Abstract
Background and Objective
Cardiovascular disease is the main cause of death in Germany and other industrialized countries. However, until now, little has been known about how people with acute coronary syndrome (ACS) value aspects of their medical treatment. The objective of this study was to evaluate patients’ preferences regarding different antiplatelet medication options following an ACS.
Method
After identification of patient-relevant treatment attributes (a literature review and qualitative interviews), a discrete-choice experiment (DCE) including five patient-relevant attributes was conducted. The DCE used a forced-choice approach in which no “opt out” was present, as no treatment is not an option after ACS. The attribute and level combinations were created using a fractional–factorial NGene design with priors. Data analysis was performed using a random-effects logit model. An additional generalized linear latent and mixed models (GLLAMM) analysis was performed to evaluate subgroup differences.
Results
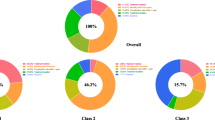
ACS patients (N = 683) participated in computer-assisted personal interviews. Preference analysis showed a clear dominance of the attribute “mortality risk” (coefficient: 0.803). Ranked second was “side effect: dyspnea” (coefficient: 0.550) followed by “risk of a new myocardial infarction” (coefficient: 0.464) and “side effect: bleeding” (coefficient: 0.400). “Frequency of intake” was less important (coefficient: 0.025). Within the 3-class GLLAMM, the variables “marital status” (p = 0.008), “highest level of education” (p = 0.003), and “body-mass index” (according to World Health Organization cluster; p = 0.014) showed a significant impact on the estimated class probabilities.
Conclusion
Our study found “mortality risk” to be of the highest value for patients. Patient-centered care and decision making requires consideration of patient preferences; moreover, the information on preferences can be used to develop effective therapies after an ACS. The data generated will enable healthcare decision makers and stakeholders to understand patient preferences to promote patients’ benefit.


Similar content being viewed by others
References
Post F, Münzel T. Das akute Koronarsyndrom. Der Internist. 2010;51(8):953–62.
Statistisches Bundesamt: Diagnosedaten der Krankenhäuser ab 2000. Krankenhausstatistik—Diagnosedaten der Patienten und Patientinnen in Krankenhäusern. 2011. https://www.gbe-bund.de/oowa921-install/servlet/oowa/aw92/WS0100/_XWD_PROC?_XWD_6/2/XWD_CUBE.DRILL/_XWD_32/D.946/14358.
Statistisches Bundesamt: Todesursachen in Deutschland. In: Gesundheit. Wiesbaden: Statistisches Bundesamt; 2012.
Kelm M, Strauer BE. Das akute Koronarsyndrom. Der Internist. 2005;46(3):265–74.
Hamm CW, Arntz HR, Bode C, Giannitsis E, Katus H, Levenson B, Nordt T, Neumann FJ, Tebbe U, Zahn R. Pocket-Leitlinien: Akutes Koronarsyndrom (ACS). Deutschen Gesellschaft für Kardiologie – Herz- und Kreislaufforschung e.V., Düsseldorf (2004).
Löwel H, Meisinger C, Schneider A, Kaup U, Gösele U, Hymer H. Koronare Herzkrankheit und akuter Myokardinfarkt. In: Gesundheitsberichterstattung des Bundes, vol 33. Berlin: Robert Koch-Institut; 2006.
Johansson G, Stallberg B, Tornling G, Andersson S, Karlsson GS, Falt K, Berggren F. Asthma treatment preference study: a conjoint analysis of preferred drug treatments. Chest. 2004;125(3):916–23.
Mühlbacher AC, Juhnke C. Patient preferences versus physicians’ judgement: does it make a difference in healthcare decision making? Appl Health Econ Health Policy. 2013;11(3): 163–80.
Mühlbacher A, Bethge S, Tockhorn A. Präferenzmessung im Gesundheitswesen: Grundlagen von Discrete-Choice-Experimenten [measuring preferences in healthcare: introduction to discrete-choice experiments]. Gesundheitsökon Qualitätsmanag. 2013;4:159–72.
Glaeske G. The dilemma between efficacy as defined by regulatory bodies and effectiveness in clinical practice. Deutsches Ärzteblatt Int. 2012;109(7):115.
Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG). Prasugrel bei akutem Koronarsyndrom, Dokumentation und Würdigung der Anhörung zum Berichtsplan. Köln: IQWiG; 2010.
Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG). Allgemeine Methoden, Entwurf für Version 4.2 vom 18.06.2014. Köln: IQWiG; 2014.
