risk of Problematic Gambling Patterns
Patient groups likely to receive a dopaminergic medication overlap with patient groups who may be at increased risk of developing gambling problems. Clinical and epidemiological review studies have suggested that patients with Parkinson’s disease may be at higher risk of poor mental health [119, 120]. Although a risk increase in GD specifically has not been extensively examined in Parkinson’s disease, comorbidity with mental health disorders may increase the risk of a secondary problematic gambling behavior, as in mental health disorders in general [8, 9, 25]. Additionally, it has been suggested that patients with Parkinson’s disease, through the disruption of their dopaminergic system, may be at higher risk of developing stereotypic and impulsive behavior, possibly even without the presence of a dopaminergic medication [121]. Thus, if patients with Parkinson’s disease are hypothesized to have an increased risk of maladaptive behaviors due to the disease itself, their exposure to dopaminergic medications may further increase their risk of GD.
Likewise, patients suffering from restless legs disorder may similarly be at higher risk of developing addictive behaviors, in particular through the suspected comorbidity with other mental health conditions [122], which in themselves are linked to an increased risk of GD [8, 9]. Fewer reports have focused on other conditions in which a dopamine agonist may be used, but such studies include the treatment with pramipexole in patients with depression [123] or fibromyalgia [124].
The clinical presentation of a GD induced by dopaminergic medications in patients with Parkinson’s disease may not necessarily differ from the one seen in other patients with GD, but patients are likely to be older, and they may have lower psychiatric comorbidity than corresponding patients with a different background than Parkinson’s disease [125]. Here, data are hitherto limited, and due to the relatively low absolute number of people with combined Parkinson’s disease and GD [125], extensive clinical and epidemiological datasets are required in order to fully highlight the characteristics in sub-groups of patients.
In addition to the suspected links between GD and dopaminergic medications in neurological diseases, mental health disorders where antipsychotic drugs are typically used are a risk factor for gambling problems [126], including schizophrenia and affective psychosis such as bipolar disorder with mania [8, 9]. Thus, patients exposed to dopamine-regulating antipsychotic drugs are likely to have a statistical risk increase of gambling problems due to their psychiatric comorbidities, over and above the potential medication effect and other socio-demographic parameters also leading to a statistically heightened risk of developing gambling problems.
Very little research has addressed whether specific gambling types are associated with an increased risk of addictive gambling in patients on dopaminergic medications. Also, studies describing gambling behaviors in patients on dopamine agonists have been conducted over many years and in many countries, such that gambling patterns of exposed individuals may have differed substantially. However, for problematic gambling in general, the increase in online gambling in recent years is likely to have caused a larger accessibility to gambling for people, avoiding the constraints and stigma associated with land-based gambling venues. Several features of online gambling confer a higher addiction potential than do physical gambling types; in addition to the accessibility, this also involves the high speed and repetitiveness of these gambling types [14]. Future studies should assess whether gambling patterns in GD patients on dopaminergic medications differ from those of other patients with GD, and whether specific gambling-type advice may need to be provided to patients at risk. In addition, the COVID-19 pandemic was suspected to influence the gambling market considerably, due to lifestyle changes and more time spent online and at home [127]. Whether or not this may have changed the risk of GD in patients on dopaminergic drugs is unknown, and longitudinal and large-scale studies to may be required to address this.
6 Clinical Guidelines and Treatment for GD in Patients on Dopaminergic Medications
Whenever gambling problems are believed to be caused by dopaminergic agonists, the treatment may likely involve the discontinuation, reduction, or adaptation of the medication. Otherwise, treatment may also involve the same treatment modalities as in other GD patients [128]. Treatment of a GD typically is delivered as a psycho-therapeutic intervention in the form of cognitive behavioral therapy. Less frequently, pharmaceutical treatment for GD is suggested, and the use of opioid antagonists (naltrexone or nalmefene) has been suggested as one part of GD treatment, although findings so far have been somewhat conflicting [129,130,131]. Other treatment strategies rely on the use of structured therapeutic interventions, including brief or more extensive motivational interventions [132, 133]. Other strategies, such as peer-support group sessions or other non-structured supportive interventions, are hitherto too sparsely documented in research [26, 27].
When a GD is suspected to be caused by a dopaminergic medication, based on a reasonable temporal association between the induction or dose increase of a dopamine agonist, decreased dosing or discontinuation of the drug is likely one of the first options chosen. This intervention may seem intuitive [15], and is likely effective in many cases. A key component of GD is the growing indebtedness caused by an increasing tolerance where gambling at initially low levels is perceived to be insufficient, and gradually replaced by a “chasing losses” behavior where gambling primarily occurs in order to try to win back money lost from gambling or money spent on interest payments from gambling-related borrowing [5,6,7]. Thus, financial support may be sought by the patient, and private financial counseling may be a key component in the management of severe GD. For patients developing a gambling problem subsequent to dopaminergic medications, in the absence of specific literature addressing the financial situation of these patient groups, typical GD counselling is also likely to be feasible and appropriate in this situation. Thus, while healthcare providers need to pay attention to this risk, screen for and diagnose gambling problems in the context of dopaminergic medications, the therapeutic guidelines may not necessarily differ from what is used in GD with other etiologies. Most likely, therapeutic methods applied in GD in general are not likely to be unfavorable in the present kind of situation.
Partly related to the financial consequences of a GD, couple therapy or other interventions addressing both the patient and the patient’s concerned significant others [134] may be of importance. In patients developing an impulse control disorder based on a dopaminergic medication, such family interventions may also be particularly needed, given the often dramatic personality changes and sometimes rapidly evolving gambling problem seen in these conditions. Thus, a number of interventions typically aimed at patients with GD in general may be of importance here, and may need to be integrated with the treatment setting in which the patient originally received the dopamine medication. Also, although the problem may have developed in a highly medical context, i.e., in the context of the dopaminergic therapy, treatment and support for the patient and the patient’s family need to be integrated and involve professionals outside of the medical sector, such as social and financial counsellors.
In the prevention of GD assumed to be caused by dopamine agonist use, psycho-educative and information approaches are already recommended in the early stages of the medication [135]. This will involve clinicians working outside the settings where behavioral addictions are typically assessed, and, therefore, this may require structured questionnaires and checklists. It has been recommended that such early risk information may not necessarily need to be the same for all patients initiated on dopamine agonists, but should be more strongly recommended in patients who display certain underlying risk factors, such as poor mental health and previous addictive behaviors [135].
