Abstract
Background
The fatigue of a muscle or muscle group can produce global responses to a variety of systems (i.e., cardiovascular, endocrine, and others). There are also reported strength and endurance impairments of non-exercised muscles following the fatigue of another muscle; however, the literature is inconsistent.
Objective
To examine whether non-local muscle fatigue (NLMF) occurs following the performance of a fatiguing bout of exercise of a different muscle(s).
Design
Systematic review and meta-analysis.
Search and Inclusion
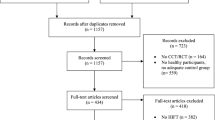
A systematic literature search using a Boolean search strategy was conducted with PubMed, SPORTDiscus, Web of Science, and Google Scholar in April 2020, and was supplemented with additional ‘snowballing’ searches up to September 2020. To be included in our analysis, studies had to include at least one intentional performance measure (i.e., strength, endurance, or power), which if reduced could be considered evidence of muscle fatigue, and also had to include the implementation of a fatiguing protocol to a location (i.e., limb or limbs) that differed to those for which performance was measured. We excluded studies that measured only mechanistic variables such as electromyographic activity, or spinal/supraspinal excitability. After search and screening, 52 studies were eligible for inclusion including 57 groups of participants (median sample = 11) and a total of 303 participants.
Results
The main multilevel meta-analysis model including all effects sizes (278 across 50 clusters [median = 4, range = 1 to 18 effects per cluster) revealed a trivial point estimate with high precision for the interval estimate [− 0.02 (95% CIs = − 0.14 to 0.09)], yet with substantial heterogeneity (Q(277) = 642.3, p < 0.01), I2 = 67.4%). Subgroup and meta-regression analyses showed that NLMF effects were not moderated by study design (between vs. within-participant), homologous vs. heterologous effects, upper or lower body effects, participant training status, sex, age, the time of post-fatigue protocol measurement, or the severity of the fatigue protocol. However, there did appear to be an effect of type of outcome measure where both strength [0.11 (95% CIs = 0.01–0.21)] and power outcomes had trivial effects [− 0.01 (95% CIs = − 0.24 to 0.22)], whereas endurance outcomes showed moderate albeit imprecise effects [− 0.54 (95% CIs = − 0.95 to − 0.14)].
Conclusions
Overall, the findings do not support the existence of a general NLMF effect; however, when examining specific types of performance outcomes, there may be an effect specifically upon endurance-based outcomes (i.e., time to task failure). However, there are relatively fewer studies that have examined endurance effects or mechanisms explaining this possible effect, in addition to fewer studies including women or younger and older participants, and considering causal effects of prior training history through the use of longitudinal intervention study designs. Thus, it seems pertinent that future research on NLMF effects should be redirected towards these still relatively unexplored areas.



Similar content being viewed by others
Data Availability Statement
All data are available in the Open Science Framework by accessing: https://osf.io/cs9e2/.
Notes
Within-participant designs are typically more statistically powerful (see [19]).
It seemed reasonable to think that performance measurements taken soon after a non-local fatiguing protocol would exhibit greater deficits, and similarly that more severe fatigue protocols would elicit greater deficits.
The term ‘power’ is commonly misused in sport, exercise, and physical activity literature considering its specific definition in Newtonian mechanics; instead, it would be more accurate to speak of the ability to generate impulse in most applications where it is used (see [21]). Indeed, power is defined as the rate of performing work. However, with respect to muscular performance, ‘power’ is often thought of as the product of force and velocity which as Winter et al. [21] note in fact refers to the impulse as derived from the impulse–momentum relationship. However, given its wide colloquial use to refer to such applications, we continue to use the term ‘power’ here for ease of communication given the primary intention of this article is not to debate terminology.
This in essence was similar to the manner in which resistance training dose is often operationalised as ‘volume-load’ relative to one-repetition maximum (1RM), i.e., sets x repetitions x %1RM [25].
