Abstract
The need for information about new and existing drugs used in children was recognized in the European Union (EU) with the implementation of the Paediatric Regulation in 2007. In 2017, the 10-year review of the Paediatric Regulation identified barriers to the conduct of clinical trials, including delays in setting up and completing paediatric trials. Across Europe, the difficulties with clinical research are compounded by variation within countries and between countries. Ethics and regulatory review have national specificities. This paper describes the Collaborative Network for European Clinical Trials for Children (conect4children, c4c), which addresses selected difficulties in the design and conduct of paediatric clinical trials. c4c is a time-limited public–private consortium funded by the Innovative Medicines Initiative (IMI2). The elements of c4c are as follows: expert advice providing input on study design and/or paediatric development programmes (including patient involvement activities); a network of sites following harmonised procedures coordinated by National Hubs and a single point of contact for Europe; a facility for education and training for sites and trial teams; and support for managing data used by the network and a common paediatric data dictionary. c4c does not sponsor trials. c4c is taking a phased approach with careful piloting through industry and non-industry studies intended to demonstrate the viability of the network (proof-of-viability studies). c4c uses a co-design approach involving industry and academics within a clearly defined scope. A sustainable, successor organization open to all potential service users will be open for business before the end of IMI2 funding in 2024.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
c4c is a time-limited public–private consortium that is co-designing a clinical trials network that will address problems with designing and executing trials that recruit babies, children and young people. |
c4c is piloting its work with a small selection of studies but will scale up when a successor organization is established before mid-2024. |
1 Introduction
The European Union (EU) has recognized the need to facilitate the development of medicines for children with the Paediatric Regulation launched in 2007. The Paediatric Regulation requires all sponsors who wish to market a new medicine, such as an application for a new indication, new pharmaceutical form or a new route of administration, to discuss a Paediatric Investigational Plan (PIP) with the European Medicines Agency (EMA) [1, 2]. The PIP may result in a paediatric development programme, or may be waived if the condition does not exist in children. Similar approaches are taken in countries that are within geographical Europe but not members of the EU and in the United States of America. Many trials are not done to support marketing so a significant number of paediatric clinical drug and biologic trials conducted across Europe take place outside the requirements of the Paediatric Regulation.
The 10-year review in 2017 of the Paediatric Regulation recognized a significant increase in the number of studies that are designed to support a paediatric indication [3]. The 10-year review also identified some barriers to the conduct of clinical trials included in PIPs. The barriers identified by the review included significant difficulties with recruitment due to scarce populations, particularly in rare diseases; difficulties with formulations; and problems with endpoints. Forty-three percent of modifications to PIPs related to timelines because of delays completing studies while 14% of modifications related to reductions in sample size. Delays in setting up and completing paediatric trials arise due to a number of challenges. These include developing contracts between sites and sponsors that are specific to each site. Templates for contracts can speed up this process; templates for clinical trial agreements are available in some, but not all European countries. Other challenges include obtaining approvals from ethics committees or institutional review boards (IRBs), and obtaining parental consent. Other difficulties are symptomatic of problems with the ‘clinical trial enterprise’ that are not unique to paediatrics and have been recognized for some time, including inadequate investigator training and recruitment, insufficient support staff and support systems for clinical trials, inconsistent data harmonization and lack of opportunities to incorporate the voices of participants and their families [4]. Across Europe, the difficulties with clinical research are compounded by variation within countries and between countries. Ethics and regulatory review have national and local specificities that relate, for example, to variation in attitudes to risk when participants cannot give consent [5].
Paediatric research needs to adapt to the specificities of babies, children and young people [6, 7]. Paediatric specificities include (1) ontogeny, so that study designs need to account for differences in drug disposition and effect between age groups; (2) practical issues such as the size of the child, behaviour during health care and capacity for assent and consent. The paediatric specificities are best addressed continuously throughout the drug development process so that study design and conduct are integrated.
There is an acute need to address the problems of the clinical trial enterprise in general, the variation across European countries and adaptation to paediatric specificities. This paper describes a time-limited consortium, the Collaborative Network for European Clinical Trials for Children (conect4children, or c4c), that addresses selected difficulties with the conduct and design of paediatric clinical trials. c4c does not aim to address all of the difficulties but focuses on difficulties that can be addressed operationally through a public–private partnership. c4c is not a lobbying organization so cannot address the nature and content of the Paediatric Regulation or variation between ethics committees/IRBs. c4c will work within the European legal and regulatory framework and will not lead on scientific issues. Rather, c4c will provide coordination between scientific groups. Clinical trial scientists and teams have been working to improve the way paediatric trials are conducted for several decades with partial success. We posit that enhanced, transnational coordination will improve the delivery of paediatric clinical trials. The aim of this review is to describe c4c’s roles in the European context and progress toward a harmonized, operational approach to the implementation and coordination of clinical trials.