Treatment is rarely reported specifically for populations where the gambling behavior is believed to be caused by dopaminergic medications. A smaller treatment study suggested, however, that treatment results of cognitive behavioral therapy (CBT) were not less successful in patients with a dopaminergic medication than in other patients with GD [128]. However, other potential interventions, such as those involving motivational or brief feedback interventions, have not been tested specifically in patients receiving dopamine agonists. Deep brain stimulation, primarily aimed at the treatment of underlying Parkinson’s disease, even has been proposed as a possible means to affect the gambling behavior assumed to have arisen from a dopaminergic medication [135, 136]. But such a potential role of surgical treatment needs to be studied further and this type of research likely requires larger study samples where a substantial sub-proportion of patients have both Parkinson’s disease and a subsequent gambling problem. In addition, results from the samples hitherto reported in the literature have been mixed, with both positive and negative findings and even suspected cases of impulse control problems arising from that intervention as such [89]. Likewise, opioid antagonists, likely the group of medications most commonly recommended for the treatment of GD, has been tested in impulse control disorders, and demonstrated promising effects in one trial [137]. Amantadine has provided conflicting findings that hitherto do not provide support for this strategy in that group specifically [89].
Little data exist on the efficacy of psycho-therapeutic interventions in GD arising from dopamine agonist treatment. One study demonstrated clearly promising effects of CBT, but in a study of limited size and in comparison to a waiting list condition [138]. Thus, few studies can guide specific therapeutic treatment in this patient group, but in the absence of data indicating that these interventions will be deleterious in a GD believed to be caused by dopamine agonists, there is reason to implement standard evidence-based GD treatment for patients with this type of etiology too. Although poorly researched so far, it could be assumed that over and above the core features of a GD, if a GD arises during dopamine agonist treatment, it may potentially be more complicated to treat than otherwise. Except for the one limited-sample treatment study cited above [128], this has not been tested. Meanwhile, therapies typically offered for GD may in some cases present challenges in the clinical setting in the patient groups addressed here; GD patients with a gambling pattern arising from a dopaminergic medication are likely older [125] compared to other GD patients. In many settings, GD therapy is typically offered in a group therapy format, both regarding CBT [139] and self-help peer support groups often offering group interventions [140]. Thus, offering GD treatment in a group format to patients with or without a dopaminergic medication-related etiology may lead to mixed groups at least with respect to age. It is unclear whether this has an effect on treatment efficacy in these groups, but the age difference, and possibly the differences in physical and psychiatric comorbidities, may present a challenge.
Other treatment strategies in GD may involve a plethora of structured interventions, brief motivational interventions [132], self-help tools, or financial or legal measures hindering the gambling in affected individuals. It has been described that treatment-seeking—or rather help-seeking—behavior in GD patients may be highly diverse [26]. One measure often taken by patients with a GD is voluntary self-exclusion, where an individual opts for a technical barrier against gambling on one specific gambling site, an internet gambling site, or on a number of operators within a jurisdiction [141, 142]. Emerging gambling problems may also be prevented by the use of motivational techniques, such as personalized normative feedback interventions.
Thus, altogether there is a lack of evidence of specific treatment strategies for a GD developed in the context of a dopaminergic medication. However, in the absence of evidence pointing to other directions, the clinical management of a dopamine agonist-induced gambling behavior may include the reduction or discontinuation of the dopaminergic medication, and may also include structured GD treatment as suggested for other patients with GD, i.e., primarily using a CBT approach.
7 Conclusion
Since the neurobiology of GD is only partly understood, the mechanism of how dopaminergic and serotonergic drugs can increase addictive behavior is still subject to speculation. Given the clear association in large study populations and many indicators from molecular research, a causal relationship is likely but has not been proven yet. Studies in animal models, where increased gambling behavior can be linked directly to drug exposure, ideally in a dose-dependent manner, would be one suitable approach to prove causality. Even if there are limitations to studying human behavior in animals, a couple of well-established tests can assess impulsivity and risk-taking behavior in rodents [59, 143].
Some drugs have an effect on gambling behavior only in patients with GD, not in the control group. In line with these findings, preclinical studies have shown that dopamine agonists increase already manifested substance addictions but will not create new addictions [144]. In general, dopaminergics seem to enhance the reinforcement of addictive behaviors especially when the habit is already formed. This could be crucial information for eventually discovering the molecular mechanism of dopaminergic medication impairing impulse control, perhaps dependent on D2 and D3 receptors in the nucleus accumbens.
The emerging knowledge in the area calls for further research in preclinical, clinical, and epidemiological study designs. In the clinical setting, treatment studies are needed in order to better outline the clinical presentation and the challenges involving other comorbidities. Likewise, evidence for the regulation of dopaminergic medications and the application of more gambling-specific pharmaceutical and psycho-therapeutic treatment strategies is hitherto limited, and this calls for treatment studies specifically in these groups of patients.
References
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 5th edn. 2013
World Health Organization. 6C50 Gambling Disorder. In: ICD-11 for Mortality and Morbidity Statistics (Version: 09/2020). World Health Organization, 2020 https://icd.who.int/browse11/l-m/en#/http%3a%2f%2fid.who.int%2ficd%2fentity%2f1041487064.
Calado F, Griffiths MD. Problem gambling worldwide: an update and systematic review of empirical research (2000–2015). J Behav Addict. 2016;5(4):592–613. https://doi.org/10.1556/2006.5.2016.073.
Potenza MN, Balodis IM, Derevensky J, Grant JE, Petry NM, Verdejo-Garcia A, et al. Gambling disorder. Nat Rev Dis Primers. 2019;5(1):51. https://doi.org/10.1038/s41572-019-0099-7.
Oksanen A, Savolainen I, Sirola A, Kaakinen M. Problem gambling and psychological distress: a cross-national perspective on the mediating effect of consumer debt and debt problems among emerging adults. Harm Reduct J. 2018;15(1):45. https://doi.org/10.1186/s12954-018-0251-9.
Håkansson A, Widinghoff C. Over-indebtedness and problem gambling in a general population sample of online gamblers. Front Psych. 2020;11:7. https://doi.org/10.3389/fpsyt.2020.00007.
Muggleton N, Parpart P, Newall P, Leake D, Gathergood J, Stewart N. The association between gambling and financial, social and health outcomes in big financial data. Nat Hum Behav. 2021;5(3):319–26. https://doi.org/10.1038/s41562-020-01045-w.
Dowling NA, Cowlishaw S, Jackson AC, Merkouris SS, Francis KL, Christensen DR. Prevalence of psychiatric co-morbidity in treatment-seeking problem gamblers: a systematic review and meta-analysis. Aust N Z J Psychiatry. 2015;49(6):519–39. https://doi.org/10.1177/0004867415575774.
Lorains FK, Cowlishaw S, Thomas SA. Prevalence of comorbid disorders in problem and pathological gambling: systematic review and meta-analysis of population surveys. Addiction (Abingdon, England). 2011;106(3):490–8. https://doi.org/10.1111/j.1360-0443.2010.03300.x.
Black DW, Shaw M, McCormick B, Allen J. Pathological gambling: relationship to obesity, self-reported chronic medical conditions, poor lifestyle choices, and impaired quality of life. Compr Psychiatry. 2013;54:97–104. https://doi.org/10.1016/j.comppsych.2012.07.001.
