Abstract
Cellular senescence, a hallmark of ageing, contributes to tissue or organ dysfunction and the pathophysiology of diverse age-related diseases (ARD) by various mechanisms. Targeting it by selective elimination of senescent cells (SCs) or blocking senescence-associated secretory phenotypes (SASP) with natural or synthetic compounds has been suggested to improve lifespan. Dietary phytochemicals possess a broad spectrum of biochemical and pharmacological effects that are beneficial to human health. Flavonoids, which are widely consumed in fruits and vegetables worldwide, are emerging as potential therapeutic agents to mitigate senescence. Naringenin, hesperetin, hesperidin, quercetin, fisetin, kaempferol, rutin, apigenin, luteolin, nobiletin, tangeretin, genistein, wogonin, epigallocatechin gallate (EGCG), theaflavin-3-gallate (TF2A), and procyanidin C1 possess potent antisenescence effects. A single biochemical process may not explain their pleiotropic pharmacological impact. Flavonoids directly modulate underlying cellular senescence processes or interact with molecular targets that regulate ageing-related pathways. This review discusses the potential use of flavonoids to mitigate senescence and consequently delay the onset of ageing-related diseases. We also highlight the underlying mechanisms of action of flavonoids as potential senotherapeutics and reflect on future perspectives and possible strategies to optimize and increase the translatability from bench to bedside in senotherapy.
Similar content being viewed by others
Polyphenolic flavonoids are emerging as potential senotherapeutic agents. |
Natural dietary flavonoids are multi-target compounds that can alleviate senescence in multiple organs by diverse mechanisms of action. |
Polyphenolic flavonoids are protective against ageing-related degenerative diseases such as cancer, diabetes and cardiovascular diseases, with potential therapeutic applications. |
Emerging preclinical evidence suggests that polyphenolic flavonoids could provide geroprotective effects. |
1 Introduction
The increased global life expectancy due to advances in healthcare has led to the increased incidence and burden of ageing and ageing-related diseases (ARDs). By 2050, the population of older people over 65 years is globally projected to be 1.6 billion, imposing a socioeconomic burden on a frail elderly population [1]. In ageing, the proliferating capacity of previously replication-competent cells decreases, leading to cellular senescence. This is a critical mechanism that contributes to ageing and ARDs, whereby cellular stress of proliferating or differentiated non-dividing cells results in a replicative arrest, apoptosis resistance, and pro-fibrotic, proinflammatory, and proinflammatory tissue-destructive senescence-associated secretory phenotype (SASP) [2]. Cellular senescence is a multifaceted process occurring in various somatic cells, and it is regulated by genetic, epigenetic, and environmental factors [3].
More specifically, senescent cells (SC) accumulate in many but not all tissues in aged mammalian organs [4]. This accumulation results from an imbalance between their generation and elimination. Senescent cells are metabolically active and capable of performing the functional roles of their original replication-competent cells. At senescence, cells are stable and persistently growth-arrested, enlarged, and activate damage response signalling pathways [e.g., p38 mitogen-activated protein kinases (p38 MAPK) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κΒ)] as well as express senescence markers [e.g., senescence-associated β-galactosidase (SA-β-gal), p16INK4A, and p21WAF1/Cip1], thereby resulting in the development of DNA segments with chromatin alterations reinforcing senescence (DNA-SCARS) [5, 6]. Senescence has beneficial as well as harmful effects on physiological and pathological processes. Acute senescence contributes to preserving cellular/tissue homeostasis, tumour suppression, wound healing, embryonic development, tissue regeneration, and the promotion of insulin secretion by pancreatic β-cells during ageing [7]. Conversely, chronic senescence, which results in the accumulation of SCs and their SASP, exerts deleterious effects on physiological processes [6, 8]. This contributes substantially to the development and progression of ageing and ARDs, including atherosclerosis, type 2 diabetes mellitus (T2DM), cancers, neurodegenerative diseases, arthritis, renal dysfunction, blindness, frailty, and sarcopenia, among many others (Fig. 1). Therefore, both pro-senescence therapies and anti-senescence treatments can be beneficial depending on the context of senescence. As a corollary, targeting cellular senescence is crucial for healthy living, especially in ageing organisms.
The use of bioactive compounds in senotherapeutics has recently emerged as a promising approach to prolong the lifespan and reduce the severity of chronic diseases. Dietary phytochemicals can enhance the lifespan by modulating metabolic pathways and cellular processes in the same manner as other antiageing interventions such as caloric restriction, intermittent fasting, and exercise [9]. These beneficial effects of dietary phytochemicals from fruits and vegetables may be attributed to the activation of stress resistance pathways. Some natural senolytic compounds and pharmaceutical drugs exert anti-senescence effects by interacting with molecular targets to affect other ageing-related courses [10]. Naturally derived senolytics may be less potent but have the advantage of low toxicity when compared to synthetic senolytics and may be promising candidates for translation into clinical settings or for the development of more specific and potent senotherapeutics.
Polyphenolic flavonoids have beneficial effects on several age-related pathologies [11, 12]; hence, they can be harnessed as senotherapeutics. However, knowledge of their potential application in senotherapy is scanty. We, therefore, provide a brief background to senescence and senotherapeutics and now review the understanding of flavonoids as potential senotherapeutic agents and their mechanisms of action from experimental animal models to clinical trials.
2 Senescent Cells
SCs abound in tissues at the late stages of life and sites of age-related pathologies, triggering disease processes through the complex cell and non-cell-autonomous effects [13]. Senescent cell phenotypes have standard core features in vitro and in vivo, even though the unique universal hallmarks of cell senescence are poorly understood. They are persistently growth-arrested and characterized by the absence of proliferation markers. SCs show altered morphology with generally enlarged and flattened cell shapes. Senescence is also characterized by high levels of lipofuscin, cell replication stimulatory proteins, SA-β-gal activity, and senescence-associated DNA markers with altered chromatin structure, such as senescence-associated DNA damage foci (SDF) and senescence-associated heterochromatin foci (SAHF) [14]. The available biomarkers developed thus far for cellular senescence are targeted towards SA-β-gal activity, telomere attrition, SAHF, cell cycle arrest, and accumulation of DNA damage with the expression of ataxia-telangiectasia mutated (ATM) kinase, p53, p16, and p21 [8]. To address the concern of not having a specific or universal biomarker, combining a collection of these biomarkers is generally accepted to define senescence in different cell types, both in cultured cells and tissues [15].
2.1 Senescence-Associated Secretory Phenotype (SASP)
The SASP is a distinctive feature that differentiates SCs from non-senescent cells and other cell cycle-arrested cells, such as quiescent and terminally differentiated cells. The SASP consists of a distinct secretion of various cytokines, chemokines, growth factors, proteases, and lipids implicated in the pathogenesis of various chronic diseases associated with ageing and thus serve as the link between cellular senescence and inflammaging [7]. The components of SASP differ in composition depending on the cell type and senescence trigger, and are activated a few days after a persistent stimulus. The SASP exerts dual effects physiologically in a context-dependent manner. It has a vital role in promoting the repair of damaged tissues, immune surveillance/clearance of SC, and exerting tumour-suppressive effects; however, SASP promotes ageing, chronic inflammation and/or tumorigenesis by spreading senescence in both an autocrine and a paracrine manner [16].
Major inducers of SASP expression include DNA damage, mitogenic signals, oxidative stress, and epigenomic disruptions [17]. In contrast to senescence and cell‐cycle arrest, SASP is independent of the ectopic expression of senescence factors such as p16Ink4a and p21Waf1/Cip1, suggesting that there are other signalling pathways in their induction [18, 19]. Although the precise mechanisms underlying SASP induction are yet to be elucidated, persistent genomic damage has been suggested to be responsible for SASP production, which is at variance with ectopic expression of p21 or p16 inhibitors [18].
3 Senotherapeutics
Pharmacological targeting of the fundamental mechanisms of ageing can reduce or delay the progression of ageing in both experimental animal models and humans. Senotherapeutics has emerged as a novel strategy in the pharmacotherapy of ageing-related diseases like cardiovascular diseases (CVD), diabetes, and cancer (Fig. 1) [4]. This new class of synthetic drugs and natural products neutralizes cellular senescence's adverse effects and ultimately prolongs lifespan. Senotherapy has gained prominence to the extent that some of these senotherapeutics are currently undergoing human clinical trials. Generally, senotherapeutic strategies include selective elimination of SC, referred to as senolysis, immune-mediated defence against SCs (immunosurveillance), and SASP neutralization. SCs are reduced by targeting networks of anti-apoptotic factors that promote their survival, suppressing the inflammatory SASP, and genetic modification using transgenic animals in activating apoptotic signals mediated by p16Ink4a or p21Cip1 promoter elements [20, 21]. However, the classification of senotherapeutic agents remains a significant problem due to possible overlap among them and an absence of knowledge of well-defined mechanism(s) of action. Nonetheless, the two prominent classes so far introduced for basic and clinical research are senolytics and senomorphics.
3.1 Senolytics
Senolytics are small molecules that act by selectively eliminating SCs either by apoptotic or nonapoptotic means. SCs typically withstand intrinsic and extrinsic proapoptotic signals, unlike normal cells using senescent cell anti-apoptotic pathways (SCAPs). This pro-survival feature enables them to stimulate diverse biological processes under stress conditions [22, 23]. Developing drugs that preferentially target these anti-apoptotic and pro-survival pathways, thereby protecting SCs from their SASP, leads to selective death of SCs and prevents their detrimental effects. Therefore, the development of senolytics focuses attention on such pro-survival (pro-senescence and anti-apoptotic) pathways, including B-cell lymphoma 2 (Bcl-2)/Bcl-xL, phosphatidylinositol 3-kinase (P13K)/protein kinase B (Akt), p53/p21/serpines, dependence receptors/tyrosine kinases, ephrins, hypoxia-inducible factor-1α (HIF-1α), and heat-shock protein 90 (Hsp90) [21, 23, 24], as well as the metabolic targets for the development of cellular senescence [25]. Many US Food and Drug Administration (FDA)-approved drugs, as well as phytochemicals and synthetic compounds, have been shown to possess potential senolytic activities [10, 26]. Table 1 summarises the identified senolytics, their sources, dosage forms, mechanisms of action, and their main effects in various pathological conditions.