2 c4c: The Initiative and its Approach
c4c is an action under the Innovative Medicines Initiative 2 (IMI2) Joint Understanding [8], Grant Agreement 777389. IMI2 is a public–private partnership that aims to improve health by speeding up the development of innovative medicines, particularly in areas where there is an unmet medical or social need. IMI is the world’s biggest public–private partnership in the life sciences. The partnership includes the European Commission (EC) (representing the EU) and the European Federation of Pharmaceutical Industries and Associations (EFPIA) (representing the pharmaceutical industry) and is funded jointly by these two parties. While industry contributes to the projects with in-kind consortium capacity and knowhow, the EC matches these contributions to fund activities provided by academia, small- to medium-sized enterprises, and other non-industry groups. The c4c consortium includes 10 large pharmaceutical companies and 34 non-industry partners including academia, hospitals, third-sector organizations and patient advocacy groups [9].
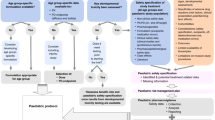
The goal of the c4c consortium is to set up and evaluate selected elements of a paediatric-focused clinical trial infrastructure that are tailored to meet the needs of children involved in clinical trials. The elements are as follows: expert advice providing input on study design and/or paediatric development programmes (including patient involvement activities); a network of sites following harmonised procedures and coordinated by national hubs (NH) and a single point of contact (SPoC) for Europe; a facility for education and training for sites and trial teams; and support for managing data used by the network and a common paediatric data dictionary. c4c does not sponsor trials. The progress and goals of c4c are shown in Fig. 1.
In order to develop a robust approach to the research infrastructure, c4c has adopted the following principles:
-
1.
A phased approach with careful piloting of the procedures used by c4c. The expert advice groups are taking requests for advice from within the consortium. The network of sites is working with studies intended to demonstrate the viability of the network (proof-of-viability [PoV] studies). c4c is working with three non-industry trials and at least four industry trials and learning from real-world experience before scale-up to sponsors outside the c4c Consortium. The educational and data workstreams are collaborating with the expert advice groups and the network of sites to facilitate their work addressing the challenges outlined in the Introduction.
-
2.
Collaboration and partnership with representatives of big pharma, NH, sites, investigators and patient experts. The development of each aspect of the network (particularly processes and workstreams) is co-led by industry and academia to ensure alignment between the two cultures and future buy-in from both.
-
3.
Focus on quality and performance management. c4c has a unified quality framework that states principles by which the consortium will ensure that it delivers what it plans to do, when it plans to do it. The principles are operationalized for each workstream. Quality management is owned by each workstream which are accountable to the Project Steering Committee (the successor organization will have similar, well-defined reporting lines). A Quality Committee ensures that each workstream has the tools to manage the quality of its work and will selectively review work to control and assure quality. The consortium has defined metrics for its activities that will inform performance management of all activities.
-
4.
Work with existing strengths when possible. c4c does not aim to replace or duplicate what is already available and effectively working, but to address specific gaps in the paediatric clinical trials enterprise. The consortium includes specialty networks Penta (HIV/AIDS and paediatric infectious diseases) [10], PRINTO (paediatric rheumatology) [11], Treat-NMD (neuromuscular disorders) [12], ECFS-CTN (cystic fibrosis) [13], SIOPE (paediatric oncology) [14] and ECNP (child and adolescent mental health) [15]. In addition, c4c is represented in the European Joint Programme on Rare Diseases [16] and the European Network of Paediatric Research at the EMA [17].
-
5.
Support sustainability. The goal of the c4c consortium is to develop a sustainable set of key services relating to scientific feasibility, (expert) advice on trial design, preparedness, planning, operational feasibility, implementation and coordination of clinical trials, education and training and the information systems that support its work. The nature of the services that become sustainable, and the mechanisms for sustainability, have not been defined in advance but will be identified based on the needs of key stakeholders (investigators, sites and trial sponsors) and the experiences gained during work with the PoV studies. Work on the business model and legal structures for a successor organization is under way. The aim is to set up a successor organization as an independent legal entity before the end of the funded Consortium in 2024.
-
6.