Kayser A. Dopamine and gambling disorder: prospects for personalized treatment. Curr Addict Rep. 2019;6(2):65–74. https://doi.org/10.1007/s40429-019-00240-8.
Zack M, St George R, Clark L. Dopaminergic signaling of uncertainty and the aetiology of gambling addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2020;99: 109853. https://doi.org/10.1016/j.pnpbp.2019.109853.
Gainsbury SM, Suhonen N, Saastamoinen J. Chasing losses in online poker and casino games: characteristics and game play of Internet gamblers at risk of disordered gambling. Psychiatry Res. 2014;217(3):220–5. https://doi.org/10.1016/j.psychres.2014.03.033.
Chóliz M. The challenge of online gambling: the effect of legalization on the increase in online gambling addiction. J Gambl Stud. 2016;32(2):749–56. https://doi.org/10.1007/s10899-015-9558-6.
Wong SH, Steiger MJ. Pathological gambling in Parkinson’s disease. BMJ. 2007;334(7598):810–1. https://doi.org/10.1136/bmj.39176.363958.80.
Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589–95. https://doi.org/10.1001/archneurol.2010.65.
Dagher A, Robbins TW. Personality, addiction, dopamine: insights from Parkinson’s disease. Neuron. 2009;61(4):502–10. https://doi.org/10.1016/j.neuron.2009.01.031.
Ronzitti S, Soldini E, Smith N, Potenza MN, Clerici M, Bowden-Jones H. Current suicidal ideation in treatment-seeking individuals in the United Kingdom with gambling problems. Addict Behav. 2017;74:33–40. https://doi.org/10.1016/j.addbeh.2017.05.032.
Karlsson A, Håkansson A. Gambling disorder, increased mortality, suicidality, and associated comorbidity: a longitudinal nationwide register study. J Behav Addict. 2018;7(4):1091–9. https://doi.org/10.1556/2006.7.2018.112.
Rojas Y. Financial indebtedness and suicide: a 1-year follow-up study of a population registered at the Swedish Enforcement Authority. Int J Soc Psychiatry. 2021. https://doi.org/10.1177/00207640211036166.
Ford M, Håkansson A. Problem gambling, associations with comorbid health conditions, substance use, and behavioural addictions: Opportunities for pathways to treatment. PLoS ONE. 2020;15(1): e0227644. https://doi.org/10.1371/journal.pone.0227644.
Haydock M, Cowlishaw S, Harvey C, Castle D. Prevalence and correlates of problem gambling in people with psychotic disorders. Compr Psychiatry. 2015;58:122–9. https://doi.org/10.1016/j.comppsych.2015.01.003.
Grall-Bronnec M, Sauvaget A, Perrouin F, Leboucher J, Etcheverrigaray F, Challet-Bouju G, et al. Pathological gambling associated with aripiprazole or dopamine replacement therapy: do patients share the same features? A review. J Clin Psychopharmacol. 2016;36(1):63–70. https://doi.org/10.1097/jcp.0000000000000444.
Lachance A, Corbeil O, Corbeil S, Chalifour G, Breault AS, Roy MA, et al. Case reports of aripiprazole and problematic gambling in schizophrenia: a critical review of the evidence. J Clin Psychopharmacol. 2019;39(4):393–7. https://doi.org/10.1097/jcp.0000000000001068.
Sundqvist K, Rosendahl I. Problem gambling and psychiatric comorbidity-risk and temporal sequencing among women and men: results from the Swelogs case-control study. J Gambl Stud. 2019;35(3):757–71. https://doi.org/10.1007/s10899-019-09851-2.
Rodda SN, Dowling NA, Lubman DI. Gamblers seeking online help are active help-seekers: time to support autonomy and competence. Addict Behav. 2018;87:272–5. https://doi.org/10.1016/j.addbeh.2018.06.001.
Gainsbury S, Hing N, Suhonen N. Professional help-seeking for gambling problems: awareness, barriers and motivators for treatment. J Gambl Stud. 2014;30(2):503–19. https://doi.org/10.1007/s10899-013-9373-x.
Slutske WS, Piasecki TM, Deutsch AR, Statham DJ, Martin NG. Telescoping and gender differences in the time course of disordered gambling: evidence from a general population sample. Addiction (Abingdon, England). 2015;110(1):144–51. https://doi.org/10.1111/add.12717.
McCarthy S, Thomas SL, Randle M, Bestman A, Pitt H, Cowlishaw S, et al. Women’s gambling behaviour, product preferences, and perceptions of product harm: differences by age and gambling risk status. Harm Reduct J. 2018;15(1):22. https://doi.org/10.1186/s12954-018-0227-9.
Tavares H, Martins SS, Lobo DS, Silveira CM, Gentil V, Hodgins DC. Factors at play in faster progression for female pathological gamblers: an exploratory analysis. J Clin Psychiatry. 2003;64(4):433–8. https://doi.org/10.4088/jcp.v64n0413.
Zakiniaeiz Y, Cosgrove KP, Mazure CM, Potenza MN. Does telescoping exist in male and female gamblers? does it matter? Front Psychol. 2017;8:1510. https://doi.org/10.3389/fpsyg.2017.01510.
Grant JE, Chamberlain SR. Expanding the definition of addiction: DSM-5 vs. ICD-11. CNS Spectr. 2016;21(4):300–3. https://doi.org/10.1017/s1092852916000183.
Grant JE, Chamberlain SR. Gambling disorder and its relationship with substance use disorders: implications for nosological revisions and treatment. Am J Addict. 2015;24(2):126–31. https://doi.org/10.1111/ajad.12112.
Victorri-Vigneau C, Spiers A, Caillet P, Bruneau M, Ignace C, Challet-Bouju G, et al. Opioid antagonists for pharmacological treatment of gambling disorder: are they relevant? Curr Neuropharmacol. 2018;16(10):1418–32. https://doi.org/10.2174/1570159x15666170718144058.
Balodis IM, Potenza MN. Common neurobiological and psychological underpinnings of gambling and substance-use disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2020;99: 109847. https://doi.org/10.1016/j.pnpbp.2019.109847.
Wise RA, Jordan CJ. Dopamine, behavior, and addiction. J Biomed Sci. 2021;28(1):83. https://doi.org/10.1186/s12929-021-00779-7.
Galaj E, Ranaldi R. Neurobiology of reward-related learning. Neurosci Biobehav Rev. 2021;124:224–34. https://doi.org/10.1016/j.neubiorev.2021.02.007.
Heinz A, Beck A, Halil MG, Pilhatsch M, Smolka MN, Liu S. Addiction as learned behavior patterns. J Clin Med. 2019. https://doi.org/10.3390/jcm8081086.