Even though senolytics are meant to be specific for SC, there is always the concern of unwanted damage/side effects since the administration is not optimized [27]. Moreover, treatment with senolytics may also potentially target physiologically relevant SCs or other types of non-senescent cells, thereby raising the question of whether the treatment with senolytics could affect normal physiological conditions. In the light of this, novel therapeutic strategies, including the encapsulation of drugs using nanocapsules that preferentially introduce the toxin (senolytic) specifically to target SCs could be employed. Other therapeutic strategies for improving the efficacy and biosafety profile of senolytics include the use of proteolysis-targeting chimera (PROTAC) technology, local administration directly to insulated sites of interest, targeting of senescence-specific molecular targets, and the combinatorial approach [21, 28,29,30]. Senolytic activity can be assessed by considering the specificity for eliminating SC, resistance to apoptosis, autophagy regulation, and prolonging lifespan.
3.2 Senomorphics
Senomorphics are small molecules that suppress/reprogram SASP or proinflammatory secretome [10, 31]. Some molecules, however, may be categorized as senomorphics based on their ability to inhibit other biomarkers of cellular senescence without killing SC. Candidates in this class include natural and synthetic compounds and approved drugs to be repurposed for senotherapy (Table 2). The primary strategic approach of senomorphics is the targeting of regulatory pathways associated with SASP expressions, such as the p38 mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase/serine/threonine protein kinase (PI3K/Akt), mechanistic target of rapamycin (mTOR), and the Janus kinase-signal transducer and activator of transcription (JAK/STAT) pathways, and transcription factors, such as NF-κΒ, CCAAT/enhancer binding protein-β (C/EBPβ) and STAT3 [32]. The other approach uses specific antibodies to target the activity and function of certain SASP factors such as interleukin (IL)-1α, IL-8, IL-6, and matrix-remodelling proteases such as A disintegrin and metalloprotease 17 (ADAM17) [27]. This strategy could provide a better way of alleviating safety concerns attributed to SASP. For instance, rapamycin, by targeting the expression of IL-6 and IL-1α, selectively inhibited the deleterious effects of senescence-associated inflammation, thereby facilitating the harmful impact of genotoxic exposures and normal ageing processes [33].
Although most senomorphics are specific in action, there is remarkable diversity in SASP occurring in the different SC populations. Understanding the biological significance of various SC types and the effects of their SASP components is needed to improve the target specificity of senomorphics in other pathological conditions [25]. However, a significant drawback is that blocking SASP leads to the inhibition of crucial pathways for the maintenance of tissue homeostasis. Another challenge is the clearing of SASP-silenced SCs by the immune system, considering that many SASP factors participate in the recruitment of immune cells. This will address the critical question as to whether the administration of senomorphics could compromise the normal function of tissues and organs [27]. However, these problems could be addressed by epigenetic control of SASP expression with chromatin modifiers. Senomorphics require improved biosafety profiles as they must be administered regularly over an extended period. Hence, consumption of dietary phytochemicals from fruits and vegetables may at least in part be a viable option to target SCs by improving stress resistance through various cellular protective mechanisms.
3.3 Immune System-Mediated Clearance of Senescent Cells
SCs elicit immunogenic responses and can undergo immune surveillance in different immune system components. Immunosurveillance of SCs potentiates the immune system for efficient recognition and elimination of SC; hence, it is another viable strategy in senotherapy. Immune-mediated clearance of SCs can be achieved either by generally boosting the immune function to prevent immunosenescence or by promoting anti-senescent cell function [27]. Since the accumulation of SCs in aged tissues is associated with an intrinsic decline of immune function [34], therapeutic strategies to boost the immune system could lead to successful clearance of SCs from aged tissues. Anti-senescent cell functions can be improved by stimulating immune recognition molecules such as natural killer (NK)-cells, macrophages, and CD4+ T cells [23, 35]. Possible immune-senotherapeutic strategies include reducing the number of senescent immune cells with specific antibodies recognizing surface senescence markers [35], facilitating the expression of these receptors on the surface of SCs [36], and increasing the binding affinity of the cell-surface receptors on the membrane of SCs [37].
Other new interventions to improve the immune system in SC clearance have emerged as effective treatments for a wide variety of diseases. Considering the immunogenic nature of SC, the restoration of immune surveillance of chronic SCs or its manipulation to promote their immunogenicity are promising clinical development strategies.
4 Molecular Aspects of Senotherapeutics
The molecular mechanisms of senotherapeutics are closely associated with the various hallmarks of aging. These fundamental aging mechanisms include genomic instability, epigenetic changes, shortening of telomeres, mitochondrial dysfunction, loss of proteostasis or autophagy, dysregulated nutrient sensing, altered cellular communication, stem cell exhaustion, and reduced regeneration capacity [38]. Epigenetic modulations such as DNA methylation and histone post-translational modifications by senotherapeutics affect genomic integrity, gene expression, and pathophysiology of organisms. Senotherapeutics target and regulate mitochondrial homeostasis, cell-cycle proteins, transcription factors, autophagy, stem cell activity, intermediary metabolism, and several signalling pathways implicated in the induction of cellular senescence (Tables 1, 2).
5 The Imprint in Degenerative Diseases
Therapeutic targeting of cellular senescence has a significant impact on disease pathogenesis. It could be more effective at alleviating degenerative diseases and frailty progression than any currently available treatment options.
5.1 Diabetes and Dyslipidaemia
Like other ageing-related diseases, the development and accumulation of SCs are underlying features of diabetes and obesity. SCs are implicated in tissue dysfunction and comorbidities associated with diabetes and obesity. They may negatively contribute to type 1 and 2 diabetes pathogenesis by directly impacting pancreatic β-cell function, SASP-mediated tissue damage, and facilitating adipose tissue dysfunction [39].
5.2 Cardiovascular Diseases
SCs are critical drivers of atherosclerotic plaque formation in the endothelium and vascular smooth muscle. Roos et al. [40] reported that depletion of SCs led to improved vasomotor dysfunction and decreased calcified plaque formation in ageing animals, indicating the importance of SCs in endothelial dysfunction, a pathologic event in developing atherosclerosis.
5.3 Cancer
Uncontrolled proliferation and metastasis are characteristic features of cancer. Like other ARDs, ageing and senescence play significant roles in cancer development [41]. SCs have been shown to promote tumorigenesis by both cell and non-cell-autonomous mechanisms, contrary to the previously established tumour-suppressive roles of senescence-associated cell cycle arrest. The pharmacological approach of combining senescence-inducing cancer therapies with senolytics would be a viable option for reducing the risk of senescent cancer cells stemness, cancer growth, and the side effects of cancer therapy [42].
5.4 Renal Diseases
Ageing and SC abundance have been implicated in renal complications such as glomerulosclerosis and nephropathies [43]. Pharmacological clearance of SCs accumulated in various kidney compartments will ameliorate renal ageing and conditions.
5.5 Liver Diseases
The onset of chronic liver diseases, such as non-alcoholic fatty liver disease (NAFLD), hepatic inflammation, steatosis, cirrhosis, jaundice, hepatic encephalopathy, portal hypertension, and hepatocellular carcinoma (HCC), are common geriatric diseases closely linked to cellular senescence [44]. Elimination of SCs using a senolytic alleviated hepatic steatosis and portal inflammation, necrosis, and fibrosis in the liver of ageing mice [45].
5.6 Osteoarthritis
Osteoarthritis (OA) is a chronic degenerative disorder involving movable joints and is the leading cause of chronic pain and disability among older people. Ageing and tissue regeneration during traumatic injuries promote the accumulation of SCs in cartilage tissues [28, 46]. Pharmacological targeting of the antiapoptotic proteins and senomorphics attenuates SASP secretion and the development of post-traumatic OA in rats [47].
5.7 Lung Diseases
Idiopathic pulmonary fibrosis (IPF), characterized by radiographically evident interstitial remodelling, is a fatal, chronic, progressive, and fibrotic age-related lung disease driven by abnormally activated alveolar epithelial cells [48]. Cellular senescence through SASP has promoted functional lung deterioration in IPF. Hence, pharmacological targeting of cell senescence with senotherapeutics would mitigate fibrotic lung disease.
5.8 Neurological Disorders
Ageing predisposes to neurological disorders, especially Alzheimer's and Parkinson's disease. A close correlation between cell senescence and ageing and age-related neurodegeneration has been observed. Cellular senescence plays a significant role in several neurodegenerative disorders. Pathological changes occurring in the brain in Alzheimer's and Parkinson's diseases share similar characteristics with cellular senescence phenotypes [49]. Pharmacological intervention with senotherapeutics improved these neurological disorders.
6 Polyphenolic Flavonoids
Flavonoids are natural polyphenolic substances commonly found in fruits and vegetables and classified into different subtypes (Fig. 2). They contain a 2-phenyl-benzo-pyrane backbone consisting of two benzene rings (A and B) bound to a 3-carbon unit heterocyclic pyran ring (C6–C3–C6), which is vital for their classification. Polyphenolic flavonoids are stored predominantly as glycosides in the cell vacuole of plants since glycosylation increases their solubility in water and decreases their reactivity. Hence, most flavonoids exist naturally as products of aglycones and sugars, mainly d-glucose and l-rhamnose, linked to the hydroxyl group at the C-3 or C-7 position.
Flavonoids possess a wide range of pharmacological properties and are promising candidates in anti-ageing research. Their effects are pleiotropic and influence cellular lifespan, SA-β-gal activity, and other markers of senescence directly or indirectly (Table 3). Such effects include maintaining SASP, inducing apoptosis in SCs, and activating different protective cellular mechanisms. Although the precise mechanisms of action have not been elucidated, their antioxidant and anti-inflammatory activities have been suggested to play a vital role since they are the main factors implicated in ageing and various ageing-related diseases [12, 50, 51]. Ample evidence from experimental animal studies has shown the anti-senescence effects of flavonoids to be senomorphic, with only a few exerting senolytic effects [4, 5, 52,53,54].