Given that the number of possible combinations (2.146988965623700007512297816 × 1027) based on the number of effect sizes extracted prohibited the computation of all possible overall effect estimates, we limited this to a random sample of 1,000,000 possible combinations.
References
Tipton CM. History of exercise physiology. Windsor: Human Kinetics Publisher Inc.; 2014. p. 16–64.
Talbott JH. The effect of fatigue. N Engl J Med. 1933;208:658–9.
Gandevia SC. Spinal and supraspinal actors in human muscle fatigue. Physiol Rev. 2001;81(4):1725–89.
Behm DG. Force maintenance with submaximal fatiguing contractions. Can J Appl Physiol. 2004;29(3):274–90.
Behm DG, St-Pierre DM. Fatigue characteristics following ankle fractures. Med Sci Sports Exerc. 1997;29(9):1115–23.
Enoka RM, Duchateau J. Translating fatigue to human performance. Med Sci Sports Exerc. 2016;48(11):2228–38.
Halperin I, Chapman DW, Behm DG. Non-local muscle fatigue: effects and possible mechanisms. Eur J Appl Physiol. 2015;115(10):2031–48.
Martin PG, Rattey J. Central fatigue explains sex differences in muscle fatigue and contralateral cross-over effects of maximal contractions. Eur J Physiol. 2007;454(6):957–69.
Rattey J, Martin PG, Kay D, Cannon J, Marino FE. Contralateral muscle fatigue in human quadriceps muscle: evidence for a centrally mediated fatigue response and cross-over effect. Eur J Physiol. 2006;452(2):199–207.
Doix AC, Lefevre F, Colson SS. Time course of the cross-over effect of fatigue on the contralateral muscle after unilateral exercise. PLoS ONE. 2013;8(5):e64910.
Aboodarda SJ, Copithorne DB, Power KE, Drinkwater E, Behm DG. Elbow flexor fatigue modulates central excitability of the knee extensors. Appl Physiol Nutr Metab. 2015;40(9):924–30.
Aboodarda SJ, Sambaher N, Millet GY, Behm DG. Knee extensors neuromuscular fatigue changes the corticospinal pathway excitability in biceps brachii muscle. Neuroscience. 2017;06(340):477–86.
Ben Othman A, Chaouachi A, Hammami R, Chaouachi MM, Kasmi S, Behm DG. Evidence of nonlocal muscle fatigue in male youth. Appl Physiol Nutr Metab. 2017;42(3):229–37.
Halperin I, Aboodarda SJ, Behm DG. Knee extension fatigue attenuates repeated force production of the elbow flexors. Eur J Sport Sci. 2014;14(8):823–9.
Halperin I, Copithorne D, Behm DG. Unilateral isometric muscle fatigue decreases force production and activation of contralateral knee extensors but not elbow flexors. Appl Physiol Nutr Metab. 2014;39(12):1338–44.
Grant MC, Robergs R, Baird MF, Baker JS. The effect of prior upper body exercise on subsequent wingate performance. Biomed Res Int. 2014;2014:329328.
Elmer SJ, Amann M, McDaniel J, Martin DT, Martin JC. Fatigue is specific to working muscles: no cross-over with single-leg cycling in trained cyclists. Eur J Appl Physiol. 2013;113(2):479–88.
Miller WK, Jeon S, Ye X. A meta-analysis of non-local heterologous muscle fatigue. J Trainology. 2019;8:9–18.
MacInnis MJ, McGlory C, Gibala MJ, Phillips SM. Investigating human skeletal muscle physiology with unilateral exercise models: when one limb is more powerful than two. Appl Physiol Nutr Metab. 2017;42(6):563–70.
Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ. 2005;331(7524):1064–5.
Winter EM, Abt G, Brookes FB, Challis JH, Fowler NE, Knudson DV, et al. Misuse of “power” and other mechanical terms in sport and exercise science research. J Strength Cond Res. 2016;30(1):292–300.
Viechtbauer W. Conducting a meta-analysis in R with metafor package. J Stat Softw. 2010;36(3):1–48.