Berlin GS, Hollander E. Compulsivity, impulsivity, and the DSM-5 process. CNS Spectr. 2014;19(1):62–8. https://doi.org/10.1017/s1092852913000722.
Ciccarelli M, Malinconico R, Griffiths MD, Nigro G, Cosenza M. Reward preferences of pathological gamblers under conditions of uncertainty: an experimental study. J Gambl Stud. 2016;32(4):1175–89. https://doi.org/10.1007/s10899-016-9593-y.
Ciccarelli M, Griffiths MD, Cosenza M, Nigro G, D’Olimpio F. Disordered gambling and attentional bias: the mediating role of risk-taking. J Affect Disord. 2020;272:496–500. https://doi.org/10.1016/j.jad.2020.03.144.
Cosenza M, Griffiths MD, Nigro G, Ciccarelli M. Risk-taking, delay discounting, and time perspective in adolescent gamblers: an experimental study. J Gambl Stud. 2017;33(2):383–95. https://doi.org/10.1007/s10899-016-9623-9.
Mishra S, Lalumière ML, Williams RJ. Gambling, risk-taking, and antisocial behavior: a replication study supporting the generality of deviance. J Gambl Stud. 2017;33(1):15–36. https://doi.org/10.1007/s10899-016-9608-8.
Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: emerging evidence. Pharmacol Ther. 2012;134(3):287–97. https://doi.org/10.1016/j.pharmthera.2012.02.004.
Weinsztok S, Brassard S, Balodis I, Martin LE, Amlung M. Delay discounting in established and proposed behavioral addictions: a systematic review and meta-analysis. Front Behav Neurosci. 2021;15: 786358. https://doi.org/10.3389/fnbeh.2021.786358.
Hønsi A, Mentzoni RA, Molde H, Pallesen S. Attentional bias in problem gambling: a systematic review. J Gambl Stud. 2013;29(3):359–75. https://doi.org/10.1007/s10899-012-9315-z.
Anselme P, Robinson MJF. From sign-tracking to attentional bias: Implications for gambling and substance use disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2020;99: 109861. https://doi.org/10.1016/j.pnpbp.2020.109861.
Boffo M, Smits R, Salmon JP, Cowie ME, de Jong DTHA, Salemink E, et al. Luck, come here! Automatic approach tendencies toward gambling cues in moderate- to high-risk gamblers. Addiction (Abingdon, England). 2018;113(2):289–98. https://doi.org/10.1111/add.14071.
Hudson A, Olatunji BO, Gough K, Yi S, Stewart SH. Eye on the prize: high-risk gamblers show sustained selective attention to gambling cues. J Gambling Issues. 2016;34:100–19. https://doi.org/10.4309/jgi.2016.34.6.
Barton KR, Yazdani A, Ayer N, Kalvapalle S, Brown S, Stapleton J, et al. The effect of losses disguised as wins and near misses in electronic gaming machines: a systematic review. J Gambl Stud. 2017;33(4):1241–60. https://doi.org/10.1007/s10899-017-9688-0.
Potenza MN. Neurobiology of gambling behaviors. Curr Opin Neurobiol. 2013;23(4):660–7. https://doi.org/10.1016/j.conb.2013.03.004.
Klein MO, Battagello DS, Cardoso AR, Hauser DN, Bittencourt JC, Correa RG. Dopamine: functions, signaling, and association with neurological diseases. Cell Mol Neurobiol. 2019;39(1):31–59. https://doi.org/10.1007/s10571-018-0632-3.
Steeves TD, Miyasaki J, Zurowski M, Lang AE, Pellecchia G, Van Eimeren T, et al. Increased striatal dopamine release in Parkinsonian patients with pathological gambling: a [11C] raclopride PET study. Brain J Neurol. 2009;132(Pt 5):1376–85. https://doi.org/10.1093/brain/awp054.
Linnet J, Mouridsen K, Peterson E, Møller A, Doudet DJ, Gjedde A. Striatal dopamine release codes uncertainty in pathological gambling. Psychiatry Res. 2012;204(1):55–60. https://doi.org/10.1016/j.pscychresns.2012.04.012.
Clark L, Boileau I, Zack M. Neuroimaging of reward mechanisms in Gambling disorder: an integrative review. Mol Psychiatry. 2019;24(5):674–93. https://doi.org/10.1038/s41380-018-0230-2.
Jones DT, Graff-Radford J. Executive dysfunction and the prefrontal cortex. Continuum (Minneap Minn). 2021;27(6):1586–601. https://doi.org/10.1212/con.0000000000001009.
Roussakis AA, Lao-Kaim NP, Piccini P. Brain imaging and impulse control disorders in Parkinson’s disease. Curr Neurol Neurosci Rep. 2019;19(9):67. https://doi.org/10.1007/s11910-019-0980-5.
Cenci MA, Francardo V, O’Sullivan SS, Lindgren HS. Rodent models of impulsive-compulsive behaviors in Parkinson’s disease: how far have we reached? Neurobiol Dis. 2015;82:561–73. https://doi.org/10.1016/j.nbd.2015.08.026.
Winstanley CA, Clark L. Translational models of gambling-related decision-making. Curr Top Behav Neurosci. 2016;28:93–120. https://doi.org/10.1007/7854_2015_5014.
Petersen K, Van Wouwe N, Stark A, Lin YC, Kang H, Trujillo-Diaz P, et al. Ventral striatal network connectivity reflects reward learning and behavior in patients with Parkinson’s disease. Hum Brain Mapp. 2018;39(1):509–21. https://doi.org/10.1002/hbm.23860.
Mosley PE, Paliwal S, Robinson K, Coyne T, Silburn P, Tittgemeyer M, et al. The structural connectivity of discrete networks underlies impulsivity and gambling in Parkinson’s disease. Brain J Neurol. 2019;142(12):3917–35. https://doi.org/10.1093/brain/awz327.
Tahmasian M, Rochhausen L, Maier F, Williamson KL, Drzezga A, Timmermann L, et al. Impulsivity is associated with increased metabolism in the fronto-insular network in Parkinson’s disease. Front Behav Neurosci. 2015;9:317. https://doi.org/10.3389/fnbeh.2015.00317.
Schmidt C, Gleesborg C, Schmidt H, Kvamme TL, Lund TE, Voon V, et al. A bias towards natural rewards away from gambling cues in gamblers undergoing active treatment. Brain Res. 2021;1764: 147479. https://doi.org/10.1016/j.brainres.2021.147479.
Dardou D, Reyrolle L, Chassain C, Durif F. Chronic pramipexole treatment induces compulsive behavior in rats with 6-OHDA lesions of the substantia nigra and ventral tegmental area. Behav Brain Res. 2017;332:327–36. https://doi.org/10.1016/j.bbr.2017.06.016.