6.1 Flavanones
6.1.1 Naringenin
Naringenin, the aglycone product of the enzymatic hydrolysis of the main bitter flavonoid naringin, has long been considered to possess various biological activities such as antioxidant, anti-inflammatory, antiproliferative, anti-dyslipidaemic, and antidiabetic effects [55]. Naringenin has been shown to improve neurogenesis in the brain of ageing mice through downregulation of tumour necrosis factor α (TNF-α) signalling pathway gene expression [56], suggesting its role in facilitating neurological impairment and cognitive ageing. Naringenin modulates oxidative stress and mitochondrial metabolic activity, leading to myoblast cells' abrogation of H2O2-induced senescence [57]. These findings suggest its importance in treating cardiometabolic disorders caused by a redox imbalance in the cell. Naringenin promotes the synthesis of the ECM of cartilage and, in turn, improves ageing in both lipopolysaccharide- and reactive oxygen species (ROS)-induced skin senescence through SIRT1-mediated inhibition of NF-κΒ, NADPH oxidase, and matrix metalloproteinases (MMPs) expression in human dermal fibroblast, suggesting regenerative and anti-ageing effects on the dermal cell structure [58]. However, Lim et al. [5] reported that naringenin did not suppress SASP production in bleomycin-induced senescence. This could be due to variation in the senescence-inducing mechanisms, which affects the composition of SASP factors. Moreover, our research group and others have reported that naringenin has known anti-inflammatory effects and shares similar pharmacological effects with the biguanide derivative metformin, which has established senomorphic effects [55, 59,60,61]. However, more evidence from preclinical and clinical studies is required to support these findings.
6.1.2 Hesperidin and Hesperetin
Hesperidin and its aglycone, hesperetin, are major flavanones found in fruits and vegetables and have known antioxidant and anti-inflammatory effects that inhibit the production of proinflammatory cytokines, leading to the blocking of SASP [62,63,64]. They exert their senomorphic effects by modulating signalling pathways involving the Nrf2, NF-κΒ, and FOXO, and increasing antioxidant enzyme activity [63, 65]. Hesperidin has been shown to stabilize SASP and exerts anabolic effects in human senescent chondrocytes by increasing cellular antioxidant capacity and decreasing proinflammatory cytokines that constitute SASP, suggesting a beneficial role in the progression of OA [63]. Similarly, hesperidin has been shown to protect against bone loss by inhibiting bone resorption, NF-kB activity, and improving bone mineral density in male senescent rats [62, 65], suggesting a role in reducing the risk of fractures. Hesperetin plays a pivotal role in lowering immune inflammation of the joints in rheumatoid arthritis by inhibiting cytokine production and c-Jun N-terminal kinase (JNK) activity in synovial fibroblasts [66]. These potential anti-SASP mechanisms of hesperidin and/or hesperetin need to be considered in future clinical studies.
6.2 Flavonols
6.2.1 Quercetin
Quercetin is recognized for its many pharmacological activities, including anticancer, antidiabetic, anti-inflammatory, anti-atherosclerotic, antithrombotic, antihypertensive effects, and benefits for human endurance exercise capacity. The antioxidant activity of quercetin is a prominent characteristic that enables it to quench the formation of resonance-stabilized phenoxyl radicals from free radicals. Besides its antioxidant activity, Chondrogianni et al. demonstrated the anti-ageing and rejuvenating effect of quercetin on senescent human fibroblasts [67], as well as prolonging organismal life expectancy in Saccharomyces cerevisiae [68] and Caenorhabditis elegans [69]. Quercetin has been shown to alleviate cellular phenotypes of Hutchinson–Gilford progeria syndrome by reducing ROS, enhancing cell proliferation, and restoring heterochromatin architecture in premature ageing human mesenchymal stem cells [70], suggesting a role in maintaining skin turgidity. It is worth mentioning that quercetin is both senolytic and senomorphic as it modulates p53/p21/serpines or PI3K/Akt/mTOR and NF-κB signalling pathways [21, 71, 72], which results in reduced ROS production. However, quercetin had to be combined with dasatinib for more effective and broad-spectrum senolytic effects [21].
The senolytic cocktail of dasatinib and quercetin (D + Q), discovered using a hypothesis-driven bioinformatics approach, had direct senolytic effects on senescent adipocytes and human umbilical vein endothelial cells by disabling the pro-survival and anti-apoptotic pathways of the SCs [21]. Clinically, the senolytic combination of D+Q is safe and effective in alleviating physical dysfunction in patients with idiopathic pulmonary fibrosis [2], reducing inflammation (in the form of circulating SASP factors) and SC abundance in the skin and adipose tissue of patients with diabetes-related kidney diseases [73]. However, the use of the senolytic regimen has not been adopted in clinical practice because of (1) inadequate knowledge of the systemic effects, (2) the precise context in which quercetin could be administered, and (3) interference with other biological pathways while inhibiting the specific SCAPs. Moreover, quercetin alone or in combination with dasatinib has been subjected to other clinical trials for coronary artery disease (NCT04907253), Alzheimer’s disease (AD) (NCT04063124, NCT04785300, NCT04685590, NCT05422885), hematopoietic stem cell transplant survivors (NCT02652052), elderly frail patients (NCT04313634), and patient's epigenetic aging rate (NCT04946383).
6.2.2 Fisetin
Fisetin is another dietary flavonol present in fruits and vegetables such as strawberries, onions, cucumbers, and grapes, with a known safety profile [74]. Fisetin, just like its analog quercetin, acts on numerous biological processes that may also contribute to its senolytic effects. For instance, because of its hydrophobic nature, fisetin penetrates and accumulates in the cell membrane to exert antioxidant and inflammatory effects [29], and also induces apoptotic effects in SCs [75] by suppressing Bcl‐2 family members and other SCAP network components. Fisetin suppressed multiple SC viability and increased lifespan by inhibiting pro-senescence effectors such as p16Ink4a and p21Cip1 in wild-type mice [76]. Fisetin showed more enhanced senotherapeutic activity than quercetin in animal and human tissues [29], and is currently undergoing several clinical trials for multiple ARD, including osteoarthritis (NCT04815902, NCT04210986, NCT04770064), coronavirus infection (NCT04771611, NCT04476953, NCT04537299), frail elderly syndrome (NCT03675724, NCT04733534, NCT03430037), chronic kidney diseases (NCT03325322), and femoroacetabular impingement (NCT05025956). Therefore, the clinical merits of fisetin in terms of feasibility, safety, tolerability, and efficacy could soon be established and employed in geriatric medicine.
6.2.3 Kaempferol
Kaempferol is considered to have strong senomorphic effects by modulating various transcription factors and stress response signalling pathways such as oxidative stress and inflammatory responses. Kaempferol has been shown to reduce SASP levels by blocking IκBζ expression in aged rats, suggesting its role in alleviating chronic low-grade inflammation associated with many ageing-related diseases [5]. Kaempferol relieves oxidative stress and acts as an anti-gerontic agent by increasing DAF-16/FOXO activity in living transgenic worms C. elegans, resulting in attenuation of lipofuscin accumulation [77]. Consequently, although there is little direct evidence of kaempferol in senotherapy, such anti-senescence effects could be expected in further studies owing to its prominent anti-inflammatory activity.
6.2.4 Rutin
Rutin, a glycoside of quercetin, attenuates atherosclerotic plaques in mice by improving metabolic disturbances and preserving oxidative stress, suggesting a role in alleviating fatal complications of diabetes associated with aberrant vascular smooth muscle cell (VSMC) proliferation, and premature senescence [78]. The anti-senescence effect of rutin appears to be because of its antioxidant, anti-inflammatory activity, and the transformation of rutin to its aglycone, quercetin, which has superior pharmacological effects [79, 80].
6.3 Flavones
6.3.1 Apigenin
Apigenin, belonging to the flavone subclass of flavonoids, is present in various fruits and vegetables. Apigenin has long-established antioxidant, anti-inflammatory, anti-mutagenic, and anti-proliferative effects [5, 81]. It appears to inhibit NF-κΒ activity by blocking translocation and phosphorylation of IκBζ, leading to the prevention of low-grade inflammation and the associated degenerative diseases [5]. The double bond and hydroxyl group substitution at the A and B ring of the flavonoid's backbone, which chelate metal ions and/or scavenges free radicals, could be responsible for the senomorphics effects of apigenin. Apigenin is now known to induce apoptosis in a p53-independent pathway by enhancing oxidative stress [82]. The p53-independent apoptotic effects of apigenin would support its use as a senolytic and in chemoprevention in neoplasm. Therefore, the potential senolytic effects of apigenin need to be considered in further studies.
6.3.2 Luteolin
Luteolin, which acts as the first-line defence system in plants against adverse photobiological effects such as protection against UV radiation, has shown promising senotherapeutic effects [83]. Luteolin regulates the expression of inflammatory cytokines, leading to the blockade of NF-κΒ's nuclear translocation and an abrogation of senescent murine embryonic fibroblast cells [76, 84], suggesting a beneficial role to patients with inflammatory disorders of the lungs. The anti-senescence effects of luteolin may be attributed in part to its anti-inflammatory, antioxidative capacities and proapoptotic effects. Luteolin suppresses proinflammatory SASP factors and regulates various signalling pathways implicated in cellular senescence, such as NF-κΒ, JAK/STAT, and toll-like receptors (TLRs) [83]. Considering the structural activity relationship, the C2–C3 double bond at the A and B rings of luteolin could enhance the senotherapeutic effects of luteolin, just like apigenin. A clinical trial investigating the therapeutic effect of luteolin combined with palmitoylethanolamide in patients with frontotemporal dementia is underway (NCT04489017).
6.3.3 Wogonin
Wogonin, the active monoflavonoid constituent of the Chinese herbal tea from Scutellari baicalensis Georgi, has known antioxidant, anti-inflammatory, antiproliferative, and antimicrobial activities [85]. Wogonin is suggested to have senolytic effects by inducing cellular apoptosis through upregulation of p53 and p21 proteins [86]. In senescent human foreskin fibroblasts, wogonin alleviated SASP by down-regulating the NF-κB pathway [5], suggesting its potential therapeutic role in skin ageing. Wogonin, just like other flavonoids, is rapidly metabolized and excreted in the liver and intestine, which affects its efficacy and absorption rates. In order to improve the bioavailability and efficacy of the poorly absorbed wogonin, analogs such as GL-V9 with a broad spectrum of action have been formulated through structural modifications. In a recent report, GL-V9 showed potential senolytic effects against senescent breast cancer cells mediated through ROS-dependent apoptotic mechanism [87]. Thus, further research on the anti-senescence effects of wogonin and GL-V9 in other senescence-associated diseases is required.
