Abstract
Background
Antithrombotic drugs are used as preventive treatment in patients with a prior myocardial infarction (MI) in both the acute and chronic phases of the disease. To support patient-centered benefit–risk assessment, it is important to understand the influence of disease stage on patient preferences.
Objective
The aim of this study was to examine patient preferences for antithrombotic treatments and whether they differ by MI disease phase.
Methods
A discrete-choice experiment was used to elicit preferences of adults in the acute (≤ 365 days before enrolment) or chronic phase (> 365 days before enrolment) of MI for key ischemic events (risk of cardiovascular [CV] death, non-fatal MI, and non-fatal ischemic stroke) and bleeding events (risk of non-fatal intracranial hemorrhage and non-fatal other severe bleeding). Preference data were analyzed using the multinomial logit model. Trade-offs between attributes were calculated as the maximum acceptable increase in the risk of CV death for a decrease in the risk of the other outcomes. To assess the potential effect of sociodemographic and clinical characteristics on patient preferences, subgroups were introduced as interaction terms in logit models.
Results
The evaluable population included 155 patients with MI in the acute phase of disease and 180 in the chronic phase. The overall population was 82% male, mean age was 64.2 ± 9.6 years, and 93% had not experienced bleeding events or key ischemic events other than MI. Patients valued reduction in the risk of non-fatal intracranial hemorrhage more than CV death (p < 0.01) and CV death more than non-fatal ischemic events (p < 0.01). Preferences were similar in the acute and chronic populations (p = 0.17). However, older patients valued reduction in risk of MI more than younger patients (p = 0.04), and patients with bleeding risk factors valued reduction in the risk of CV death (p = 0.01) and MI (p = 0.01) less than patients without bleeding risk factors. Also, patients who were at high risk of future ischemic events valued reduction of the risk of CV death less than those at low risk (p = 0.01).
Conclusion
Patient preferences for antithrombotic treatments were unaffected by disease stage but varied by bleeding risk and other factors. This heterogeneity in preferences is an important consideration because it can affect the benefit–risk balance and the acceptability of antithrombotic treatments to patients.
Similar content being viewed by others
Patient preferences for antithrombotic treatment attributes were similar between the acute and chronic phases of myocardial infarction. |
Patients with myocardial infarction are willing to accept significant increases in the mortality risk to avoid increases in non-fatal bleeding events. |
1 Introduction
Antithrombotic treatments reduce the risk of cardiovascular (CV) events, including stroke and myocardial infarction (MI) [1, 2], but also increase the risk of bleeding episodes [3]. Historically, benefit–risk decision making for antithrombotic treatments has been based on expert opinion and has not included patient perspectives on the importance of key outcomes [4,5,6]. However, the growing consensus among regulators and other key stakeholders is that patient preferences should be incorporated into benefit–risk decisions [5, 7,8,9], especially when preferences of patients may differ from those of clinicians or other healthcare providers [10, 11]. This patient-centered approach is becoming an important part of decision making by regulatory authorities [12, 13], and data collected on patient preferences can form the basis for shared decision making between patients and their healthcare professionals [14].
Antithrombotic drugs are used as preventive treatment in patients with a prior MI in both the acute and chronic phases of disease [15]. To support patient-centered benefit–risk assessment, it is important to understand the influence of disease stage on patient preferences. Although patients generally underestimate their risk of recurring cardiac events, especially after an intervention such as coronary artery bypass surgery [16,17,18,19,20], we hypothesized that disease chronicity may affect a patient’s tolerance for an increased probability of adverse events. The relative importance of benefit and risk attributes of antithrombotic therapies in various CV diseases, including MI, has been explored [11, 21,22,23,24,25,26] but the influence of disease phase has not.
This study examined whether patient preferences for antithrombotic treatments differ by MI disease phase. The findings from this study should help inform drug development decisions as antithrombotic drugs are investigated and approved for initial administration at different stages of the disease process. The findings from this study should also support efforts of the IMI-PREFER consortia [27], a collaborative research project of the Innovative Medicines Initiative, which is developing recommendations to industry, regulatory authorities, and health technology assessment bodies in the European Union on how and when to include patient preferences for benefits and risks of medicinal products.
2 Methods
2.1 Study Design
An online discrete-choice experiment (DCE) survey was conducted between February 2019 and May 2020 to elicit preferences for antithrombotic treatment attributes from patients with MI during the acute and chronic phases of disease. The study followed best practice guidelines on preference-based methods from the International Society for Pharmacoeconomics and Outcomes Research [28, 29].