Filip P, Linhartová P, Hlavatá P, Šumec R, Baláž M, Bareš M, et al. Disruption of multiple distinctive neural networks associated with impulse control disorder in Parkinson’s disease. Front Hum Neurosci. 2018;12:462. https://doi.org/10.3389/fnhum.2018.00462.
Hammes J, Theis H, Giehl K, Hoenig MC, Greuel A, Tittgemeyer M, et al. Dopamine metabolism of the nucleus accumbens and fronto-striatal connectivity modulate impulse control. Brain J Neurol. 2019;142(3):733–43. https://doi.org/10.1093/brain/awz007.
Rao H, Mamikonyan E, Detre JA, Siderowf AD, Stern MB, Potenza MN, et al. Decreased ventral striatal activity with impulse control disorders in Parkinson’s disease. Mov Disord. 2010;25(11):1660–9. https://doi.org/10.1002/mds.23147.
van Eimeren T, Pellecchia G, Cilia R, Ballanger B, Steeves TD, Houle S, et al. Drug-induced deactivation of inhibitory networks predicts pathological gambling in PD. Neurology. 2010;75(19):1711–6. https://doi.org/10.1212/WNL.0b013e3181fc27fa.
Markovic V, Agosta F, Canu E, Inuggi A, Petrovic I, Stankovic I, et al. Role of habenula and amygdala dysfunction in Parkinson disease patients with punding. Neurology. 2017;88(23):2207–15. https://doi.org/10.1212/wnl.0000000000004012.
Potenza MN. Searching for replicable dopamine-related findings in gambling disorder. Biol Psychiat. 2018;83(12):984–6. https://doi.org/10.1016/j.biopsych.2018.04.011.
Elsinga PH, Hatano K, Ishiwata K. PET tracers for imaging of the dopaminergic system. Curr Med Chem. 2006;13(18):2139–53. https://doi.org/10.2174/092986706777935258.
Akdemir Ü, Bora Tokçaer A, Atay L. Dopamine transporter SPECT imaging in Parkinson’s disease and parkinsonian disorders. Turk J Med Sci. 2021;51(2):400–10. https://doi.org/10.3906/sag-2008-253.
van Holst RJ, Sescousse G, Janssen LK, Janssen M, Berry AS, Jagust WJ, et al. Increased striatal dopamine synthesis capacity in gambling addiction. Biol Psychiat. 2018;83(12):1036–43. https://doi.org/10.1016/j.biopsych.2017.06.010.
O’Sullivan SS, Wu K, Politis M, Lawrence AD, Evans AH, Bose SK, et al. Cue-induced striatal dopamine release in Parkinson’s disease-associated impulsive-compulsive behaviours. Brain J Neurol. 2011;134(Pt 4):969–78. https://doi.org/10.1093/brain/awr003.
Wu K, Politis M, O’Sullivan SS, Lawrence AD, Warsi S, Bose S, et al. Single versus multiple impulse control disorders in Parkinson’s disease: an 11C-raclopride positron emission tomography study of reward cue-evoked striatal dopamine release. J Neurol. 2015;262(6):1504–14. https://doi.org/10.1007/s00415-015-7722-7.
Linnet J, Møller A, Peterson E, Gjedde A, Doudet D. Inverse association between dopaminergic neurotransmission and Iowa Gambling Task performance in pathological gamblers and healthy controls. Scand J Psychol. 2011;52(1):28–34. https://doi.org/10.1111/j.1467-9450.2010.00837.x.
Linnet J, Møller A, Peterson E, Gjedde A, Doudet D. Dopamine release in ventral striatum during Iowa Gambling Task performance is associated with increased excitement levels in pathological gambling. Addiction (Abingdon, England). 2011;106(2):383–90. https://doi.org/10.1111/j.1360-0443.2010.03126.x.
Joutsa J, Johansson J, Niemelä S, Ollikainen A, Hirvonen MM, Piepponen P, et al. Mesolimbic dopamine release is linked to symptom severity in pathological gambling. Neuroimage. 2012;60(4):1992–9. https://doi.org/10.1016/j.neuroimage.2012.02.006.
Clark L, Stokes PR, Wu K, Michalczuk R, Benecke A, Watson BJ, et al. Striatal dopamine D2/D3 receptor binding in pathological gambling is correlated with mood-related impulsivity. Neuroimage. 2012;63(1):40–6. https://doi.org/10.1016/j.neuroimage.2012.06.067.
Boileau I, Payer D, Chugani B, Lobo D, Behzadi A, Rusjan PM, et al. The D2/3 dopamine receptor in pathological gambling: a positron emission tomography study with [11C]-(+)-propyl-hexahydro-naphtho-oxazin and [11C]raclopride. Addiction (Abingdon, England). 2013;108(5):953–63. https://doi.org/10.1111/add.12066.
Payer DE, Guttman M, Kish SJ, Tong J, Strafella A, Zack M, et al. [11C]-(+)-PHNO PET imaging of dopamine D(2/3) receptors in Parkinson’s disease with impulse control disorders. Mov Disord. 2015;30(2):160–6. https://doi.org/10.1002/mds.26135.
Ray NJ, Miyasaki JM, Zurowski M, Ko JH, Cho SS, Pellecchia G, et al. Extrastriatal dopaminergic abnormalities of DA homeostasis in Parkinson’s patients with medication-induced pathological gambling: a [11C] FLB-457 and PET study. Neurobiol Dis. 2012;48(3):519–25. https://doi.org/10.1016/j.nbd.2012.06.021.
Stark AJ, Smith CT, Lin YC, Petersen KJ, Trujillo P, van Wouwe NC, et al. Nigrostriatal and mesolimbic D(2/3) receptor expression in Parkinson’s disease patients with compulsive reward-driven behaviors. J Neurosci. 2018;38(13):3230–9. https://doi.org/10.1523/jneurosci.3082-17.2018.
Joutsa J, Voon V, Johansson J, Niemelä S, Bergman J, Kaasinen V. Dopaminergic function and intertemporal choice. Transl Psychiatry. 2015;5(1): e491. https://doi.org/10.1038/tp.2014.133.
Vriend C, Nordbeck AH, Booij J, van der Werf YD, Pattij T, Voorn P, et al. Reduced dopamine transporter binding predates impulse control disorders in Parkinson’s disease. Mov Disord. 2014;29(7):904–11. https://doi.org/10.1002/mds.25886.
Voon V, Rizos A, Chakravartty R, Mulholland N, Robinson S, Howell NA, et al. Impulse control disorders in Parkinson’s disease: decreased striatal dopamine transporter levels. J Neurol Neurosurg Psychiatry. 2014;85(2):148–52. https://doi.org/10.1136/jnnp-2013-305395.
Lee JY, Seo SH, Kim YK, Yoo HB, Kim YE, Song IC, et al. Extrastriatal dopaminergic changes in Parkinson’s disease patients with impulse control disorders. J Neurol Neurosurg Psychiatry. 2014;85(1):23–30. https://doi.org/10.1136/jnnp-2013-305549.