Lancaster KJ. A new approach to consumer theory. J Polit Econ. 1966;74(2):132–57.
McFadden D. Conditional logit analysis of qualitative choice behavior. Zarembka. 1974;1974:105–42.
Ben-Akiva ME, Lerman SR. Discrete choice analysis: theory and application to travel demand. MIT Press series in transportation studies. Cambridge: MIT Press; 1985.
Hensher DA, Rose JM, Greene WH. Applied choice analysis: a primer. Cambridge: Cambridge University Press; 2005.
Johnson FR. Why not ask?: Measuring patient preferences for healthcare decision making. Patient. 2008;1(4):245–8.
Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user’s guide. Pharmacoeconomics. 2008;26(8):661–78.
Ryan M, Gerard K, Amaya-Amaya M. Using discrete choice experiments to value health and health care. The economics of non-market goods and resources, vol. 11. Dordrecht: Springer; 2008.
Bridges JFP, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, et al. Conjoint analysis use in health studies—a checklist: a report of the ISPOR conjoint analysis in health good research practices task force. ISPOR TF Report. 2011;14:403–13.
Höer A, Behrendt S. Versorgung und Kosten von Versicherten mit akutem Koronarsyndrom (ACS) in Deutschland (berichtigte Fassung). Berlin: IGES Institut GmbH; 2012.
Orme BK. Sample size issues for conjoint analysis. In: Getting started with conjoint analysis: strategies for product design and pricing research. 2nd ed. Madison: Research Publishers LLC; 2010. pp. 57–66.
US Department of Health and Human Services, Office of Human Research Protection. Guidance on reviewing and reporting unanticipated problems involving risks to subjects or others and adverse events. 2007. http://www.hhs.gov/ohrp/policy/advevntguid.html. Accessed 1 Dec 2013.
Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–57.
Behan MW, Chew DP, Aylward PE. The role of antiplatelet therapy in the secondary prevention of coronary artery disease. Curr Opin Cardiol. 2010;25(4):321–8. doi:10.1097/HCO.0b013e328338f7b5.
Storey RF, Becker RC, Cannon CP, Cools F, Emanuelsson H, Harrington RA, et al. Ticagrelor does not affect pulmonary function tests compared to clopidogrel in acute coronary syndromes: results of the PLATO pulmonary substudy. J Am Coll Cardiol. 2010;55(10 Suppl 1):A108.E1007.
Storey RF, Becker RC, Harrington RA, Husted S, James SK, Cools F, et al. Characterization of dyspnoea in PLATO study patients treated with ticagrelor or clopidogrel and its association with clinical outcomes. Eur Heart J. 2011;32(23):2945–53.
Storey RF, Becker RC, Harrington RA, Husted S, James SK, Cools F, et al. Pulmonary function in patients with acute coronary syndrome treated with ticagrelor or clopidogrel (from the platelet inhibition and patient outcomes [PLATO] pulmonary function substudy). Am J Cardiol. 2011;108(11):1542–6.
Storey RF, Bliden KP, Patil SB, Karunakaran A, Ecob R, Butler K, et al. Incidence of dyspnea and assessment of cardiac and pulmonary function in patients with stable coronary artery disease receiving ticagrelor, clopidogrel, or placebo in the ONSET/OFFSET study. J Am Coll Cardiol. 2010;56(3):185–93.
Helm R, Steiner M. Präferenzmessung: Methodengestützte Entwicklung zielgruppenspezifischer Produktinnovationen. Stuttgart: W. Kohlhammer; 2008.
Louviere JJ, Islam T, Wasi N, Street D, Burgess L. Designing discrete choice experiments: do optimal designs come at a price? J Consum Res. 2008;35(2):360–75.
Acknowledgments
The authors would like to thank E. Piletzki and Dr. H. Brauer (Rehaklinik Silbermühle, Plau am See, Germany) for their help organizing the pre-test interviews and all of the participants taking part in the study.
Author’s declaration
AM developed, designed and coordinated the study, contributed to data analysis, data interpretation and revised the paper. SB carried out the questionnaire studies, contributed to data analysis, study design and drafted the paper.
Conflict of interest
This study was funded by AstraZeneca GmbH, Germany. AM and SB declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mühlbacher, A.C., Bethge, S. Reduce Mortality Risk Above All Else: A Discrete-Choice Experiment in Acute Coronary Syndrome Patients. PharmacoEconomics 33, 71–81 (2015). https://doi.org/10.1007/s40273-014-0223-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-014-0223-1