Adults (≥ 18 years of age) living in England with a previous hospitalization for MI were recruited. Patients in the acute phase of MI (≤ 365 days before enrolment) were recruited by clinicians and research staff from five participating National Health Service organizations across England. Patients in the chronic phase of MI (> 365 days before enrolment) were recruited from online patient databases. Patients were excluded if they could not read and understand English, had an acute psychopathology, or a had a potential cognitive, visual, or hearing impairment that, in the opinion of the investigator, could interfere with their ability to complete the survey. Study participants were remunerated for completing the study.
Potential attributes and levels to be included in the DCE were identified through a systematic literature review of Embase and MEDLINE [11, 21,22,23,24,25,26, 30, 31], consultation with clinicians, and based on events with irreversible harm traditionally selected by regulators and other stakeholders for making benefit–risk decisions about antithrombotic drugs. Accordingly, the key benefit and risk attributes included CV death, non-fatal MI, non-fatal ischemic stroke (IS), non-fatal intracranial hemorrhage (ICH), and non-fatal other severe bleeding (as defined by Global Use of Strategies to Open Occluded Arteries [GUSTO] [32]). Given the relatively low annual risk of severe bleeding events associated with the use of antithrombotic drugs, 3-year risk levels were applied to ensure sufficient variation in attribute levels. The 3-year risk levels were based on the range of estimated differences in extrapolated risks observed in the Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2°P)-TIMI 50 trial of an antiplatelet medication [33].
Semi-structured pilot interviews were conducted in two rounds of five patients each to test the attributes and levels, confirm comprehension of the wording used in the DCE, and refine the DCE survey, attributes, and levels. Next, a quantitative pilot survey was conducted with 40 patients in the chronic phase of MI. The main survey was planned to include approximately 200 patients in the acute phase of MI and 200 in the chronic phase of MI. Attributes and levels included in the final DCE are summarized in Table 1. The risks of bleeding events (i.e., non-fatal ICH and non-fatal GUSTO) were presented as an increase in risk associated with the use of antithrombotic drugs, whereas the risks of CV death, non-fatal MI, and non-fatal stroke were presented as a reduction in risk (i.e., a treatment benefit). This was done to help patients distinguish between treatment benefits and risks and based on feedback obtained during the pilot interviews. In addition to completing the DCE survey, patients completed a questionnaire assessing their health literacy [34] and numeracy [35] skills.
2.2 Discrete-Choice Experiment Design
The DCE was designed with the dp-optimal method using Ngene software version 1.1.2 (ChoiceMetrics, Sydney, Australia). Results from the quantitative pilot were used as priors to update the DCE design for the main survey. Each choice task in the DCE presented patients with two hypothetical scenarios representing trade-offs on the probability of occurrence of clinical events. An example choice task is shown in Fig. 1. Patients first completed a practice choice task, after which they completed 14 experimental choice tasks, a repeat of the first choice task placed at a random location between the 7th and the 12th choice tasks, and a fixed-choice task placed at a random location between the 2nd and 15th choice task. The repeated choice task was intended to test preference stability. The fixed-choice task had one option described by the most favorable level of one or more attributes and the other by the least favorable level of the same attributes, and it was intended as a dominance test, wherein patients are expected to choose the better alternative. Attributes were grouped into benefits (favorable outcomes) and risks (unfavorable outcomes) to minimize error variance, and the order of the groups and the order of the attributes within the groups were randomized between patients to minimize attribute ordering effects [36].
2.3 Statistical Analysis
Statistical analysis was conducted using Stata version 15.1 (StataCorp LLC, College Station, TX, USA). The DCE preference data for all attributes were analyzed using a multinomial logit (MNL) model within the framework of random utility maximization [37, 38]. Although presented as discrete levels in the experiment, the attributes were coded as continuous variables in the model with a linear specification. An MNL model with dummy coding for all attributes was also estimated and used to test the linearity assumptions. The linear-coded MNL model was used to derive a meaningful marginal rate of substitution as the maximum acceptable increase in the risk of CV death for a decrease in the risk of the other outcomes. In addition, a heteroscedastic MNL model was estimated to assess the presence of scale heterogeneity between acute and chronic samples that could affect validity of pooling data from the two samples. The presence of serial correlation of choices within each patient was tested by estimating an MNL model with individual-specific error component.