Smith KM, Xie SX, Weintraub D. Incident impulse control disorder symptoms and dopamine transporter imaging in Parkinson disease. J Neurol Neurosurg Psychiatry. 2016;87(8):864–70. https://doi.org/10.1136/jnnp-2015-311827.
Weintraub D, Mamikonyan E. Impulse control disorders in Parkinson’s disease. Am J Psychiatry. 2019;176(1):5–11. https://doi.org/10.1176/appi.ajp.2018.18040465.
Cilia R, van Eimeren T. Impulse control disorders in Parkinson’s disease: seeking a roadmap toward a better understanding. Brain Struct Funct. 2011;216(4):289–99. https://doi.org/10.1007/s00429-011-0314-0.
Lee JY, Kim JM, Kim JW, Cho J, Lee WY, Kim HJ, et al. Association between the dose of dopaminergic medication and the behavioral disturbances in Parkinson disease. Parkinsonism Relat Disord. 2010;16(3):202–7. https://doi.org/10.1016/j.parkreldis.2009.12.002.
Piercey MF. Pharmacology of pramipexole, a dopamine D3-preferring agonist useful in treating Parkinson’s disease. Clin Neuropharmacol. 1998;21(3):141–51.
Jost WH, Angersbach D. Ropinirole, a non-ergoline dopamine agonist. CNS Drug Rev. 2005;11(3):253–72. https://doi.org/10.1111/j.1527-3458.2005.tb00046.x.
Seeman P. Parkinson’s disease treatment may cause impulse-control disorder via dopamine D3 receptors. Synapse (New York, NY). 2015;69(4):183–9. https://doi.org/10.1002/syn.21805.
Zengin-Toktas Y, Authier N, Denizot H, Chassain C, Hafidi A, Llorca PM, et al. Motivational properties of D2 and D3 dopamine receptors agonists and cocaine, but not with D1 dopamine receptors agonist and L-dopa, in bilateral 6-OHDA-lesioned rat. Neuropharmacology. 2013;70:74–82. https://doi.org/10.1016/j.neuropharm.2012.12.011.
Martinkova J, Trejbalova L, Sasikova M, Benetin J, Valkovic P. Impulse control disorders associated with dopaminergic medication in patients with pituitary adenomas. Clin Neuropharmacol. 2011;34(5):179–81. https://doi.org/10.1097/WNF.0b013e3182281b2f.
U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA warns about new impulse-control problems associated with mental health drug aripiprazole (Abilify, Abilify Maintena, Aristada). U.S. Food and Drug Administration, 2016. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-about-new-impulse-control-problems-associated-mental-health.
Tuplin EW, Holahan MR. Aripiprazole, a drug that displays partial agonism and functional selectivity. Curr Neuropharmacol. 2017;15(8):1192–207. https://doi.org/10.2174/1570159x15666170413115754.
Chen HY, Ma CH, Liu CC. Two cases of de novo pathological gambling associated with aripiprazole. Clin Neuropharmacol. 2019;42(3):101–2. https://doi.org/10.1097/wnf.0000000000000342.
Ceylan M, Evrensel A, Ünsalver B, Cömert G. Pathological gambling due to aripiprazole: two cases. West Indian Med J. 2015. https://doi.org/10.7727/wimj.2015.107.
Etminan M, Sodhi M, Samii A, Procyshyn RM, Guo M, Carleton BC. Risk of gambling disorder and impulse control disorder with aripiprazole, pramipexole, and ropinirole: a pharmacoepidemiologic study. J Clin Psychopharmacol. 2017;37(1):102–4. https://doi.org/10.1097/jcp.0000000000000634.
Wolfschlag M, Håkansson A. Increased risk for developing gambling disorder under the treatment with pramipexole, ropinirole, and aripiprazole: a nationwide register study in Sweden. PLoS ONE. 2021;16(6): e0252516. https://doi.org/10.1371/journal.pone.0252516.
Menon R, Cribb L, Sarris J, Ng CH. A case of acute problem gambling associated with agomelatine. J Clin Psychopharmacol. 2018;38(2):153–5. https://doi.org/10.1097/jcp.0000000000000846.
Egorov AY. The use of agomelatine (valdoxan) in gambling therapy: a pilot study. Zh Nevrol Psikhiatr Im S S Korsakova. 2015;115(9):28–31. https://doi.org/10.17116/jnevro20151159128-31.
Tsutsui-Kimura I, Ohmura Y, Izumi T, Yamaguchi T, Yoshida T, Yoshioka M. The effects of serotonin and/or noradrenaline reuptake inhibitors on impulsive-like action assessed by the three-choice serial reaction time task: a simple and valid model of impulsive action using rats. Behav Pharmacol. 2009;20(5–6):474–83. https://doi.org/10.1097/FBP.0b013e3283305e65.
Ye Z, Altena E, Nombela C, Housden CR, Maxwell H, Rittman T, et al. Improving response inhibition in Parkinson’s disease with atomoxetine. Biol Psychiatry. 2015;77(8):740–8. https://doi.org/10.1016/j.biopsych.2014.01.024.
Rae CL, Nombela C, Rodríguez PV, Ye Z, Hughes LE, Jones PS, et al. Atomoxetine restores the response inhibition network in Parkinson’s disease. Brain J Neurol. 2016;139(8):2235–48. https://doi.org/10.1093/brain/aww138.
Hinson VK, Delambo A, Elm J, Turner T. A randomized clinical trial of atomoxetine for mild cognitive impairment in Parkinson’s disease. Mov Disord Clin Pract. 2017;4(3):416–23. https://doi.org/10.1002/mdc3.12455.
Ding YS, Naganawa M, Gallezot JD, Nabulsi N, Lin SF, Ropchan J, et al. Clinical doses of atomoxetine significantly occupy both norepinephrine and serotonin transports: implications on treatment of depression and ADHD. Neuroimage. 2014;86:164–71. https://doi.org/10.1016/j.neuroimage.2013.08.001.
Sakurada K, Nibuya M, Yamada K, Nakagawa S, Suzuki E. Kleptomania induced by venlafaxine. Case Rep Psychiatry. 2021;2021:8470045. https://doi.org/10.1155/2021/8470045.
Camardese G, Picello A, Bria P. Venlafaxine: successful treatment in impulsive disorders. Psychiatry Clin Neurosci. 2008;62(2):241–2. https://doi.org/10.1111/j.1440-1819.2008.01763.x.
Smart K, Desmond RC, Poulos CX, Zack M. Modafinil increases reward salience in a slot machine game in low and high impulsivity pathological gamblers. Neuropharmacology. 2013;73:66–74. https://doi.org/10.1016/j.neuropharm.2013.05.015.
Zack M, Poulos CX. Amphetamine primes motivation to gamble and gambling-related semantic networks in problem gamblers. Neuropsychopharmacology. 2004;29(1):195–207. https://doi.org/10.1038/sj.npp.1300333.