6.4 Polymethoxyflavones
6.4.1 Nobiletin
Nobiletin (5, 6, 7, 8, 3′, 4′-hexamethoxyfavone) is a polyethoxylated flavonoid found in fruits and vegetables and has a broad range of pharmacological activities such as pro-apoptotic, anti-inflammatory, anti-tumour, antioxidant, and anti-diabetes [88]. Nobiletin has known promising anti-ageing and lifespan-extending effects [89, 90]. This could be because of the ability of nobiletin to interact with other molecular targets to produce extra bioactivities other than senescence. For instance, nobiletin interacts with cyclooxygenases, inducible NO synthase proteins, prostaglandin E2, MAPK, AMPK, and MMPs, exerting proapoptotic, anti-inflammatory, and anti-cancer effects [91,92,93]. By enhancing autophagy and mitochondrial recovery through the SIRT-1/FOXO3a and PGC-1α pathways, respectively, nobiletin reverses hepatic tissue damage in mice [94], suggesting a role in protecting the liver from ischaemia–reperfusion injury. In human chondrocytes, nobiletin treatment improved the synthesis of ECM proteins. It abrogated articular cartilage degradation by reducing the expression of inflammatory cytokines and preventing the activation of PI3K/Akt and NF-κΒ [95]. This suggests that nobiletin could confer structural support and enhance physical function in patients with OA. Collectively, these findings suggest potential senotherapeutic effects of nobiletin but need to be corroborated in further preclinical and clinical studies.
6.4.2 Tangeretin
The senotherapeutic effects of the polymethoxyflavone tangeretin appear to depend on its anti-inflammatory and apoptotic effects [96, 97]. Tangeretin has known apoptotic effects of inhibiting anti-apoptotic protein Bcl-2 and Bcl-xl and modulating the PI3K/Akt signalling pathway [96], which can be argued to be senolytic by making SCs susceptible to their pro-apoptotic microenvironment. Tangeretin prevented ischaemic and reperfusion-induced neuronal damage by inhibiting NF-κB activity, reducing oxidative stress, and decreasing proinflammatory cytokines in the brain of rats [97], suggesting its beneficial role in patients with ischaemic stroke. Tangeretin alleviates activated microglia-induced neuroinflammation by reducing proinflammatory cytokines and suppressing NF-κB and MAPK signalling [98], suggesting its valuable role in patients with neurodegenerative diseases.
6.5 Isoflavones
6.5.1 Genistein
The isoflavone genistein, which acts as a selective estrogen receptor modulator, is found in citrus fruits [99, 100]. Genistein attenuates senescence in human vascular smooth muscle cells (VSMCs) by inhibiting mTOR activity and activating autophagy [101], supporting its therapeutic potential in age-related vascular diseases such as atherosclerosis. The genistein-dependent autophagy induction in vascular cells was mediated by liver kinase B1 (LKB1)–AMPK signalling pathways. Genistein could function as a potential rapamycin analog (a known senomorphic agent) with little or no side effects by inhibiting mTOR. A clinical trial on the therapeutic effect of genistein in AD patients is ongoing (NCT01982578). Hence, further preclinical and clinical evidence on the senotherapeutic effects of genistein is necessary to promote its translation to geriatric medicine.
6.6 Flavanols
6.6.1 Epigallocatechin Gallate
The phytochemical epigallocatechin gallate (EGCG) is the major catechin found in green tea, with many potential benefits on health outcomes, including cell senescence, ageing, and ARD. Preclinical evidence suggests that EGCG exhibits promising senolytic and senomorphic effects through multi-faceted mechanisms, including targeting apoptotic pathways, cell cycle regulation, nutrient sensing pathways, SASP, and oxi-inflammatory stress pathways [102, 103]. EGCG attenuated DNA damage, cell cycle arrest, SASP formation, and induced apoptosis by inhibiting Bcl-2 and mTOR pathway in senescent preadipocytes [102], suggesting a central role in ageing and senescence-associated diseases. By activating the regulatory transcription factors Nrf2 and SIRT3, EGCG enhanced the antioxidant defences and alleviated SASP production in senescent preadipocytes [103], suggesting a beneficial role in both aging and obesity. The senotherapeutic mechanisms of action of EGCG are far from being fully understood and require further preclinical and clinical data validation.
6.7 Other Polyphenolic Flavonoids
Other polyphenolic flavonoids, including the oxidation and polymerization products of flavonoids such as theaflavin 3-gallate (TF2A) and procyanidin C1, have been shown to possess senotherapeutic effects in animals [104,105,106]. Theaflavins, the core functional polyphenols responsible for the red colour of black tea, have various biological activities, such as free radical scavenging and antioxidant, antiapoptotic, and anti-inflammatory activities [107]. TF2A exerted anti-senescence effect by mimicking and modulating the gene expression of long non-coding RNAs (LncRNAs). TF2A alleviated senescence of hypothalamic neural stem cells [104], bone marrow mesenchymal stem cells [105], and respectively improved aging-related phenotypes and bone regeneration in mice by stabilizing or delaying the degradation of Y-box protein 1 (YB-1), a transcriptional repressor of the senescence marker gene p16INK4A. Thus, TF2A could be a promising candidate for stem cell regenerative application in patients with osteoporosis. Moreover, TF2A has been shown as a natural antagonist for Hsp90 [107], a known pharmacological target for senolytics, suggesting its prospects as a lead compound in the design of new senotherapeutics, after rigorous validation approaches.
The polyphenolic flavonoid procyanidin C1 (PCC1) isolated from grape seed extract has been shown as a novel phytochemical senotherapeutic with superior specificity and efficiency for a wider range of SC types and senescence inducers than many reported senolytics [106]. PCC1 was reported to be senomorphic at low concentrations and senolytic upon treatment at higher concentrations, which could be suggested to be responsible for the elimination of SC, increased lifespan, and improved physiological functions in preclinical studies [106]. The senotherapeutic mechanism of PCC1 could, at least to some extent, be suggested to be by downregulating proinflammatory gene expression and promoting ROS and mitochondrial-dependent apoptosis induction.
7 Clinical Considerations
Due to their cell-type specificity and unknown safety profile, currently available senotherapeutics should not be used to treat the various multimorbidities associated with cellular senescence. Natural dietary flavonoids are multi-target compounds that can alleviate senescence in multiple organs by diverse mechanisms of action. Unlike toxic synthetic agents, polyphenolic flavonoids have an improved tolerance profile and are cheaper in most cases.
Several epidemiological studies have reported that increased consumption of flavonoids is inversely correlated with the risk of CVD and T2DM [108, 109]. The pharmacological effects of flavonoids on atherosclerosis and CVD may be attributed to their ability to lower oxidative stress, hyperlipidaemia, and inflammation, and alleviate endothelial function, arterial blood pressure, and lipid metabolism [110]. For these experimental observations to be translated into clinical practice, optimization of bioavailability, and determination of dosage forms, delivery systems have to be determined. Epidemiologic data from 34,489 postmenopausal women demonstrated that consuming foods rich in flavonoids was inversely associated with all-cause mortality due to coronary heart disease and CVD [111]. Consumption of naringenin in the form of naringin has been associated with a reduced risk of CVD by improving the lipid profile in hypercholesterolemic patients [112] and exerting significant antihypertensive effects in patients with stage I hypertension [113].
Flavonoids regulate molecular events involved in the process of atherogenesis. Daily oral intake of hesperidin improved vascular reactivity and endothelial function, reduced inflammation, and favourably altered lipid profiles in patients with metabolic syndrome [64]. Moreover, hesperidin abrogated the development of atherosclerotic plaque formation by reducing adhesion molecules of endothelial cells [114]. These findings may reduce the risk of developing stroke in patients with metabolic syndrome.
Preclinical evidence suggests the beneficial effects of flavonoids on glucose intolerance, insulin secretion and sensitivity, insulin resistance [115], hepatic glucose output and intestinal glucose absorption [116], peripheral glucose uptake [117], inflammation [115], and the activity of enzymes and transporters involved in glucose and lipid metabolism [115, 118], suggesting their potential as an antidiabetic treatment regimen, especially among older people. They can cross the blood–brain barrier to exert antioxidative, anti-inflammatory, and neuroprotective effects on neural cells and hence are promising candidates for alleviating age-related neurodegenerative disorders. Apigenin improves AD-associated learning and memory impairment by reducing amyloid-β peptides (Aβ) accumulation, inhibiting oxidative stress, and restoring cerebral BDNF levels [119], suggesting a role in preserving brain function in ageing. Naringenin has been shown to inhibit acetylcholinesterase activity and reduce neurotoxic effects of Aβ deposition in animals [120], suggesting its potential role in treating patients with dementia. Treatment with hesperidin or hesperetin ameliorated the development of AD and improved glucose metabolism by downregulating Aβ-induced autophagy in insulin-stimulated neuronal cells [121], suggesting a beneficial role in elderly patients with diabetes and AD comorbidities.
Several preclinical studies have demonstrated the chemopreventive effects of flavonoids, such as flavanones and polymethoxyflavones, on both cancer and non-cancer diseases [122]. Research on the anti-cancer activity of flavonoids has mainly focused on elucidating anti-proliferative effects, enzyme inhibition, and cytotoxicity in vitro and in vivo. They have consequently been shown to exert anticancer activity by alleviating chemotherapy resistance and synergizing with chemotherapeutic drugs to induce apoptosis of sensitive cancer cells [123, 124]. Their ability to halt cell cycle modulation, antiangiogenic effects, apoptosis induction, inflammation, and oxidative stress may be responsible for their beneficial roles in various types of cancers such as gastric cancer, colon cancer, breast cancer, lung cancer, and hepatomas. Furthermore, the structural similarities between flavonoids and 17β-estradiol could be associated with anti-estrogenic effects that could mitigate breast cancer.
A population-based, case–control study of lung cancer showed that dietary intake of quercetin and naringin conferred protection against lung cancer in Hawaiian patients [125]. Flavonoids are effective inhibitors of cancer invasion and metastasis by interfering with metastatic cascades, including cell–cell attachment, tissue-barrier degradation, migration, invasion, cell-matrix adhesion, and angiogenesis. In brain tumour cells isolated as surgical samples from patients, nobiletin and tangeretin suppressed brain tumour invasion by reducing gelatinase secretion, adhesion, and migration [126], indicating a role in patients with malignant gliomas.