Cohen J. Statistical power analysis for the behavioural sciences. In: Hillside NJ, editor. Erbraum associates. London: Academic Press; 1988. p. 24–92.
Hedges LV, Tipton E, Johnson MC. Robust variance estimation in meta-regression with dependent effect size estimates. Res Synth Methods. 2010;1(1):39–65.
Scott BR, Duthie GM, Thornton HR, Dascombe BJ. Training monitoring for resistance exercise: theory and applications. Sports Med. 2016;46(5):687–98.
McShane BB, Gal D, Gelman A, Robert C, Tackett JL. Abandon statistical significance. Proc Am Stat Assoc. 2019;73:235–45.
Amrhein V, Greenland S, McShane B. Scientists rise up against statistical significance. Nature. 2019;567(7748):305–7.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. British Med J. 2003;327(7414):557–60.
Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. London: Academic Press; 2011. p. 38–111.
Olkin I, Dahabreh IJ, Trikalinos TA. GOSH: a graphical display of study heterogeniety. Res Synth Methods. 2012;3(3):214–23.
Andrews SK, Horodyski JM, MacLeod DA, Whitten J, Behm DG. The interaction of fatigue and potentiation following an acute bout of unilateral squats. J Sports Sci Med. 2016;15(4):625–32.
Bouhlel E, Chelly MS, Gmada N, Tabka Z, Shephard R. Effect of a prior force-velocity test performed with legs on subsequent peak power output measured with arms or vice versa. J Strength Cond Res. 2010;24(4):992–8.
Ciccone AB, Brown LE, Coburn JW, Galpin AJ. Effects of traditional vs. alternating whole-body strength training on squat performance. J Strength Cond Res. 2014;28(9):2569–77.
Decorte N, Lafaix PA, Millet GY, Wuyam B, Verges S. Central and peripheral fatigue kinetics during exhaustive constant-load cycling. Scand J Med Sci Sports. 2012;22(3):381–91.
Ross EZ, Middleton N, Shave R, George K, Nowicky A. Corticomotor excitability contributes to neuromuscular fatigue following marathon running in man. Exp Physiol. 2007;92(2):417–26.
Sambaher N, Aboodarda SJ, Behm DG. Bilateral knee extensor fatigue modulates force and responsiveness of the corticospinal pathway in the non-fatigued, dominant elbow flexors. Front Hum Neurosci. 2016;10:18.
Prieske O, Aboodarda SJ, Benitez Sierra JA, Behm DG, Granacher U. Slower but not faster unilateral fatiguing knee extensions alter contralateral limb performance without impairment of maximal torque output. Eur J Appl Physiol. 2017;117(2):323–34.
Kennedy DS, Fitzpatrick SC, Gandevia SC, Taylor JL. Fatigue-related firing of muscle nociceptors reduces voluntary activation of ipsilateral but not contralateral lower limb muscles. J Appl Physiol. 2015;118(4):408–18.
Kawamoto JE, Aboodarda SJ, Behm DG. Effect of differing intensities of fatiguing dynamic contractions on contralateral homologous muscle performance. J Sports Sci Med. 2014;13(4):836–45.
Sidhu SK, Weavil JC, Venturelli M, Garten RS, Rossman MJ, Richardson RS, et al. Spinal mu-opioid receptor-sensitive lower limb muscle afferents determine corticospinal responsiveness and promote central fatigue in upper limb muscle. J Physiol. 2014;592(22):5011–24.
Nordsborg N, Mohr M, Pedersen LD, Nielsen JJ, Langberg H, Bangsbo J. Muscle interstitial potassium kinetics during intense exhaustive exercise: effect of previous arm exercise. Am J Physiol Regul Integr Comp Physiol. 2003;285(1):R143–8.
Bogdanis GC, Nevill ME, Lakomy HK. Effects of previous dynamic arm exercise on power output during repeated maximal sprint cycling. J Sports Sci. 1994;12(4):363–70.