Develop processes for work within c4c that can be adapted to the needs of service providers and customers as the organization grows.
-
7.
Facilitate patient-centric clinical trials. c4c will provide services along the drug development pathway that will give sponsors opportunities to engage with patients, families and caregivers to work on the co-design of clinical trials according to patients’ needs.
-
8.
Coordination and synergy within the consortium, with cross-cutting work between different workstreams, such as education for investigators (network of sites and education team), well specified and tested information systems (information team and all other workstreams), quality assurance of expert advice (quality team and expert advice team).
-
9.
Contribute to global interoperability for paediatric clinical trials by working with similar initiatives outside the EU.
As an IMI project, c4c services can only be used by members of the consortium. This is because the consortium is funded by commitments from IMI2 with in-kind contributions from the industry members of the Consortium. After end of the IMI funding, when network operations are transferred to an independent new legal entity, services will be available to all sponsors, industry, academia, advocate-driven groups and to intermediary organizations such as contract research organizations (CROs). The final organizational and revenue structure of the successor organization has not yet been defined, as it will be developed as part of the work of the IMI2-funded consortium.
3 The Work of c4c
The work of c4c is summarised in Table 1, which describes the selected difficulties with paediatric clinical trials that c4c is addressing.
3.1 Design of Paediatric Investigational Plans (PIPs) and Clinical Trial Protocols
Some PIPs and protocols do not succeed because they do not fit with clinical practice or because assumptions about participant availability are flawed. In addition, methodology may be suboptimal: extrapolation, adaptive designs and innovative methodologies are underutilized. In order to address these problems, c4c has set up a database of > 300 experts in methodology and clinical subspecialties, as well patient and parent representatives. This database is managed by the advisory group secretariat, which reports to the c4c Network Management Committee (Fig. 2). The clinical experts are organized in subspecialty groups, as much as possible in connection with the relevant learned society or existing research network. The innovative methodology experts are organized in groups that include study methodology (classical and innovative), pharmacometrics, health technology assessment (HTA), developmental pharmacology, formulations, ethics, pharmacogenomics and other omics technologies and pharmacovigilance. Parent and patient experts are connected by a network of young patient advisory groups (YPAGs), disease-oriented patient organizations (PO), as well as individual patients. When academic or industry sponsors seek advice from c4c on their paediatric studies or programmes, the advisory group secretariat forms an ad-hoc strategic feasibility group including two to eight relevant clinical, methodology and/or parent/patient experts. The ad-hoc expert group then meets face to face or online to address the sponsor’s specific questions. These questions can be simple, such as evaluation of an informed consent form or a specific outcome measure, or more complicated such as advice on strategic decisions for PIPs. To optimize the contracting process of experts, a centralised contracting process will be implemented with a single master agreement between each industry or academic sponsor and c4c advisory group secretariat. With respect to transparency and compliance related to working with health care professionals, c4c has implemented processes where payment in line with fair market value and adherence to the EFPIA Code of Practice for industry sponsors is ensured. Since the inclusion of our experts in the standing expert groups in 2019, c4c has handled 12 advice requests, addressing different issues with different combinations of expertise. For example, one completed advice request asked for c4c advice on (1) the proposed research population, (2) ethical challenges of the proposed design, (3) clinical perspective on risk and benefits as well as feasibility of the proposal, (4) methodologies and possible approaches to study design and (5) the patient parent perspective on the proposed trial design including assessments and follow-up. c4c provided the requester with written advice following meetings with experts and several patient/parent interviews.
The organization of the c4c trials network and strategic feasibility service across and within countries. c4c Governance groups are shown as rounded rectangles. Countries are shown as circles. Sites are shown as triangles and experts (scientific, clinical or participants) are shown as small squares. Sites are only shown for two countries in the interests of clarity. c4c conect4children, SPoC single point of contact
To share innovative methodology approaches and their potential application in paediatric drug development, the expert groups will prepare and publish white papers during the course of the IMI project.