Apart from their beneficial metabolic effects, flavonoids could be considered suitable adjuvants in treating human immunodeficiency virus type (HIV) infection-induced immune senescence [127]. Some naturally occurring flavonoids and several senotherapeutics, especially the tyrosine kinase inhibitors and Bcl-2 inhibitors, which are commonly used to treat cancer, hold therapeutic promise of suppressing viral replication of latently infected HIV cells [50, 51, 128], as well as immunomodulatory effects of reducing proinflammatory mediators [52], leading to abrogation of neuroinflammation and persistent immune activation associated with HIV infection. By inhibiting CDK9 and Tat transactivation, chrysin, apigenin, and luteolin reinforce HIV latency and induce transcriptional quiescence [51], suggesting a role in silencing HIV reservoirs in humans. Therefore, combining flavonoids with these senotherapeutics could be a viable therapeutic strategy for mitigating ageing, metabolic derangement, and complications associated with HIV treatment. Considering the cost, safety profile, and beneficial effects of polyphenolic flavonoids on various ARDs, research should focus on paving the way for these flavonoids in the clinic as senotherapeutics.
8 Conclusion and Future Perspectives
As cellular senescence is linked to the development and progression of ageing and ARDs, it is imperative to use natural compounds to protect organisms against the effects of excessive accumulation of SC. This review focused on the senotherapeutic effects of flavonoids and their potential mechanisms. Several preclinical shreds of evidence demonstrate that flavonoids can improve cellular senescence by modulating several ageing-associated signalling pathways and cellular protective mechanisms (Fig. 3). The effect of flavonoids in lowering systemic inflammation and oxidative stress and targeting SCs and their secreted SASP mark their potential as therapeutic agents for senescence.
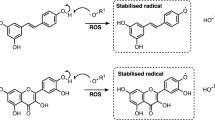
Model illustrating the potential effects of citrus flavonoids on cellular senescence. Citrus flavonoids are pleotropic molecules that target several pathways including those involved in maintaining SASP, inducing apoptosis of senescent cells by inhibiting pro-senescence pathways or by inhibiting antiapoptotic signalling, in a direct or indirect manner, and activating different cellular protective mechanisms. AMPK AMP-activated protein kinase, Akt protein kinase-β, Bcl B-cell lymphoma, C/EBP β CAAT/enhancer binding protein, FOXO Forkhead box protein, IL-1R interleukin-1 alpha, IL-6R interleukin-6, JAK Janus kinase, NF-κB nuclear factor kappa-light-chain enhancer of activated B cells, PI3K phosphoinositide 3-kinase, ROS reactive oxygen species, STAT signal transducer and activator of transcription, SIRTs silent information regulators (sirtuins), TGFβ transforming growth factor beta. Figure created with BioRender.com
Ageing and ARDs have a redox-regulated component, which participates actively in the mode of action of several senomorphics. A strategy of specifically targeting the signalling pathways of these redox intermediates of SASP in SCs, such as the redox-sensitive transcription factors NF-κΒ, Nrf2, or FOXO, instead of the unspecific elimination of ROS, may be a boon for the identification of effective senomorphics in the future. Another factor is the induction of senescence in experimental studies by single stress, whereas, in the physiological state of the organisms, individual cells experience multiple stresses [24]. Therefore, to ensure the reliability of polyphenolic flavonoids on senescence, an approach to mimic the physiological milieu of senescence would be advisable by inducing senescence in cells with various types of stresses. In addition, it is essential to study the effects of these flavonoids in their combined form since they do not exist in plants in isolation.
The bioavailability of polyphenolic flavonoids is generally low due to poor solubility, low permeability, and inferior stability as the liver and intestine metabolize them mostly to glucuronides and sulphates, which severely reduces their effectiveness as therapeutic agents. Moreover, different people have different genetic predispositions towards the bioavailability of these polyphenolic flavonoids, which might affect the different results for studies with polyphenolic flavonoids. Therefore, it is imperative to develop strategies to abrogate the absorption barriers of these polyphenolic flavonoids, such as co-ingestion with enhancers or other macro- and micro-constituents, improving metabolic stability, structural modifications, and novel delivery systems such as carrier complexes, nanotechnology, and cocrystals. Further studies should be geared toward understanding the interactions between genetic variability and flavonoid bioavailability, which could assist in elucidating the role of genetic factors on polyphenolic flavonoid metabolism.
Furthermore, the multi-target effects of these flavonoids also raise a concern about their potential adverse effects and drug interactions in clinical applications. Therefore, it is vital to determine and match their pharmacokinetic profiles with conventional drugs. However, the specific SC types and SASP targeted by polyphenolic flavonoids could be identified, and the mechanisms of action elucidated using in silico and experimental approaches. Therefore, further studies on specific indications of these flavonoids on SC type and SASP are required, which could be used as templates for designing new leads for combinatory therapy in senescence-associated diseases. There is also the need for large-scale randomized clinical trials to validate the clinical effectiveness of flavonoids as senotherapeutics since much of the information available is from preclinical studies. Flavonoids may be developed as nutraceuticals, food supplements, or complementary and alternative medicines in senotherapeutics and geroprotection.
References
Song S, Lam EWF, Tchkonia T, Kirkland JL, Sun Y. Senescent cells: emerging targets for human aging and age-related diseases. Trends Biochem Sci. 2020;45(7):578–92.
Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–63.
Campisi J. Replicative senescence: an old lives’ tale? Cell. 1996;84(4):497–500.
Lagoumtzi SM, Chondrogianni N. Senolytics and senomorphics: natural and synthetic therapeutics in the treatment of aging and chronic diseases. Free Radic Biol Med. 2021;171(8):169–90.
Lim H, Park H, Kim HP. Effects of flavonoids on senescence-associated secretory phenotype formation from bleomycin-induced senescence in BJ fibroblasts. Biochem Pharmacol. 2015;96(4):337–48.
He S, Sharpless NE. Senescence in health and disease. Cell. 2017;169(6):1000–11.
Childs BG, Gluscevic M, Baker DJ, Laberge R-M, Marquess D, Dananberg J, et al. Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov. 2017;16(10):718–35.
Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15(7):482–96.
Martel J, Ojcius DM, Ko Y-F, Ke P-Y, Wu C-Y, Peng H-H, et al. Hormetic effects of phytochemicals on health and longevity. Trends Endocrinol Metab. 2019;30(6):335–46.
Martel J, Ojcius DM, Wu C-Y, Peng H-H, Voisin L, Perfettini J-L, et al. Emerging use of senolytics and senomorphics against aging and chronic diseases. Med Res Rev. 2020;40(6):2114–31.
Tripoli E, Guardia ML, Giammanco S, Majo DD, Giammanco M. Citrus flavonoids: molecular structure, biological activity and nutritional properties: a review. Food Chem. 2007;104(2):466–79.
Yi L, Ma S, Ren D. Phytochemistry and bioactivity of citrus flavonoids: a focus on antioxidant, anti-inflammatory, anticancer and cardiovascular protection activities. Phytochem Rev. 2017;16(3):479–511.
Soto-Gamez A, Demaria M. Therapeutic interventions for aging: the case of cellular senescence. Drug Discov. 2017;22(5):786–95.
Narita M, Nuñez S, Heard E, Narita M, Lin AW, Hearn SA, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113(6):703–16.
Collado M, Serrano M. The power and the promise of oncogene-induced senescence markers. Nat Rev Cancer. 2006;6(6):472–6.
Lopes-Paciencia S, Saint-Germain E, Rowell M-C, Ruiz AF, Kalegari P, Ferbeyre G. The senescence-associated secretory phenotype and its regulation. Cytokine. 2019;117:15–22.
Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75(1):685–705.
Coppé J-P, Rodier F, Patil CK, Freund A, Desprez P-Y, Campisi J. Tumor suppressor and aging biomarker p16INK4a induces cellular senescence without the associated inflammatory secretory phenotype. J Biol Chem. 2011;286(42):36396–403.
Watanabe S, Kawamoto S, Ohtani N, Hara E. Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases. Cancer Sci. 2017;108(4):563–9.
Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–6.
Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644–58.
Wang E. Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer Res. 1995;55(11):2284–92.
Ovadya Y, Krizhanovsky V. Strategies targeting cellular senescence. J Clin Investig. 2018;128(4):1247–54.
Kirkland JL, Tchkonia T. Cellular senescence: a translational perspective. EBioMedicine. 2017;21:21–8.
Romashkan S, Chang H, Hadley EC. National Institute on Aging Workshop: repurposing drugs or dietary supplements for their senolytic or senomorphic effects: considerations for clinical trials. J Gerontol A Biol Sci Med Sci J. 2021;76(6):1144–52.
Ngoi NYL, Liew AQX, Chong SJF, Davids MS, Clement M-V, Pervaiz S. The redox-senescence axis and its therapeutic targeting. Redox Biol. 2021;45: 102032.
von Kobbe C. Targeting senescent cells: approaches, opportunities, challenges. Aging. 2019;11(24):12844–61.
Jeon OH, Kim C, Laberge R-M, Demaria M, Rathod S, Vasserot AP, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23(6):775–81.
Zhu Y, Doornebal EJ, Pirtskhalava T, Giorgadze N, Wentworth M, Fuhrmann-Stroissnigg H, et al. New agents that target senescent cells: the flavone, fisetin, and the BCL-X(L) inhibitors, A1331852 and A1155463. Aging. 2017;9(3):955–63.
He Y, Zhang X, Chang J, Kim H-N, Zhang P, Wang Y, et al. Using proteolysis-targeting chimera technology to reduce navitoclax platelet toxicity and improve its senolytic activity. Nat Commun. 2020;11(1):1996.
Myrianthopoulos V, Evangelou K, Vasileiou PVS, Cooks T, Vassilakopoulos TP, Pangalis GA, et al. Senescence and senotherapeutics: a new field in cancer therapy. Pharmacol Ther. 2019;193:31–49.
Song S, Tchkonia T, Jiang J, Kirkland JL, Sun Y. Targeting senescent cells for a healthier aging: challenges and opportunities. Adv Sci. 2020;7(23):2002611.
Laberge R-M, Sun Y, Orjalo AV, Patil CK, Freund A, Zhou L, et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol. 2015;17(8):1049–61.
Ovadya Y, Landsberger T, Leins H, Vadai E, Gal H, Biran A, et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat Commun. 2018;9(1):5435.
von Kobbe C. Cellular senescence: a view throughout organismal life. Cell Mol Life Sci. 2018;75(19):3553–67.
Sagiv A, Burton DGA, Moshayev Z, Vadai E, Wensveen F, Ben-Dor S, et al. NKG2D ligands mediate immunosurveillance of senescent cells. Aging. 2016;8(2):328–44.