Amann M, Venturelli M, Ives SJ, McDaniel J, Layec G, Rossman MJ, et al. Peripheral fatigue limits endurance exercise via a sensory feedback-mediated reduction in spinal motoneuronal output. J Appl Physiol. 2013;115(3):355–64.
Aboodarda SJ, Iannetta D, Emami N, Varesco G, Murias JM, Millet GY. Effects of pre-induced fatigue vs. concurrent pain on exercise tolerance, neuromuscular performance and corticospinal responses of locomotor muscles. J Physiol. 2020;598(2):285–302.
Doix AM, Wachholz F, Marterer N, Immler L, Insam K, Federolf PA. Is the cross-over effect of a unilateral high-intensity leg extension influenced by the sex of the participants? Biol Sex Differ. 2018;9(1):29.
Arora S, Budden S, Byrne JM, Behm DG. Effect of unilateral knee extensor fatigue on force and balance of the contralateral limb. Eur J Appl Physiol. 2015;115(10):2177–87.
Aboodarda S, Šambaher N, Behm D. Unilateral elbow flexion fatigue modulates corticospinal responsiveness in non-fatigued contralateral biceps brachii. Scand J Med Sci Sports. 2016;26(11):1301–12.
Aboodarda SJ, Zhang CXY, Sharara R, Cline M, Millet GY. Exercise-induced fatigue in one leg does not impair the neuromuscular performance in the contralateral leg but improves the excitability of the ipsilateral corticospinal pathway. Brain Sci. 2019;9(10):250.
Grant MC, Robergs R, Baird MF, Baker JS. The effect of prior upper body exercise on subsequent wingate performance. BioMed Res Intern. 2014. https://doi.org/10.1155/2014/329328.
Behm DG, Colwell EM, Power GMJ, Ahmadi H, Behm ASM, Bishop A, et al. Transcutaneous electrical nerve stimulation improves fatigue performance of the treated and contralateral knee extensors. Eur J Appl Physiol. 2019;119(11–12):2745–55.
Chen TC, Chen HL, Lin MJ, Yu HI, Nosaka K. Contralateral repeated bout effect of eccentric exercise of the elbow flexors. Med Sci Sports Exerc. 2016;48(10):2030–9.
Grabiner MD, Owings TM. Effects of eccentrically and concentrically induced unilateral fatigue on the involved and uninvolved limbs. J Electromyogr Kinesiol. 1999;9(3):185–9.
Hamilton AR, Behm DG. The effect of prior knowledge of test endpoint on non-local muscle fatigue. Eur J Appl Physiol. 2017;117(4):651–63.
Humphry AT, Lloyd-Davies EJ, Teare RJ, Williams KE, Strutton PH, Davey NJ. Specificity and functional impact of post-exercise depression of cortically evoked motor potentials in man. Eur J Appl Physiol. 2004;92(1–2):211–8.
Kavanagh JJ, Feldman MR, Simmonds MJ. Maximal intermittent contractions of the first dorsal interosseous inhibits voluntary activation of the contralateral homologous muscle. J Neurophysiol. 2016;116(5):2272–80.
Morgan PT, Bailey SJ, Banks RA, Fulford J, Vanhatalo A, Jones AM. Contralateral fatigue during severe-intensity single-leg exercise: influence of acute acetaminophen ingestion. Am J Physiol Regul Integr Comp Physiol. 2019;317(2):R346–54.
Pethick J, Winter SL, Burnley M. Effects of ipsilateral and contralateral fatigue and muscle blood flow occlusion on the complexity of knee-extensor torque output in humans. Exp Physiol. 2018;103(7):956–67.
Post M, Bayrak S, Kernell D, Zijdewind I. Contralateral muscle activity and fatigue in the human first dorsal interosseous muscle. J Appl Physiol. 2008;105(1):70–82.
Regueme SC, Barthelemy J, Nicol C. Exhaustive stretch-shortening cycle exercise: no contralateral effects on muscle activity in maximal motor performances. Scand J Med Sci Sports. 2007;17(5):547–55.