Boileau I, Payer D, Chugani B, Lobo DS, Houle S, Wilson AA, et al. In vivo evidence for greater amphetamine-induced dopamine release in pathological gambling: a positron emission tomography study with [(11)C]-(+)-PHNO. Mol Psychiatry. 2014;19(12):1305–13. https://doi.org/10.1038/mp.2013.163.
Zack M, Poulos CX. A D2 antagonist enhances the rewarding and priming effects of a gambling episode in pathological gamblers. Neuropsychopharmacology. 2007;32(8):1678–86. https://doi.org/10.1038/sj.npp.1301295.
Tremblay AM, Desmond RC, Poulos CX, Zack M. Haloperidol modifies instrumental aspects of slot machine gambling in pathological gamblers and healthy controls. Addict Biol. 2011;16(3):467–84. https://doi.org/10.1111/j.1369-1600.2010.00208.x.
Fong T, Kalechstein A, Bernhard B, Rosenthal R, Rugle L. A double-blind, placebo-controlled trial of olanzapine for the treatment of video poker pathological gamblers. Pharmacol Biochem Behav. 2008;89(3):298–303. https://doi.org/10.1016/j.pbb.2007.12.025.
McElroy SL, Nelson EB, Welge JA, Kaehler L, Keck PE Jr. Olanzapine in the treatment of pathological gambling: a negative randomized placebo-controlled trial. J Clin Psychiatry. 2008;69(3):433–40. https://doi.org/10.4088/jcp.v69n0314.
Weintraub D, Mamikonyan E. The neuropsychiatry of parkinson disease: a perfect storm. Am J Geriatr Psychiatry. 2019;27(9):998–1018. https://doi.org/10.1016/j.jagp.2019.03.002.
Ravina B, Camicioli R, Como PG, Marsh L, Jankovic J, Weintraub D, et al. The impact of depressive symptoms in early Parkinson disease. Neurology. 2007;69(4):342–7. https://doi.org/10.1212/01.wnl.0000268695.63392.10.
Weintraub D. Impulse control disorders in Parkinson’s disease: a 20-year odyssey. Mov Disord. 2019;34(4):447–52. https://doi.org/10.1002/mds.27668.
Tully PJ, Kurth T, Elbaz A, Tzourio C. Convergence of psychiatric symptoms and restless legs syndrome: a cross-sectional study in an elderly French population. J Psychosom Res. 2020;128: 109884. https://doi.org/10.1016/j.jpsychores.2019.109884.
Aiken CB. Pramipexole in psychiatry: a systematic review of the literature. J Clin Psychiatry. 2007;68(8):1230–6.
Holman AJ. Impulse control disorder behaviors associated with pramipexole used to treat fibromyalgia. J Gambl Stud. 2009;25(3):425–31. https://doi.org/10.1007/s10899-009-9123-2.
Sauvaget A, Jiménez-Murcia S, Fernández-Aranda F, Granero R, Grall-Bronnec M, Victorri-Vigneau C, et al. A comparison of treatment-seeking behavioral addiction patients with and without Parkinson’s disease. Front Psych. 2017;8:214. https://doi.org/10.3389/fpsyt.2017.00214.
Vita A, Bastiani L, Turrina C, Benedetti E, Bergamini A, Molinaro S. At-risk gambling in patients with severe psychiatric illness and in community subjects matched for age and sex. Psychiatry Res. 2021;304: 114142. https://doi.org/10.1016/j.psychres.2021.114142.
Brodeur M, Audette-Chapdelaine S, Savard AC, Kairouz S. Gambling and the COVID-19 pandemic: a scoping review. Prog Neuropsychopharmacol Biol Psychiatry. 2021;111: 110389. https://doi.org/10.1016/j.pnpbp.2021.110389.
Jiménez-Murcia S, Bove FI, Israel M, Steiger H, Fernández-Aranda F, Alvarez-Moya E, et al. Cognitive-behavioral therapy for pathological gambling in Parkinson’s disease: a pilot controlled study. Eur Addict Res. 2012;18(6):265–74. https://doi.org/10.1159/000337442.
Di Nicola M, De Crescenzo F, D’Alò GL, Remondi C, Panaccione I, Moccia L, et al. Pharmacological and psychosocial treatment of adults with gambling disorder: a meta-review. J Addict Med. 2020;14(4):e15–23. https://doi.org/10.1097/adm.0000000000000574.
Cowlishaw S, Merkouris S, Dowling N, Anderson C, Jackson A, Thomas S. Psychological therapies for pathological and problem gambling. Cochrane Database Syst Rev. 2012;11: CD008937. https://doi.org/10.1002/14651858.CD008937.pub2.
Goslar M, Leibetseder M, Muench HM, Hofmann SG, Laireiter AR. Pharmacological treatments for disordered gambling: a meta-analysis. J Gambl Stud. 2019;35(2):415–45. https://doi.org/10.1007/s10899-018-09815-y.
Yakovenko I, Quigley L, Hemmelgarn BR, Hodgins DC, Ronksley P. The efficacy of motivational interviewing for disordered gambling: systematic review and meta-analysis. Addict Behav. 2015;43:72–82. https://doi.org/10.1016/j.addbeh.2014.12.011.
Quilty LC, Wardell JD, Thiruchselvam T, Keough MT, Hendershot CS. Brief interventions for problem gambling: a meta-analysis. PLoS ONE. 2019;14(4): e0214502. https://doi.org/10.1371/journal.pone.0214502.
Tremblay J, Dufour M, Bertrand K, Blanchette-Martin N, Ferland F, Savard AC, et al. The experience of couples in the process of treatment of pathological gambling: couple vs. individual therapy. Front Psychol. 2017;8:2344. https://doi.org/10.3389/fpsyg.2017.02344.
Wu K, Politis M, Piccini P. Parkinson disease and impulse control disorders: a review of clinical features, pathophysiology and management. Postgrad Med J. 2009;85(1009):590–6. https://doi.org/10.1136/pgmj.2008.075820.
Yip SW, Potenza MN. Treatment of gambling disorders. Curr Treat Options Psychiatry. 2014;1(2):189–203. https://doi.org/10.1007/s40501-014-0014-5.
Papay K, Xie SX, Stern M, Hurtig H, Siderowf A, Duda JE, et al. Naltrexone for impulse control disorders in Parkinson disease: a placebo-controlled study. Neurology. 2014;83(9):826–33. https://doi.org/10.1212/wnl.0000000000000729.
Okai D, Askey-Jones S, Samuel M, O’Sullivan SS, Chaudhuri KR, Martin A, et al. Trial of CBT for impulse control behaviors affecting Parkinson patients and their caregivers. Neurology. 2013;80(9):792–9. https://doi.org/10.1212/WNL.0b013e3182840678.