Amor C, Feucht J, Leibold J, Ho Y-J, Zhu C, Alonso-Curbelo D, et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature. 2020;583(7814):127–32.
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217.
Tian Y, Zhang Y, Fu X. β cell senescence as a common contributor to type 1 and type 2 diabetes. Trends Mol Med. 2019;25(9):735–7.
Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15(5):973–7.
Calcinotto A, Kohli J, Zagato E, Pellegrini L, Demaria M, Alimonti A. Cellular senescence: aging, cancer, and injury. Physiol Rev. 2019;99(2):1047–78.
Demaria M, O’Leary MN, Chang J, Shao L, Liu S, Alimirah F, et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 2017;7(2):165–76.
Valentijn FA, Falke LL, Nguyen TQ, Goldschmeding R. Cellular senescence in the aging and diseased kidney. Cell Commun Signal. 2018;12(1):69–82.
Aravinthan AD, Alexander GJM. Senescence in chronic liver disease: is the future in aging? J Hepatol. 2016;65(4):825–34.
Ogrodnik M, Miwa S, Tchkonia T, Tiniakos D, Wilson CL, Lahat A, et al. Cellular senescence drives age-dependent hepatic steatosis. Nat Commun. 2017;8(1):15691.
Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31(6):722–33.
Peilin W, Songsong T, Chengyu Z, Zhi C, Chunhui M, Yinxian Y, et al. Directed elimination of senescent cells attenuates development of osteoarthritis by inhibition of c-IAP and XIAP. Biochim Biophys Acta Mol Basis Dis. 2019;1865(10):2618–32.
King TE, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378(9807):1949–61.
Tan FCC, Hutchison ER, Eitan E, Mattson MP. Are there roles for brain cell senescence in aging and neurodegenerative disorders? Biogerontology. 2014;15(6):643–60.
Cummins NW, Sainski-Nguyen AM, Natesampillai S, Aboulnasr F, Kaufmann S, Badley AD, et al. Maintenance of the HIV reservoir is antagonized by selective BCL2 inhibition. J Virol. 2017;91(11):e00012-17.
Schonhofer C, Yi J, Sciorillo A, Andrae-Marobela K, Cochrane A, Harris M, et al. Flavonoid-based inhibition of cyclin-dependent kinase 9 without concomitant inhibition of histone deacetylases durably reinforces HIV latency. Biochem Pharmacol. 2021;186: 114462.
Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24(8):1246–56.
El-Otmani M, Ait-Oubahou A, Zacarías L. 21—Citrus spp.: orange, mandarin, tangerine, clementine, grapefruit, pomelo, lemon and lime. In: Yahia EM, editor. Postharvest biology and technology of tropical and subtropical fruits. Sawston: Woodhead Publishing; 2011. p. 437e–516e.
Wang M, Zhao H, Wen X, Ho C-T, Li S. Citrus flavonoids and the intestinal barrier: interactions and effects. Compr Rev Food Sci Food Saf. 2021;20(1):225–51.
Nyane NA, Tlaila TB, Malefane TG, Ndwandwe DE, Owira PMO. Metformin-like antidiabetic, cardio-protective and non-glycemic effects of naringenin: molecular and pharmacological insights. Eur J Pharmacol. 2017;803:103–11.
Gao J, Wu Y, He D, Zhu X, Li H, Liu H, et al. Anti-aging effects of Ribes meyeri anthocyanins on neural stem cells and aging mice. Aging. 2020;12(17):17738–53.
Da Pozzo E, Costa B, Cavallini C, Testai L, Martelli A, Calderone V, et al. The citrus flavanone naringenin protects myocardial cells against age-associated damage. Oxid Med Cell Longev. 2017;2017:9536148.
Lim KH, Kim GR. Inhibitory effect of naringenin on LPS-induced skin senescence by SIRT1 regulation in HDFs. Biomed Dermatol. 2018;2(1):26.
Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4(1):2192.
Mbara KC, Mofo Mato PE, Driver C, Nzuza S, Mkhombo NT, Gcwensa SKP, et al. Metformin turns 62 in pharmacotherapy: emergence of non-glycaemic effects and potential novel therapeutic applications. Eur J Pharmacol. 2021;898: 173934.
Manchope MF, Casagrande R, Verri WA Jr. Naringenin: an analgesic and anti-inflammatory citrus flavanone. Oncotarget. 2017;8(3):3766–7.
Habauzit V, Sacco SM, Gil-Izquierdo A, Trzeciakiewicz A, Morand C, Barron D, et al. Differential effects of two citrus flavanones on bone quality in senescent male rats in relation to their bioavailability and metabolism. Bone. 2011;49(5):1108–16.
Tsai Y-F, Chen Y-R, Chen J-P, Tang Y, Yang K-C. Effect of hesperidin on anti-inflammation and cellular antioxidant capacity in hydrogen peroxide-stimulated human articular chondrocytes. Process Biochem. 2019;85:175–84.
Rizza S, Muniyappa R, Iantorno M, Kim J, Chen H, Pullikotil P, et al. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J Clin Endocr Metab. 2011;96(5):E782–92.
Fu Z, Chen Z, Xie Q, Lei H, Xiang S. Hesperidin protects against IL-1β-induced inflammation in human osteoarthritis chondrocytes. Exp Ther Med. 2018;16(4):3721–7.
Choi EM, Lee YS. Effects of hesperetin on the production of inflammatory mediators in IL-1β treated human synovial cells. Cell Immunol. 2010;264(1):1–3.
Chondrogianni N, Kapeta S, Chinou I, Vassilatou K, Papassideri I, Gonos ES. Anti-ageing and rejuvenating effects of quercetin. Exp Gerontol. 2010;45(10):763–71.
Belinha I, Amorim MA, Rodrigues P, de Freitas V, Moradas-Ferreira P, Mateus N, et al. Quercetin increases oxidative stress resistance and longevity in Saccharomyces cerevisiae. J Agric Food Chem. 2007;55(6):2446–51.
Kampkötter A, Timpel C, Zurawski RF, Ruhl S, Chovolou Y, Proksch P, et al. Increase of stress resistance and lifespan of Caenorhabditis elegans by quercetin. Comp Biochem Physiol B Biochem Mol Biol. 2008;149(2):314–23.
Geng L, Liu Z, Zhang W, Li W, Wu Z, Wang W, et al. Chemical screen identifies a geroprotective role of quercetin in premature aging. Protein Cell. 2019;10(6):417–35.
Kim SR, Jiang K, Ogrodnik M, Chen X, Zhu X-Y, Lohmeier H, et al. Increased renal cellular senescence in murine high-fat diet: effect of the senolytic drug quercetin. Transl Res. 2019;213:112–23.
Shao Z, Wang B, Shi Y, Xie C, Huang C, Chen B, et al. Senolytic agent Quercetin ameliorates intervertebral disc degeneration via the Nrf2/NF-κB axis. Osteoarthr Cartil. 2021;29(3):413–22.
Hickson LJ, Langhi Prata LGP, Bobart SA, Evans TK, Giorgadze N, Hashmi SK, et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019;47:446–56.
Currais A, Prior M, Dargusch R, Armando A, Ehren J, Schubert D, et al. Modulation of p25 and inflammatory pathways by fisetin maintains cognitive function in Alzheimer’s disease transgenic mice. Aging Cell. 2014;13(2):379–90.
Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8(1):14532.
Yousefzadeh MJ, Zhu Y, McGowan SJ, Angelini L, Fuhrmann-Stroissnigg H, Xu M, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18–28.
Kampkötter A, Gombitang Nkwonkam C, Zurawski RF, Timpel C, Chovolou Y, Wätjen W, et al. Effects of the flavonoids kaempferol and fisetin on thermotolerance, oxidative stress and FoxO transcription factor DAF-16 in the model organism Caenorhabditis elegans. Arch Toxicol. 2007;81(12):849–58.
Li Y, Qin R, Yan H, Wang F, Huang S, Zhang Y, et al. Inhibition of vascular smooth muscle cells premature senescence with rutin attenuates and stabilizes diabetic atherosclerosis. J Nutr Biochem. 2018;51:91–8.
Williamson G, Plumb GW, Uda Y, Price KR, Rhodes MJC. Dietary quercetin glycosides: antioxidant activity and induction of the anticarcinogenic phase II marker enzyme quinone reductase in Hepalclc7 cells. Carcinogenesis. 1996;17(11):2385–7.
Wang L, Wang L, Wang T, Li Z, Gao Y, Cui SW, et al. Comparison of quercetin and rutin inhibitory influence on Tartary buckwheat starch digestion in vitro and their differences in binding sites with the digestive enzyme. Food Chem. 2022;367: 130762.
Perrott KM, Wiley CD, Desprez P-Y, Campisi J. Apigenin suppresses the senescence-associated secretory phenotype and paracrine effects on breast cancer cells. GeroScience. 2017;39(2):161–73.
Banerjee K, Mandal M. Oxidative stress triggered by naturally occurring flavone apigenin results in senescence and chemotherapeutic effect in human colorectal cancer cells. Redox Biol. 2015;5:153–62.
Gendrisch F, Esser PR, Schempp CM, Wölfle U. Luteolin as a modulator of skin aging and inflammation. BioFactors. 2021;47(2):170–80.
Chen C-Y, Peng W-H, Tsai K-D, Hsu S-L. Luteolin suppresses inflammation-associated gene expression by blocking NF-κB and AP-1 activation pathway in mouse alveolar macrophages. Life Sci. 2007;81(23):1602–14.
Chen L-G, Hung L-Y, Tsai K-W, Pan Y-S, Tsai Y-D, Li Y-Z, et al. Wogonin, a bioactive flavonoid in herbal tea, inhibits inflammatory cyclooxygenase-2 gene expression in human lung epithelial cancer cells. Mol Nutr Food Res. 2008;52(11):1349–57.
Chen Y-C, Shen S-C, Lee W-R, Lin H-Y, Ko C-H, Shih C-M, et al. Wogonin and fisetin induction of apoptosis through activation of caspase 3 cascade and alternative expression of p21 protein in hepatocellular carcinoma cells SK-HEP-1. Arch Toxicol. 2002;76(5):351–9.
Yang D, Tian X, Ye Y, Liang Y, Zhao J, Wu T, et al. Identification of GL-V9 as a novel senolytic agent against senescent breast cancer cells. Life Sci. 2021;272: 119196.