Li Y, Power KE, Marchetti PH, Behm DG. The effect of dominant first dorsal interosseous fatigue on the force production of a contralateral homologous and heterologous muscle. Appl Physiol Nutr Metab. 2019;44(7):704–12. https://doi.org/10.1139/apnm-2018-0583.
Gastin PB. Energy system interaction and relative contribution during maximal exercise. Sports Med. 2001;31(10):725–41.
Pageaux B, Lepers R. Fatigue induced by physical and mental exertion increases perception of effort and impairs subsequent endurance performance. Front Physiol. 2016;7:587.
Cady EB, Jones DA, Lynn J, Newham DJ. Changes in force and intracellular metabolites during fatigue of human skeletal muscle. J Physiol. 1989;418:311–25.
Kent-Braum JA. Central and peripheral contributions to muscle fatigue in humans during sustained maximal effort. Eur J Appl Physiol. 1999;80:57–63.
Kowalchuk JM, Heigenhauser GJ, Jones NL. Effect of pH on metabolic and cardiorespiratory responses during progressive exercise. J Appl Physiol Respir Environ Exerc Physiol. 1984;57(5):1558–63.
Hultman E, Del Canale S, Sjoholm H. Effect of induced metabolic acidosis on intracellular pH, buffer capacity and contraction force of human skeletal muscle. Clin Sci (Lond). 1985;69(5):505–10.
Johnson MA, Mills DE, Brown PI, Sharpe GR. Prior upper body exercise reduces cycling work capacity but not critical power. Med Sci Sports Exerc. 2014;46(4):802–8.
Bangsbo J, Madsen K, Kiens B, Richter EA. Effect of muscle acidity on muscle metabolism and fatigue during intense exercise in man. J Physiol. 1996;495(Pt 2):587–96.
Lamb GD, Stephenson DG. Point: lactic acid accumulation is an advantage during muscle activity. J Appl Physiol. 2006;100(4):1410–2.
Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88(1):287–332.
Fitts RH. The cross-bridge cycle and skeletal muscle fatigue. J Appl Physiol. 2008;104(2):551–8.
Knuth ST, Dave H, Peters JR, Fitts RH. Low cell pH depresses peak power in rat skeletal muscle fibres at both 30 degrees C and 15 degrees C: implications for muscle fatigue. J Physiol. 2006;575(Pt 3):887–99.
Allen DG, Lee JA, Westerblad H. Intracellular calcium and tension during fatigue in isolated single muscle fibres from Xenopus laevis. J Physiol. 1989;415:433–58.
Sejersted OM, Sjogaard G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol Rev. 2000;80(4):1411–81.
Juel C. Potassium and sodium shifts during in vitro isometric muscle contraction, and the time course of the ion-gradient recovery. Pflug Arch. 1986;406(5):458–63.
Koh TJ. Do small heat shock proteins protect skeletal muscle from injury? Exerc Sport Sci Rev. 2002;30(3):117–21.
Jammes Y, Steinberg JG, By Y, Brerro-Saby C, Condo J, Olivier M, et al. Fatiguing stimulation of one skeletal muscle triggers heat shock protein activation in several rat organs: the role of muscle innervation. J Exp Biol. 2012;215(Pt 22):4041–8.
Thomas JA, Noble EG. Heat shock does not attenuate low-frequency fatigue. Can J Physiol Pharmacol. 1999;77(1):64–70.
Amann M. Central and peripheral fatigue: interaction during cycling exercise in humans. Med Sci Sports Exerc. 2011;43(11):2039–45.
Amann M. Significance of group III and IV muscle afferents for the endurance exercising human. Clin Exp Pharmacol Physiol. 2012;39(9):831–5.
Nybo L, Rasmussen P. Inadequate cerebral oxygen delivery and central fatigue during strenuous exercise. Exerc Sport Sci Rev. 2007;35(3):110–8.
Rasmussen P, Nybo L, Volianitis S, Moller K, Secher NH, Gjedde A. Cerebral oxygenation is reduced during hyperthermic exercise in humans. Acta Physiol (Oxf). 2010;199(1):63–70.