Choi SW, Shin YC, Youn H, Lim SW, Ha J. Pharmacotherapy and group cognitive behavioral therapy enhance follow-up treatment duration in gambling disorder patients. Ann Gen Psychiatry. 2016;15:20. https://doi.org/10.1186/s12991-016-0107-1.
Binde P. A Swedish mutual support society of problem gamblers. Int J Ment Heal Addict. 2012;10:1–12. https://doi.org/10.1007/s11469-011-9335-4.
Kotter R, Kräplin A, Pittig A, Bühringer G. A systematic review of land-based self-exclusion programs: demographics, gambling behavior, gambling problems, mental symptoms, and mental health. J Gambl Stud. 2019;35(2):367–94. https://doi.org/10.1007/s10899-018-9777-8.
Håkansson A, Widinghoff C. Gambling despite nationwide self-exclusion—a survey in online gamblers in Sweden. Front Psych. 2020;11: 599967. https://doi.org/10.3389/fpsyt.2020.599967.
van den Bos R, Koot S, de Visser L. A rodent version of the Iowa Gambling Task: 7 years of progress. Front Psychol. 2014;5:203. https://doi.org/10.3389/fpsyg.2014.00203.
Collins GT, Cunningham AR, Chen J, Wang S, Newman AH, Woods JH. Effects of pramipexole on the reinforcing effectiveness of stimuli that were previously paired with cocaine reinforcement in rats. Psychopharmacology. 2012;219(1):123–35. https://doi.org/10.1007/s00213-011-2382-5.
Bastiaens J, Dorfman BJ, Christos PJ, Nirenberg MJ. Prospective cohort study of impulse control disorders in Parkinson’s disease. Mov Disord. 2013;28(3):327–33. https://doi.org/10.1002/mds.25291.
Poletti M, Logi C, Lucetti C, Del Dotto P, Baldacci F, Vergallo A, et al. A single-center, cross-sectional prevalence study of impulse control disorders in Parkinson disease: association with dopaminergic drugs. J Clin Psychopharmacol. 2013;33(5):691–4. https://doi.org/10.1097/JCP.0b013e3182979830.
Garcia-Ruiz PJ, Martinez Castrillo JC, Alonso-Canovas A, Herranz Barcenas A, Vela L, Sanchez Alonso P, et al. Impulse control disorder in patients with Parkinson’s disease under dopamine agonist therapy: a multicentre study. J Neurol Neurosurg Psychiatry. 2014;85(8):840–4. https://doi.org/10.1136/jnnp-2013-306787.
Rodríguez-Violante M, González-Latapi P, Cervantes-Arriaga A, Camacho-Ordoñez A, Weintraub D. Impulse control and related disorders in Mexican Parkinson’s disease patients. Parkinsonism Relat Disord. 2014;20(8):907–10. https://doi.org/10.1016/j.parkreldis.2014.05.014.
Sharma A, Goyal V, Behari M, Srivastva A, Shukla G, Vibha D. Impulse control disorders and related behaviours (ICD-RBs) in Parkinson’s disease patients: assessment using “Questionnaire for impulsive-compulsive disorders in Parkinson’s disease” (QUIP). Ann Indian Acad Neurol. 2015;18(1):49–59. https://doi.org/10.4103/0972-2327.144311.
Rizos A, Sauerbier A, Antonini A, Weintraub D, Martinez-Martin P, Kessel B, et al. A European multicentre survey of impulse control behaviours in Parkinson’s disease patients treated with short- and long-acting dopamine agonists. Eur J Neurol. 2016;23(8):1255–61. https://doi.org/10.1111/ene.13034.
Erga AH, Alves G, Larsen JP, Tysnes OB, Pedersen KF. Impulsive and compulsive behaviors in Parkinson’s disease: the Norwegian Parkwest study. J Parkinsons Dis. 2017;7(1):183–91. https://doi.org/10.3233/jpd-160977.
Corvol JC, Artaud F, Cormier-Dequaire F, Rascol O, Durif F, Derkinderen P, et al. Longitudinal analysis of impulse control disorders in Parkinson disease. Neurology. 2018;91(3):e189–201. https://doi.org/10.1212/wnl.0000000000005816.
El Otmani H, Mouni FZ, Abdulhakeem Z, Attar Z, Rashad L, Saali I, et al. Impulse control disorders in Parkinson disease: a cross-sectional study in Morocco. Revue neurologique. 2019;175(4):233–7. https://doi.org/10.1016/j.neurol.2018.07.009.
Vargas AP, Vaz LS, Reuter A, Couto CM, Costa Cardoso FE. Impulse control symptoms in patients with Parkinson’s disease: the influence of dopaminergic agonist. Parkinsonism Relat Disord. 2019;68:17–21. https://doi.org/10.1016/j.parkreldis.2019.06.019.
Cornelius JR, Tippmann-Peikert M, Slocumb NL, Frerichs CF, Silber MH. Impulse control disorders with the use of dopaminergic agents in restless legs syndrome: a case-control study. Sleep. 2010;33(1):81–7.
Moore TJ, Glenmullen J, Mattison DR. Reports of pathological gambling, hypersexuality, and compulsive shopping associated with dopamine receptor agonist drugs. JAMA Intern Med. 2014;174(12):1930–3. https://doi.org/10.1001/jamainternmed.2014.5262.
Lanteri PF, Leguia A, Doladé NG, García GC, Figueras A. Drug-induced gambling disorder: a not so rare but underreported condition. Psychiatry Res. 2018;269:593–5. https://doi.org/10.1016/j.psychres.2018.09.008.
Scavone C, Stelitano B, Rafaniello C, Rossi F, Sportiello L, Capuano A. Drugs-induced pathological gambling: an analysis of Italian spontaneous reporting system. J Gambl Stud. 2020;36(1):85–96. https://doi.org/10.1007/s10899-019-09828-1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by Lund University. Anders Håkansson has a position at Lund University that is sponsored by the state-owned gambling operator AB Svenska Spel, and he has funding acquired from the research council of Svenska Spel. Mirjam Wolfschlag is part of Håkansson’s research group and has obtained funding from the research council of Svenska Spel. Håkansson’s time invested in the present research is thereby supported by the position sponsored by Svenska Spel, although no specific funding has been obtained for the present study. No other organization than the authors themselves were involved in the idea or content of the present paper.
Conflicts of interest/competing interest
The authors declare no other conflicts of interest besides the ones mentioned in the Funding declaration.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
AH and MW contributed equally to this review article regarding the literature search and writing the manuscript. All authors have read and approved the final submitted manuscript and agree to be accountable for the work.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wolfschlag, M., Håkansson, A. Drug-Induced Gambling Disorder: Epidemiology, Neurobiology, and Management. Pharm Med 37, 37–52 (2023). https://doi.org/10.1007/s40290-022-00453-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40290-022-00453-9