Huang H, Li L, Shi W, Liu H, Yang J, Yuan X, et al. The multifunctional effects of nobiletin and its metabolites in vivo and in vitro. Evid Based Complement Altern Med. 2016;2016:2918796.
Nohara K, Mallampalli V, Nemkov T, Wirianto M, Yang J, Ye Y, et al. Nobiletin fortifies mitochondrial respiration in skeletal muscle to promote healthy aging against metabolic challenge. Nat Commun. 2019;10(1):3923.
Keshtkar S, Kaviani M, Jabbarpour Z, Geramizadeh B, Motevaseli E, Nikeghbalian S, et al. Protective effect of nobiletin on isolated human islets survival and function against hypoxia and oxidative stress-induced apoptosis. Sci Rep. 2019;9(1):11701.
Murakami A, Nakamura Y, Torikai K, Tanaka T, Koshiba T, Koshimizu K, et al. Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Res. 2000;60(18):5059–66.
Hsiao P-C, Lee W-J, Yang S-F, Tan P, Chen H-Y, Lee L-M, et al. Nobiletin suppresses the proliferation and induces apoptosis involving MAPKs and caspase-8/-9/-3 signals in human acute myeloid leukemia cells. Tumor Biol. 2014;35(12):11903–11.
Ma X, Jin S, Zhang Y, Wan L, Zhao Y, Zhou L. Inhibitory effects of nobiletin on hepatocellular carcinoma in vitro and in vivo. Phytother Res. 2014;28(4):560–7.
Dusabimana T, Kim SR, Kim HJ, Park SW, Kim H. Nobiletin ameliorates hepatic ischemia and reperfusion injury through the activation of SIRT-1/FOXO3a-mediated autophagy and mitochondrial biogenesis. Exp Mol Med. 2019;51(4):1–16.
Xie L, Xie H, Chen C, Tao Z, Zhang C, Cai L. Inhibiting the PI3K/AKT/NF-κB signal pathway with nobiletin for attenuating the development of osteoarthritis: in vitro and in vivo studies. Food Funct. 2019;10(4):2161–75.
Raza W, Luqman S, Meena A. Prospects of tangeretin as a modulator of cancer targets/pathways. Pharmacol Res. 2020;161: 105202.
Yang T, Feng C, Wang D, Qu Y, Yang Y, Wang Y, et al. Neuroprotective and anti-inflammatory effect of tangeretin against cerebral ischemia-reperfusion injury in rats. Inflammation. 2020;43(6):2332–43.
Lee YY, Lee E-J, Park J-S, Jang S-E, Kim D-H, Kim H-S. Anti-inflammatory and antioxidant mechanism of tangeretin in activated microglia. J Neuroimmune Pharmacol. 2016;11(2):294–305.
Mária J, Ingrid Ž. Effects of bioactive compounds on senescence and components of senescence associated secretory phenotypes in vitro. Food Funct. 2017;8(7):2394–418.
Lu Q, Lv S, Peng Y, Zhu C, Pan S. Characterization of phenolics and antioxidant abilities of red navel orange “Cara Cara” harvested from five regions of China. Int J Food Prop. 2018;21(1):1107–16.
Lee KY, Kim J-R, Choi HC. Genistein-induced LKB1–AMPK activation inhibits senescence of VSMC through autophagy induction. Vascul Pharmacol. 2016;81:75–82.
Kumar R, Sharma A, Kumari A, Gulati A, Padwad Y, Sharma R. Epigallocatechin gallate suppresses premature senescence of preadipocytes by inhibition of PI3K/Akt/mTOR pathway and induces senescent cell death by regulation of Bax/Bcl-2 pathway. Biogerontology. 2019;20(2):171–89.
Lilja S, Oldenburg J, Pointner A, Dewald L, Lerch M, Hippe B, et al. Epigallocatechin gallate effectively affects senescence and anti-SASP via <i>SIRT3</i> in 3T3-L1 preadipocytes in comparison with other bioactive substances. Oxid Med Cell Longev. 2020;2020:4793125.
Xiao Y-Z, Yang M, Xiao Y, Guo Q, Huang Y, Li C-J, et al. Reducing hypothalamic stem cell senescence protects against aging-associated physiological decline. Cell Metab. 2020;31(3):534-48.e5.
Cai G, Xiao Y, Yang M, Guo Q, Su T, Liu Y, et al. Long noncoding RNA Gm31629 promotes bone regeneration by maintaining bone marrow mesenchymal stem cells activity. PeerJ. 2022;10: e13475.
Xu Q, Fu Q, Li Z, Liu H, Wang Y, Lin X, et al. The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice. Nat Metab. 2021;3(12):1706–26.
Bhadresha K, Kumar SP, Brahmbhatt J, Patel C, Pandya P, Jain N, et al. Theaflavin-3-gallate, a natural antagonist for Hsp 90: in-silico and in-vitro approach. Chem Biol Interact. 2022;353: 109774.
Cassidy A, Rimm EB, O’Reilly ÉJ, Logroscino G, Kay C, Chiuve SE, et al. Dietary flavonoids and risk of stroke in women. Stroke. 2012;43(4):946–51.
Joshipura KJ, Hu FB, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med. 2001;134(12):1106–14.
Mahmoud AM, Hernández Bautista RJ, Sandhu MA, Hussein OE. Beneficial effects of citrus flavonoids on cardiovascular and metabolic health. Oxid Med Cell Longev. 2019;2019:5484138.
Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong C-P, Nettleton JA, et al. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr. 2007;85(3):895–909.
Jung UJ, Kim HJ, Lee JS, Lee MK, Kim HO, Park EJ, et al. Naringin supplementation lowers plasma lipids and enhances erythrocyte antioxidant enzyme activities in hypercholesterolemic subjects. Clin Nutr. 2003;22(6):561–8.
Reshef N, Hayari Y, Goren C, Boaz M, Madar Z, Knobler H. Antihypertensive effect of sweetie fruit in patients with stage I hypertension. Am J Hypertens. 2005;18(10):1360–3.
Kim S-W, Kim CE, Kim MH. Flavonoids inhibit high glucose-induced up-regulation of ICAM-1 via the p38 MAPK pathway in human vein endothelial cells. Biochem Biophys Res Commun. 2011;415(4):602–7.
Zhang Q-Y, Pan Y, Wang R, Kang L-L, Xue Q-C, Wang X-N, et al. Quercetin inhibits AMPK/TXNIP activation and reduces inflammatory lesions to improve insulin signaling defect in the hypothalamus of high fructose-fed rats. J Nutr Biochem. 2014;25(4):420–8.
Mahmoud AM, Ahmed OM, Ashour MB, Abdel-Moneim A. In vivo and in vitro antidiabetic effects of citrus flavonoids; a study on the mechanism of action. Int J Diabetes Dev Ctries. 2015;35(3):250–63.
Fang X-K, Gao J, Zhu D-N. Kaempferol and quercetin isolated from Euonymus alatus improve glucose uptake of 3T3-L1 cells without adipogenesis activity. Life Sci. 2008;82(11):615–22.
Prince PSM, Kamalakkannan N. Rutin improves glucose homeostasis in streptozotocin diabetic tissues by altering glycolytic and gluconeogenic enzymes. J Biochem Mol. 2006;20(2):96–102.
Zhao L, Wang J-L, Liu R, Li X-X, Li J-F, Zhang L. Neuroprotective, anti-amyloidogenic and neurotrophic effects of apigenin in an Alzheimer’s disease mouse model. Molecules. 2013;18(8):9949–65.
Heo HJ, Kim MJ, Lee JM, Choi SJ, Cho HY, Hong B, et al. Naringenin from Citrus junos has an inhibitory effect on acetylcholinesterase and a mitigating effect on amnesia. Dement Geriatr Cogn Disord. 2004;17(3):151–7.
Huang S-M, Tsai S-Y, Lin J-A, Wu C-H, Yen G-C. Cytoprotective effects of hesperetin and hesperidin against amyloid β-induced impairment of glucose transport through downregulation of neuronal autophagy. Mol Nutr Food Res. 2012;56(4):601–9.
Cirmi S, Ferlazzo N, Lombardo GE, Maugeri A, Calapai G, Gangemi S, et al. Chemopreventive agents and inhibitors of cancer hallmarks: may citrus offer new perspectives? Nutrients. 2016;8(11):698.
Rawson NE, Ho C-T, Li S. Efficacious anti-cancer property of flavonoids from citrus peels. Food Sci Hum Wellness. 2014;3(3):104–9.
Meiyanto E, Hermawan A, Anindyajati A. Natural products for cancer-targeted therapy: citrus flavonoids as potent chemopreventive agents. Asian Pac J Cancer Prev. 2012;13(2):427–36.
Le Marchand LC, Murphy SP, Hankin JH, Wilkens LR, Kolonel LN. Intake of flavonoids and lung cancer. J Natl Cancer Inst. 2000;92(2):154–60.
Rooprai HK, Kandanearatchi A, Maidment SL, Christidou M, Trillo-Pazos G, Dexter DT, et al. Evaluation of the effects of swainsonine, captopril, tangeretin and nobiletin on the biological behaviour of brain tumour cells in vitro. Neuropathol Appl Neurobiol. 2001;27(1):29–39.
Szaniawski MA, Spivak AM. Senotherapeutics for HIV and aging. Curr Opin HIV AIDS. 2020;15(2):83–93.
Bermejo M, López-Huertas MR, García-Pérez J, Climent N, Descours B, Ambrosioni J, et al. Dasatinib inhibits HIV-1 replication through the interference of SAMHD1 phosphorylation in CD4+ T cells. Biochem Pharmacol. 2016;106:30–45.
Yosef R, Pilpel N, Tokarsky-Amiel R, Biran A, Ovadya Y, Cohen S, et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun. 2016;7(1):11190.
Chang J, Wang Y, Shao L, Laberge R-M, Demaria M, Campisi J, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22(1):78–83.
Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. 2016;15(3):428–35.
Pan J, Li D, Xu Y, Zhang J, Wang Y, Chen M, et al. Inhibition of Bcl-2/xl with ABT-263 selectively kills senescent type II pneumocytes and reverses persistent pulmonary fibrosis induced by ionizing radiation in mice. Int J Radiat Oncol Biol Phys. 2017;99(2):353–61.