To assess a potential effect of sociodemographic and clinical characteristics on preference heterogeneity, the following subgroups were introduced as an interaction term with the treatment attributes in the MNL model: disease stage (acute vs chronic), bleeding risk factors (0 vs ≥ 1), past MIs (> 1 vs 1), family member or close friend with health outcomes of interest (yes vs no), age (< 65 vs ≥ 65 years), gender (male vs female), and risk of future ischemic events (low vs medium vs high). Bleeding risk factors included low body weight, prior use of antithrombotic drugs, and prior CV disease. Risk of future ischemic events was scored using a validated TIMI (thrombolysis in myocardial infarction) risk prediction algorithm [39]. The TIMI risk score is calculated based on the following nine risk factors: age ≥ 75 years, diabetes mellitus, hypertension, current smoking, peripheral artery disease, prior stroke, prior coronary artery bypass grafting, history of heart failure, and renal dysfunction. The presence of each risk factor is given a score of 1. Patients are categorized as low (risk score = 0–1), medium (risk score = 2), and high (risk score ≥ 3) risk. Details of the MNL model specifications are provided in Online Resource 1 (see electronic supplementary material [ESM]).
2.4 Ethics
Patients had to provide electronic consent before taking part in the study. The study was approved by the Bloomsbury Research Ethics Committee and Health Research Authority (reference no. 17/LO/2076) and was conducted in accordance with the General Data Protection Regulation.
3 Results
3.1 Sample Characteristics
Of 1042 patients with MI screened (206 in the acute phase, 836 in the chronic phase), 335 (155 in the acute phase, 180 in the chronic phase) completed the survey (Fig. 2). Patients were predominantly male (82%), the mean age was 64.2 ± 9.6 years, and 39% had a college degree or higher (Table 2). Most patients (93%) had not experienced comorbidities related to the treatment attributes (IS, ICH, or other bleeding events). The most common CV comorbidities were placement of a coronary stent (50%), hypertension (44%), high cholesterol (43%), and diabetes (21%). Most patients had a history of smoking (58%), although few (6%) were current smokers. The most common currently or previously used medications for MI or stroke were antithrombotic drugs, followed by cholesterol-lowering drugs. Most patients had adequate health literacy (77%) and numeracy (89%).
Patients in the acute phase of MI were younger than those in the chronic phase (mean 61.2 vs 66.8 years; p < 0.001). Patients in the acute phase were also less likely than patients in the chronic phase to have high cholesterol (52% vs 32%; p < 0.001), less likely to have had a coronary artery bypass graft (21% vs 8%; p = 0.002), but more likely to have had placement of a coronary stent (40% vs 62%; p < 0.001). Furthermore, patients in the acute phase less often previously used antithrombotic drugs (46%) than those in the chronic phase (79%), although antithrombotic drugs were used as current treatment by most patients in both the acute (99%) and chronic phases (95%).
3.2 Validity Assessments
Of the 335 patients included, 313 (93%) passed the dominance test and 276 (82%) demonstrated consistency in answering repeated choice questions (Online Resource 2, see ESM). All but one participant were considering all the alternatives presented (i.e., did not choose either all option A or all option B) when making decisions across the choice questions, and only 21% made decisions based solely on a single attribute. Most patients (79%) took at least 20 minutes to complete the survey.
3.3 Model Selection
Significant correlations of choices within patients and scale heterogeneity were not observed between acute and chronic MI patients (Online Resource 3, see ESM). The latter finding indicated that data from these two populations could be pooled. Because the dummy-coded MNL model showed preferences for attributes with four levels to be highly linear (R2 = 0.85–0.99; Online Resource 4, see ESM), a linear-coded MNL model was used for analysis of attribute preferences.
3.4 Preferences for Attributes
All attributes were significantly valued by patients (Fig. 3). Patients most valued a 1% reduction in the risk of non-fatal ICH (coefficient = 0.20 [95% CI 0.17–0.23]), followed by a 1% reduction in the risk of non-fatal other severe bleeding (coefficient = 0.15 [95% CI 0.13–0.18]), a 1% reduction in the risk of CV death (coefficient = 0.14 [95% CI 0.12–0.16]), a 1% reduction in the risk of non-fatal MI (coefficient = 0.08 [95% CI 0.07–0.1]), and a 1% reduction in the risk of non-fatal IS (coefficient = 0.06 [95% CI 0.02–0.11]).