Patient and public involvement (PPI) activities are usually set up as an ad-hoc service that is mainly performed using an online format. c4c is also ready to conduct face-to-face activities with patients. PPI experts contribute during all of the advice process and facilitate appropriate PPI activities. Young adult patients, caregivers, experts from POs and facilitators of YPAGs are part of a dedicated patient involvement database of experts that is consulted once the c4c advisory group secretariat receives a request for patient involvement. Liaison with disease-specific and umbrella POs is also done with the aim to select the right experts to be involved in these activities. All the results are shared with the sponsor who will decide how or whether to include the advisory outcomes in the final design of their project (protocol design, patient information documents, etc.). A dedicated analysis of the return on investment and the return on engagement will be performed during the project to help in the final definition of the services portfolio of the future c4c legal successor entity in the field of PPI activities. The definition of engagement used by c4c is “the direct and constructive interaction with patients in various roles at all appropriate points within the medical product life cycle to ensure the best possible alignment between patient needs and products and services that improve health outcomes for patients” [18]. Return on engagement considers the impact of initiatives that are participant-centred on the participant and on the financial and operational aspects of clinical trials. For a specific participant-centred initiative, return on engagement considers the impact the initiative has on the experience of the participant (or other desirable objective for the participant) and the impact on the quality, speed, or other key performance indicator of the trial. The assessment of return on engagement takes account of the cost and ease of conducting the participant-centred initiative [18].
3.2 Set Up and Conduct of Studies
Table 1 describes the challenges and delays experienced by many sponsors that will be addressed by c4c. c4c will not be able to address the ‘structural’ reasons for these delays. For example, c4c will not be able to change a hospital’s policy on intellectual property or electronic signatures. c4c will address ‘modifiable’ contributions to these delays, with a key contribution from c4c NH. NH comprise a scientific lead with support and administrative staff. The roles of NH include acting as a national point of contact for c4c; supporting sponsors in coordinating trial conduct in their country, they may support sites in discussions about contracts, regulatory and ethics approvals by aligning templates and providing mediation when needed; managing c4c feasibility requests at country level and supporting sites during clinical trials. NH are hosted by one organization. Depending on national circumstances, NH can be hosted by a hospital, university, or a legal entity that is specific to the hub. NH can be distributed or based in a single location. NH have ongoing relationships with most or all of the sites in their country. These relationships extend beyond individual studies or companies. NH capture ‘national corporate memory’ of clinical capacity and context for research in ways that commercial entities cannot do. NH can clarify topics under discussion and facilitate steps to study set up as part of formal or informal negotiation; for example, the basis for variation in costings, contracts, ethics, etc. within a country or in comparison with other countries. Furthermore, NH will troubleshoot within a metrics-driven performance management and quality framework, intervening when needed in a flexible, efficient and coordinated way. NH can promote buddying and mentoring for inexperienced sites and sharing practice when some sites have more success with recruitment than other sites. Standards for sites, NH and the pan-European coordination of the network will indicate what service users can expect. Standards will facilitate training and quality improvement. Investigators will have access to training about how to contribute to industry trials and support during feasibility assessments. Investigator preparedness will also include intelligence about future research and training about drug development and how to work with children, young people and their advocates during all stages of drug development.
4 Elements of c4c
The c4c SPoC provides one e-mail address and one online form to direct the requestor to relevant sources of help for paediatric research in Europe. This includes cascading requests to all NH, sites, the advisory group secretariat and expert groups. SPoC does not resolve queries but will manage the process to resolve queries.
4.1 Information Systems
A c4c information system (c4c-IS) is being implemented to support the establishment of the performance management and quality framework of the network. c4c-IS is a distributed system designed to support the governance of the network, to virtually aggregate all the operational levels (SPoC, NH, sites, experts and PPI), to support the execution of each incoming service request collected by the SPoC and to empower interactions between elements of the network.
The c4c-IS will sustain c4c daily operations, support for trial conduct, and coordination of the information flow across the network by facilitating the collection of datapoints that feed the business intelligence engine. The business intelligence engine will populate the dashboards that will allow performance monitoring and effective decision making.
Moreover, the c4c-IS will promote the set-up of a virtual community, in which communication, collaboration and information sharing will be strongly enhanced by means of a dedicated set of IT tools.
4.2 Patient and Public Involvement
c4c will provide sponsors with the right services to ensure that they can involve patient experts in the design and performance of clinical trials. Several activities are underway:
-
On-line training for researchers and sponsors to educate them about PPI and to ensure they know the potential benefits of co-designing studies with patients and families.
-
Training for POs to introduce patient involvement in c4c and to ensure that a train-the-trainers programme can be performed within the POs to set up the right participatory research culture among their members.
-
PPI experts are included in any activity with patients and their families. The PPI cross-cutting theme is formed by YPAG facilitators and senior experts in PPI and umbrella POs.
An early discussion between the PPI c4c experts, the advisory group secretariat of c4c, and the sponsors will facilitate the optimal selection of the patient involvement activities in each request to c4c.