Muñoz-Espín D, Rovira M, Galiana I, Giménez C, Lozano-Torres B, Paez-Ribes M, et al. A versatile drug delivery system targeting senescent cells. EMBO Mol Med. 2018;10(9): e9355.
Galiana I, Lozano-Torres B, Sancho M, Alfonso M, Bernardos A, Bisbal V, et al. Preclinical antitumor efficacy of senescence-inducing chemotherapy combined with a nanoSenolytic. J Control Release. 2020;323:624–34.
Ekpenyong-Akiba AE, Canfarotta F, Abd HB, Poblocka M, Casulleras M, Castilla-Vallmanya L, et al. Detecting and targeting senescent cells using molecularly imprinted nanoparticles. Nanoscale Horiz. 2019;4(3):757–68.
Fuhrmann-Stroissnigg H, Ling YY, Zhao J, McGowan SJ, Zhu Y, Brooks RW, et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun. 2017;8(1):422.
Palmer AK, Xu M, Zhu Y, Pirtskhalava T, Weivoda MM, Hachfeld CM, et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell. 2019;18(3): e12950.
Zhang P, Kishimoto Y, Grammatikakis I, Gottimukkala K, Cutler RG, Zhang S, et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat Neurosci. 2019;22(5):719–28.
Ozsvari B, Nuttall JR, Sotgia F, Lisanti MP. Azithromycin and Roxithromycin define a new family of “senolytic” drugs that target senescent human fibroblasts. Aging. 2018;10(11):3294–307.
He Y, Li W, Lv D, Zhang X, Zhang X, Ortiz YT, et al. Inhibition of USP7 activity selectively eliminates senescent cells in part via restoration of p53 activity. Aging Cell. 2020;19(3): e13117.
Baar MP, Brandt RMC, Putavet DA, Klein JDD, Derks KWJ, Bourgeois BRM, et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell. 2017;169(1):132-47.e16.
Li W, He Y, Zhang R, Zheng G, Zhou D. The curcumin analog EF24 is a novel senolytic agent. Aging. 2019;11(2):771–82.
Nogueira-Recalde U, Lorenzo-Gómez I, Blanco FJ, Loza MI, Grassi D, Shirinsky V, et al. Fibrates as drugs with senolytic and autophagic activity for osteoarthritis therapy. EBioMedicine. 2019;45:588–605.
Triana-Martínez F, Picallos-Rabina P, Da Silva-Álvarez S, Pietrocola F, Llanos S, Rodilla V, et al. Identification and characterization of cardiac glycosides as senolytic compounds. Nat Commun. 2019;10(1):4731.
Wang Y, Chang J, Liu X, Zhang X, Zhang S, Zhang X, et al. Discovery of piperlongumine as a potential novel lead for the development of senolytic agents. Aging. 2016;8(11):2915–26.
Zhang X, Zhang S, Liu X, Wang Y, Chang J, Zhang X, et al. Oxidation resistance 1 is a novel senolytic target. Aging Cell. 2018;17(4): e12780.
Samaraweera L, Adomako A, Rodriguez-Gabin A, McDaid HM. A novel indication for panobinostat as a senolytic drug in NSCLC and HNSCC. Sci Rep. 2017;7(1):1900.
Laberge R-M, Zhou L, Sarantos MR, Rodier F, Freund A, de Keizer PLJ, et al. Glucocorticoids suppress selected components of the senescence-associated secretory phenotype. Aging Cell. 2012;11(4):569–78.
Cherif H, Bisson DG, Jarzem P, Weber M, Ouellet JA, Haglund L. Curcumin and o-vanillin exhibit evidence of senolytic activity in human IVD Cells in vitro. J Clin Med. 2019;8(4):433.
Jiang L, Jin Y, Wang H, Jiang Y, Dong J. Glucosamine protects nucleus pulposus cells and induces autophagy via the mTOR-dependent pathway. J Orthop Res. 2014;32(11):1532–42.
Kao C-L, Chen L-K, Chang Y-L, Yung M-C, Hsu C-C, Chen Y-C, et al. Resveratrol protects human endothelium from H2O2-induced oxidative stress and senescence via SirT1 activation. J Atheroscler Thromb. 2010;17(9):970–9.
Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191–6.
Moiseeva O, Deschênes-Simard X, St-Germain E, Igelmann S, Huot G, Cadar AE, et al. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activation. Aging Cell. 2013;12(3):489–98.
Jadhav KS, Dungan CM, Williamson DL. Metformin limits ceramide-induced senescence in C2C12 myoblasts. Mech Ageing Dev. 2013;134(11):548–59.
Noren Hooten N, Martin-Montalvo A, Dluzen DF, Zhang Y, Bernier M, Zonderman AB, et al. Metformin-mediated increase in DICER1 regulates microRNA expression and cellular senescence. Aging Cell. 2016;15(3):572–81.
Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci USA. 2015;112(46):E6301–10.
Cao K, Graziotto JJ, Blair CD, Mazzulli JR, Erdos MR, Krainc D, et al. Rapamycin reverses cellular phenotypes and enhances mutant protein clearance in Hutchinson–Gilford progeria syndrome cells. Sci Transl Med. 2011;3(89):89ra58.
Iglesias-Bartolome R, Patel V, Cotrim A, Leelahavanichkul K, Molinolo Alfredo A, Mitchell James B, et al. mTOR inhibition prevents epithelial stem cell senescence and protects from radiation-induced mucositis. Cell Stem Cell. 2012;11(3):401–14.
Wang R, Yu Z, Sunchu B, Shoaf J, Dang I, Zhao S, et al. Rapamycin inhibits the secretory phenotype of senescent cells by a Nrf2-independent mechanism. Aging Cell. 2017;16(3):564–74.
Thapa RK, Nguyen HT, Jeong J-H, Kim JR, Choi H-G, Yong CS, et al. Progressive slowdown/prevention of cellular senescence by CD9-targeted delivery of rapamycin using lactose-wrapped calcium carbonate nanoparticles. Sci Rep. 2017;7(1):43299.
Nguyen HT, Thapa RK, Shin BS, Jeong J-H, Kim J-R, Yong CS, et al. CD9 monoclonal antibody-conjugated PEGylated liposomes for targeted delivery of rapamycin in the treatment of cellular senescence. Nanotechnology. 2017;28(9): 095101.
Liu S, Uppal H, Demaria M, Desprez P-Y, Campisi J, Kapahi P. Simvastatin suppresses breast cancer cell proliferation induced by senescent cells. Sci Rep. 2015;5(1):17895.
Kang HT, Park JT, Choi K, Kim Y, Choi HJC, Jung CW, et al. Chemical screening identifies ATM as a target for alleviating senescence. Nat Chem Biol. 2017;13(6):616–23.
Tilstra JS, Robinson AR, Wang J, Gregg SQ, Clauson CL, Reay DP, et al. NF-κB inhibition delays DNA damage–induced senescence and aging in mice. J Clin Investig. 2012;122(7):2601–12.
Palmer AK, Tchkonia T, LeBrasseur NK, Chini EN, Xu M, Kirkland JL. Cellular senescence in type 2 diabetes: a therapeutic opportunity. Diabetes. 2015;64(7):2289–98.
Ghorbani A, Nazari M, Jeddi-Tehrani M, Zand H. The citrus flavonoid hesperidin induces p53 and inhibits NF-κB activation in order to trigger apoptosis in NALM-6 cells: involvement of PPARγ-dependent mechanism. Eur J Nutr. 2012;51(1):39–46.
Elavarasan J, Velusamy P, Ganesan T, Ramakrishnan SK, Rajasekaran D, Periandavan K. Hesperidin-mediated expression of Nrf2 and upregulation of antioxidant status in senescent rat heart. J Pharm Pharmacol. 2012;64(10):1472–82.
Lee YR, Cho HM, Park EJ, Zhang M, Doan TP, Lee BW, et al. Metabolite profiling of Rambutan (Nephelium lappaceum L.) seeds using UPLC-qTOF-MS/MS and senomorphic effects in aged human dermal fibroblasts. Nutrients. 2020;12(5):1430.
Wölfle U, Heinemann A, Esser PR, Haarhaus B, Martin SF, Schempp CM. Luteolin prevents solar radiation-induced matrix metalloproteinase-1 activation in human fibroblasts: a role for p38 mitogen-activated protein kinase and interleukin-20 released from keratinocytes. Rejuvenation Res. 2012;15(5):466–75.
Martinez RM, Pinho-Ribeiro FA, Steffen VS, Caviglione CV, Vignoli JA, Barbosa DS, et al. Naringenin inhibits UVB irradiation-induced inflammation and oxidative stress in the skin of hairless mice. J Nat Prod. 2015;78(7):1647–55.
Kometsi L, Govender K, Mofo Mato EP, Hurchund R, Owira PMO. By reducing oxidative stress, naringenin mitigates hyperglycaemia-induced upregulation of hepatic nuclear factor erythroid 2-related factor 2 protein. J Pharm Pharmacol. 2020;72(10):1394–404.
Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med. 2017;23(9):1072–9.
Hohmann MS, Habiel DM, Coelho AL, Waldiceu A, Verri J, Hogaboam CM. Quercetin enhances ligand-induced apoptosis in senescent idiopathic pulmonary fibrosis fibroblasts and reduces lung fibrosis in vivo. Am J Respir Cell Mol Biol. 2019;60(1):28–40.
Sohn E-J, Kim JM, Kang S-H, Kwon J, An HJ, Sung J-S, et al. Restoring effects of natural anti-oxidant quercetin on cellular senescent human dermal fibroblasts. Am J Chin Med. 2018;46(04):853–73.
Lust S, Vanhoecke B, Van Gele M, Philippé J, Bracke M, Offner F. The flavonoid tangeretin activates the unfolded protein response and synergizes with imatinib in the erythroleukemia cell line K562. Mol Nutr Food Res. 2010;54(6):823–32.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this paper.
Conflict of interest
None to declare.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Author contributions
MKC reviewed the literature, and wrote and edited the manuscript. DN reviewed and edited the manuscript. OPMO conceptualized, reviewed, and critically edited the manuscript.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mbara, K.C., Devnarain, N. & Owira, P.M.O. Potential Role of Polyphenolic Flavonoids as Senotherapeutic Agents in Degenerative Diseases and Geroprotection. Pharm Med 36, 331–352 (2022). https://doi.org/10.1007/s40290-022-00444-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40290-022-00444-w