Bonato C, Zanette G, Manganotti P, Tinazzi M, Bongiovanni G, Polo A, et al. ‘Direct’ and ‘crossed’ modulation of human motor cortex excitability following exercise. Neurosci Letters. 1996;216(2):97–100.
Takahashi K, Maruyama A, Maeda M, Etoh S, Hirakoba K, Kawahira K, et al. Unilateral grip fatigue reduces short interval intracortical inhibition in ipsilateral primary motor cortex. Clin Neurophysiol. 2009;120(1):198.
Takahashi K, Maruyama A, Hirakoba K, Maeda M, Etoh S, Kawahira K, et al. Fatiguing intermittent lower limb exercise influences corticospinal and corticocortical excitability in the non-exercised upper limb. Brain Stim. 2011;4(2):90.
Baumer T, Munchau A, Weiller C, Liepert J. Fatigue suppresses ipsilateral intracortical facilitation. Exper Brain Res. 2002;146(4):467–73.
Samii A, Caños M, Ikoma K, Wassermann EM, Hallett M. Absence of facilitation or depression of motor evoked potentials after contralateral homologous muscle activation. Electroencephal Clin Neurophysiol. 1997;105(3):241–5.
Hess C, Mills K, Murray N. Magnetic stimulation of the human brain: facilitation of motor responses by voluntary contraction of ipsilateral and contralateral muscles with additional observations on an amputee. Neurosci Lett. 1986;71(2):235–40.
Brasil-Neto JP, Araújo VP, Carneiro CR. Postexercise facilitation of motor evoked potentials elicited by ipsilateral voluntary contraction. Muscle Nerve. 1999;22(12):1710–2.
Todd G, Petersen NT, Taylor JL, Gandevia SC. The effect of a contralateral contraction on maximal voluntary activation and central fatigue in elbow flexor muscles. Exp Brain Res. 2003;15(3):308–13.
Samii A, Canos M, Ikoma K, Wassermann EM, Hallett M. Absence of facilitation or depression of motor evoked potentials after contralateral homologous muscle activation. Electroencephal Clin Neurophysiol. 1997;105(3):241–5.
Holgado DS, Sanabria D. Does self-paced exercise depend on executive processing? A narrative review of the current evidence. Inter Rev Sport Exerc Psychol. 2020;13:1–24.
Herold F, Hamacher D, Torpel A, Goldschmidt L, Muller NG, Schega L. Does squatting need attention?-A dual-task study on cognitive resources in resistance exercise. PLoS ONE. 2020;15(1):e0226431.
Pageaux B, Lepers R, Dietz KC, Marcora SM. Response inhibition impairs subsequent self-paced endurance performance. Eur J Appl Physiol. 2014;114(5):1095–105.
Pageaux B, Marcora SM, Lepers R. Prolonged mental exertion does not alter neuromuscular function of the knee extensors. Med Sci Sports Exerc. 2013;45(12):2254–64.
Marcora SM, Staiano W, Manning V. Mental fatigue impairs physical performance in humans. J Appl Physiol. 2009;106(3):857–64.
Holgado D, Sanabria D, Perales JC, Vadillo M. Mental fatigue might not be so bad for exercise performance after all: a systematic review and bias-sensitive meta-analysis. SportRχiv. 2020;3(1):38. https://doi.org/10.5334/joc.126.
Steele J. What is (perception of) effort? Objective and subjective effort during task performance. PsyArχiv. 2020. https://doi.org/10.31234/osf.io/kbyhm
Greenhouse-Tucknott A, Wrightson JG, Raynsford M, Harrison NA, Dekerle J. Interactions between perceptions of fatigue, effort, and affect decrease knee extensor endurance performance following upper body motor activity, independent of changes in neuromuscular function. Psychophysiology. 2020;57(9):e13602.
Gandevia SC, Smith JL, Crawford M, Proske U, Taylor JL. Motor commands contribute to human position sense. J Physiol. 2006;571(3):703–10.