Preferences for attributes of antithrombotics overall and by MI disease phase. Preferences for changes in the attributes are expressed as preference weights. Markers indicate maximum likelihood estimates and the bars indicate 95% CI. The preference weights were obtained from separate MNL model results for the overall, acute, and chronic MI patients. The acute phase of MI disease was defined as last MI ≤ 365 days before enrolment, and the chronic phase of MI disease was defined as last MI > 365 days before enrolment. CI confidence interval, CV cardiovascular, ICH intracranial hemorrhage, IS ischemic stroke, MI myocardial infarction; *p < 0.05, **p < 0.01, ***p < 0.001
3.5 Willingness to Make Trade-Offs Between Attributes
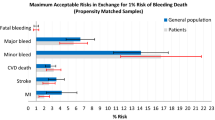
Willingness to make trade-offs between attributes was estimated as the maximum acceptable risk of CV death. On average, patients were willing to accept an increase in the risk of CV death of 1.39% (95% CI 1.14–1.64) to reduce the risk of non-fatal ICH by 1%, 1.06% (95% CI 0.85–1.26) to reduce the risk of non-fatal other severe bleeding by 1%, 0.57% (95% CI 0.48–0.66) to reduce the risk of non-fatal MI by 1%, and 0.41% (95% CI 0.10–0.73) to reduce the risk of non-fatal IS by 1% (Table 3).
3.6 Differences in Preferences Between Participant Subgroups
Patients aged ≥ 65 years were more likely than those aged < 65 years to choose a treatment that reduces the risk of MI (odds ratio [OR] 1.03, 95% CI 1.00–1.06). On the other hand, patients with one or more bleeding risk factors were less likely than those without any bleeding risk factors to choose a treatment that reduces the risk of CV death by 1% (OR 0.94, 95% CI 0.90–0.98) and to choose a treatment that reduces the risk of MI by 1% (OR 0.95, 95% CI 0.92–0.99) (Fig. 4). Also, patients who were at high risk of developing a future ischemic event (TIMI risk score of ≥ 3) were less likely than those who were at low risk (TIMI risk score ≤ 1) to choose a treatment that reduces the risk of CV death by 1% (OR 0.93, 95% CI 0.88–0.98). No significant difference in preferences were observed according to gender (p = 0.32), family history of MI (p = 0.12), having experienced more than one MI in the past (p = 0.35), or disease stage (p = 0.17) (Online Resource 5, see ESM).
4 Discussion
The results demonstrated that preferences for antithrombotic treatment attributes did not vary between patients with a prior MI during the acute and chronic phases of disease. The most valued attribute was reducing the risk of non-fatal ICH, followed by reducing the risk of non-fatal other severe bleeding, CV death, non-fatal MI, and non-fatal IS. The study also showed that patients with MI were willing to accept a significant increase in CV mortality risk in exchange for a reduced risk of non-fatal bleeding and ischemic events.
Preference for a reduced risk of bleeding over a reduced risk of IS and further CV complications was also reported in patients treated for atrial fibrillation [40,41,42]. Similar to the current study, these studies presented the risk of bleeding as an increase in treatment risk associated with the use of antithrombotic drugs, and the risk of stroke as a reduction in risk as a result of antithrombotic treatment. Framing the risks in this way, however, could have resulted in the patients avoiding risks in favor of gaining benefits, a common phenomenon known as ‘loss aversion’ [43].
Patient preferences for alternative approaches to antithrombotic prophylaxis can vary substantially depending on disease context [21, 24]. Factors influencing preference heterogeneity between studies include prior experience with the treatments, type of health outcomes and treatment benefits considered, and the methods used for preference elicitation. In the current study, patients aged ≥ 65 years valued reduction in the risk of nonfatal MI more than those aged < 65 years, and those without any bleeding risk factors valued reduction in the risk of CV death and nonfatal MI more than those who had at least one bleeding risk factor, whereas patients who were at high risk of developing a future ischemic event valued the risk of CV death less than those who were at low risk. Although previous MI or stroke can influence perceptions about antithrombotic therapies [11, 12], prior CV events other than MI, having experienced more than one previous MI, and having family or close friends with CV disease did not affect preferences in the current study. Importantly, the study also found that preferences were similar between patients in the acute and chronic phases of MI, suggesting that the amount of time since the last MI did not influence patient preferences. Had differences in patient preferences been found between patients with acute and chronic MI disease, they could alter the benefit–risk profile for a given drug and influence decisions about prioritization of assets and product development. Heterogeneity of preferences for a particular drug can also indicate subgroups of patients for whom a drug has an acceptable benefit–risk profile even when it is unfavorable for the full population [44]. Such information can also help support regulatory decision making.
A potential limitation of this study is that it adopted a between-sample rather than a within-sample approach to compare preferences between patients with MI in the acute and chronic phases of disease. Because the results were obtained from two independent groups of patients, it could not account for the variation in individual-specific effects or assess whether preferences of each individual change over time. Although a within-sample approach may have been more statistically rigorous, it would have required substantially more resources to measure preferences longitudinally and would have been subject to losses to follow-up and missing data.