The c4c patient involvement model is also focused on the inclusion of innovative activities with patients. In this sense, it is expected that two innovative activities will be performed before the end of the project:
-
Electronic assent.
-
Simulation of clinical trials. This will allow the inclusion of patients’ needs, feedback and improvements before the trial is initiated.
4.3 Education and Training
Site and investigator preparation needs to include training and education. The c4c paediatric medicine academy will develop a learning environment targeting the level of expertise according to the learners' needs. c4c has established a portal (virtual learning environment) to host the training courses.
Courses will include:
-
Generic training
-
Good Clinical Practice (GCP) for paediatric trials,
-
Key issues in paediatric trials for sites, monitors, ethics committees, hospital administrators,
-
Advanced Certified Course on Paediatric Drug Development and Paediatric Clinical Trials.
-
-
Trial-specific training
-
Each non-industry trial will generate courses about the protocol and assessments that sites will be able to access remotely in a secure manner.
-
PPI training (see above).
-
In addition to hosting an efficient, rapid and high throughput portal, c4c operates robust governance to assure the relevance and quality of c4c courses. An international Educational Board oversees the development of courses from a pedagogic perspective and reviews feedback about the courses and the portal.
4.4 Trial Data
The lack of interoperable and standardized data is a fundamental issue within the field of paediatric research. The significant variability in the data items collected—and how they are collected—makes future re-use of the data challenging, thus limiting its potential to inform and improve future research. One of the key outputs of c4c will be the cross-cutting paediatric data dictionary (CCPDD). A data dictionary is a centralized repository of information about specific data; for instance, showing meaning, relationships to other data, origin, usage and format. This CCPDD is based around a list of data items that are particularly relevant to paediatric studies and that tend to be collected in most trials, regardless of the disease area. They are generally items from demographics, vital signs, and some developmental scales. Where possible, the c4c CCPDD is aligned to the Clinical Data Interchange Standards Consortium (CDISC) and the controlled paediatric terminology hosted by the US National Cancer Institute Enterprise Vocabulary Service. CDISC is an important standards development organisation since its foundational standards are mandated for submission to both the US FDA and Japanese Pharmaceuticals and Medical Devices Agency. In addition to the CCPDD, c4c is working closely with CDISC to develop a Therapeutic Area User Guide (TAUG) for paediatrics, which will help all paediatric studies using CDISC to generate more standardised and interoperable data.
5 Discussion
The potential for c4c to improve European clinical research into drugs for diseases that affect children relates to specific activities based on relationship-driven implementation of private–public business processes developed within a public–private partnership. Whether this potential is realized depends on the efficacy and efficiency of processes that aim to deliver intended benefits. The use of a small number of studies to test the network will allow us to assess the viability of a successor to c4c.
One of the key goals of c4c is to reach sustainability and to seamlessly transfer the network built during the IMI project into a sustainable stand-alone legal entity after the end of IMI funding. In some ways, the IMI2 project works like an accelerator for creation and start-up of the network. It provides the academic partners with stable funding needed to concentrate on building the networks and allows industry partners to cooperate in a non-competitive environment. It is important to note that the current IMI consortium will dissolve after the end of the IMI funding period (2024) and the to-be-created, new legal entity will be independent from the current consortium and will cooperate with public and private partners and receive funding from different sources. The network will need to expand its reach and grow its customer base over time.
c4c will not address all challenges to paediatric trials. The variation in ethics/regulators is beyond the scope of the network. Pharmaceutical policy such as access and pricing is beyond the scope of the network. c4c works with many external stakeholders: each stakeholder could develop divergent expectations of the consortium and its work. Significant work has been done, and will continue, to learn about the needs of stakeholders and identify how c4c’s work aligns with those needs. It will be important to identify opportunities for synergistic work with similar initiatives and threats to c4c due to competition from other organizations working on paediatric clinical trials. Once the key elements of the successor organization have been identified, including services and revenue structure, then a marketing strategy will underpin extension of access to c4c services beyond the consortium’s current membership.
6 Conclusion
c4c is a novel, multidisciplinary, multisectoral approach to improving the paediatric clinical trial environment in Europe. The staged approach to establish the network’s viability underpins robust performance and quality. By 2024 a single point of contact will provide pan-European access to multiple, coordinated services to facilitate research into drugs for the paediatric population.
References
EMA. The European Paediatric Initiative: history of the paediatric regulation. 2007. https://www.ema.europa.eu/en/documents/other/european-paediatric-initiative-history-paediatric-regulation_en.pdf. Accessed 13 July 2020.