Hureau TJ, Romer LM, Amann M. The “sensory tolerance limit”: a hypothetical construct determining exercise performance? Eur J Sport Sci. 2018;18(1):13–24.
Holgado D, Troya E, Perales JC, Vadillo MA, Sanabria D. Does mental fatigue impair physical performance? A replication study. Eur J Sport Sci. 2020;30:1–9.
Danneels LA, Vanderstraeten GG, Cambier DC, Witvrouw EE, Stevens VK, De Cuyper HJ. A functional subdivision of hip, abdominal, and back muscles during asymmetric lifting. Spine. 2001;26(6):E114–21.
Baker JS, Davies B. Additional considerations and recommendations for the quantification of hand-grip strength in the measurement of leg power during high-intensity cycle ergometry. Res Sports Med. 2009;17(3):145–55.
Tarnanen SP, Ylinen JJ, Siekkinen KM, Mälkiä EA, Kautiainen HJ, Häkkinen AH. Effect of isometric upper-extremity exercises on the activation of core stabilizing muscles. Arch Physical Med Rehabil. 2008;89(3):513–21.
Kibler WB, Press J, Sciascia A. The role of core stability in athletic function. Sports Med. 2006;36(3):189–98.
Ye X, Beck TW, Wages NP, Carr JC. Sex comparisons of non-local muscle fatigue in human elbow flexors and knee extensors. J Musculoskelet Neuronal Interact. 2018;18(1):92–9.
Hunter SK. Sex differences and mechanisms of task-specific muscle fatigue. Exerc Sport Sci Rev. 2009;37(3):113–22.
Stuart C, Steele J, Gentil P, Giessing J, Fisher JP. Fatigue and perceptual responses of heavier- and lighter-load isolated lumbar extension resistance exercise in males and females. PeerJ. 2018;6:e4523.
Hicks AL, Kent-Braun J, Ditor DS. Sex differences in human skeletal muscle fatigue. Exerc Sport Sci Rev. 2001;29(3):109–12.
Triscott S, Gordon J, Kuppuswamy A, King N, Davey N, Ellaway P. Differential effects of endurance and resistance training on central fatigue. J Sports Sci. 2008;26(9):941–51.
Lattier G, Millet GY, Maffiuletti NA, Babault N, Lepers R. Neuromuscular differences between endurance-trained, power-trained, and sedentary subjects. J Strength Cond Res. 2003;17(3):514–21.
Avin KG, Law LA. Age-related differences in muscle fatigue vary by contraction type: a meta-analysis. Phys Ther. 2011;91(8):1153–65.
Bontemps B, Piponnier E, Chalchat E, Blazevich AJ, Julian V, Bocock O, et al. Children exhibit a more comparable neuromuscular fatigue profile to endurance athletes than untrained adults. Front Physiol. 2019;10:119.
Author information
Authors and Affiliations
Contributions
DB wrote the first draft of the manuscript. DB, SHA, CH, ER, and MMIM performed the literature search. JS performed the meta-analyses. All authors were involved in the interpretation of the meta-analyses, read, revised, and approved the final manuscript.
Corresponding author
Ethics declarations
Funding
Partial financial support for graduate students (ER, MMIM, and JW) was received from the Natural Science and Engineering Research Council of Canada.
Conflict of Interest
David Behm, Shahab Alizadeh, Saman Hadjizedah Anvar, Courtney Hanlon, Emma Ramsay, Mohamed Mamdouh Ibrahim Mahmoud, Joseph Whitten, James Fisher, Olaf Prieske, Helmi Chaabene, Urs Granacher, and James Steele declare that they have no conflicts of interest relevant to the content of this review.
Rights and permissions
About this article
Cite this article
Behm, D.G., Alizadeh, S., Hadjizedah Anvar, S. et al. Non-local Muscle Fatigue Effects on Muscle Strength, Power, and Endurance in Healthy Individuals: A Systematic Review with Meta-analysis. Sports Med 51, 1893–1907 (2021). https://doi.org/10.1007/s40279-021-01456-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-021-01456-3