EMA. Paediatric investigation plans. 2020. https://www.ema.europa.eu/en/human-regulatory/research-development/paediatric-medicines/paediatric-investigation-plans. Accessed 13 July 2020.
EC. State of Paediatric Medicines in the EU: 10 years of the EU Paediatric Regulation. 2017. https://ec.europa.eu/health/sites/health/files/files/paediatrics/docs/2017_childrensmedicines_report_en.pdf. Accessed 13 July 2020.
Weisfeld NE, English RA, Claiborne AB. Envisioning a transformed clinical trials enterprise in the United States: establishing an agenda for 2020. Washington: National Academies Press; 2012.
Lepola P, Needham A, Mendum J, Sallabank P, Neubauer D, de Wildt S. Informed consent for paediatric clinical trials in Europe. Arch Dis Child. 2016;101(11):1017–25. https://doi.org/10.1136/archdischild-2015-310001.
van den Anker J, Reed MD, Allegaert K, Kearns GL. Developmental changes in pharmacokinetics and pharmacodynamics. J Clin Pharmacol. 2018;58(Suppl 10):S10-s25. https://doi.org/10.1002/jcph.1284.
Ward RM, Benjamin D, Barrett JS, Allegaert K, Portman R, Davis JM, et al. Safety, dosing, and pharmaceutical quality for studies that evaluate medicinal products (including biological products) in neonates. Pediatr Res. 2017;81(5):692–711. https://doi.org/10.1038/pr.2016.221.
IMI2. https://www.imi.europa.eu. Accessed 25 Oct 2020.
c4c. https://conect4children.org. Accessed 25 Oct 2020.
PENTA. https://penta-id.org. Accessed 25 Oct 2020.
PRINTO. https://printo.it/. Accessed 25 Oct 2020.
Treat-NMD. https://treat-nmd.org. Accessed 25 Oct 2020.
Network ECFSCT. https://www.ecfs.eu/ctn. Accessed 25 Oct 2020.
SIOPE. https://siope.eu. Accessed 25 Oct 2020.
Neuropsychopharmacology ECo. https://www.ecnp.eu/research-innovation/ECNP-networks/List-ECNP-Networks/Child-and-adolescent-neuropsychopharmacology. Accessed 25 Oct 2020.
Diseases EJPoR. https://www.ejprarediseases.org. Accessed 25 Oct 2020.
EMA. https://www.ema.europa.eu/en/partners-networks/networks/european-network-paediatric-research-european-medicines-agency-enpr-ema. Accessed 25 Oct 2020.
Michaels DL, Lamberti MJ, Peña Y, Kunz BL, Getz K. Assessing biopharmaceutical company experience with patient-centric initiatives. Clin Ther. 2019;41(8):1427–38. https://doi.org/10.1016/j.clinthera.2019.07.018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The Collaborative Network for European Clinical Trials for Children (conect4children or c4c) is an action under the Innovative Medicines Initiative 2 (IMI2) Joint Understanding (https://www.imi.europa.eu), Grant Agreement 777389. The open access fee was paid by the University of Liverpool.
Conflicts of interest/competing interests
All the authors are associated with the c4c project because their employers are Beneficiaries of the c4c Consortium. Cheng is an employee of Johnson and Johnson; Hildebrand is an employee of Bayer AG.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
MAT drafted the paper and contributes to the leadership of c4c. HH reviewed the paper and contributes to the leadership of c4c. RMF reviewed the paper and contributes to the leadership of c4c. SdW reviewed the paper and contributes to the leadership of c4c. FM reviewed the paper and contributes to the leadership of c4c. RH reviewed the paper and contributes to the leadership of c4c. RL reviewed the paper and contributes to the leadership of c4c. FB reviewed the paper and contributes to the leadership of c4c. PN reviewed the paper and contributes to the leadership of c4c. KC reviewed the paper and contributes to the leadership of c4c. SA reviewed the paper and contributes to the leadership of c4c. PR reviewed the paper and contributes to the leadership of c4c. FR reviewed the paper and contributes to the leadership of c4c. JC reviewed the paper and contributes to the leadership of c4c. BN reviewed the paper and contributes to the leadership of c4c. CG reviewed the paper and contributes to the leadership of c4c.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Turner, M.A., Hildebrand, H., Fernandes, R.M. et al. The conect4children (c4c) Consortium: Potential for Improving European Clinical Research into Medicines for Children. Pharm Med 35, 71–79 (2021). https://doi.org/10.1007/s40290-020-00373-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40290-020-00373-6