A related limitation was that this study compared two different samples recruited from two different sources (i.e., NHS sites and online patient databases). Chronic-phase patients were identified from online patient databases because early feasibility assessment suggested that they are difficult to recruit from the cardiology specialist clinics at the NHS site as they are more likely to attend follow-ups in a primary care setting once their conditions have stabilized. Although an effort was made to recruit acute- and chronic-phase patients with similar characteristics, there were some differences. Notably, patients in the chronic phase of disease were older and had more co-morbidities (e.g., heart failure and hypercholesterolemia) than those in the acute phase. Analysis controlling for the effect of disease stage, however, confirmed the robustness of the findings from the subgroup analysis. This suggests that the differences in sociodemographic and clinical characteristics between disease phases did not affect overall preferences for the attributes of antithrombotic drugs in this study.
Another potential limitation is that although the study was designed to assess patient preferences for both acute and chronic MI, it was not specifically powered to detect differences between groups. Although no obvious differences in preferences were detected between acute and chronic MI, it is possible that some small differences could have been detected with a larger sample. However, the current results indicate that the effect size would likely be too small to affect benefit–risk assessment.
In this study, a separate qualitative research was not conducted to identify and formulate the attributes and levels included in the DCE [45]. The initial list of treatment attributes included CV effects with irreversible clinical harm (MI, IS, ICH, severe bleeding), which have been traditionally used by regulators and other key stakeholders for benefit–risk decision making. However, to ensure that the attribute definitions and their levels were relevant and comprehensible, they were discussed in qualitative pilot interviews, and the survey instrument was updated based on review of the feedback. As part of this, patients were asked for their opinion about possible additional attributes that may be included. Therefore, although the attributes and levels were not selected from separate qualitative research, the qualitative pilot testing helped ensure that all attributes included in this study were relevant and meaningful for patients.
A final potential limitation of this study was that no reference was made to baseline risks of developing the CV or bleeding events when framing the DCE choice question. This could have influenced the way patients interpreted the treatment benefits and risks and therefore had some effect on their preferences. Including baseline risk in an opt-out alternative in the DCE choice tasks may help standardize interpretation of risks in future studies.
5 Conclusion
This study provided information that should help inform regulatory decisions about antithrombotic drugs. Our findings suggest that phase of MI is not an important consideration for patient-centered benefit–risk assessments of antithrombotic drugs, although age, bleeding risk factors, and risk of future ischemic events are. The study also indicated that patients with MI are willing to accept increases in the mortality risk in exchange for avoiding increases in non-fatal bleeding events. This suggests that patients are averse to the risk of bleeding events and, as such, when assessing the benefits and risks of antithrombotic drugs, a treatment will need to achieve a greater reduction in risk of further ischemic events to have a positive benefit–risk balance. The results of this study can also support decisions about drug development, such as whether to seek to seek approval for an antithrombotic drug for both phases of MI disease. Finally, although undertaken primarily as an industry-sponsored preference study, the current results will be used to support development of guidelines by the PREFER project [46] on how and when to include patient perspectives on benefits and risks of medicinal products.
References
Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33(21):2719–47. https://doi.org/10.1093/eurheartj/ehs253.
Soliman EZ, Safford MM, Muntner P, Khodneva Y, Dawood FZ, Zakai NA, et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med. 2014;174(1):107–14. https://doi.org/10.1001/jamainternmed.2013.11912.
Pasea L, Chung SC, Pujades-Rodriguez M, Shah AD, Alvarez-Madrazo S, Allan V, et al. Bleeding in cardiac patients prescribed antithrombotic drugs: electronic health record phenotyping algorithms, incidence, trends and prognosis. BMC Med. 2019;17(1):206. https://doi.org/10.1186/s12916-019-1438-y.
Holmes EAF, Plumpton C, Baker GA, Jacoby A, Ring A, Williamson P, et al. Patient-focused drug development methods for benefit-risk assessments: a case study using a discrete choice experiment for antiepileptic drugs. Clin Pharmacol Ther. 2019;105(3):672–83. https://doi.org/10.1002/cpt.1231.
Tervonen T, Angelis A, Hockley K, Pignatti F, Phillips LD. Quantifying preferences in drug benefit-risk decisions. Clin Pharmacol Ther. 2019;106(5):955–9. https://doi.org/10.1002/cpt.1447.
Tervonen T, Naci H, van Valkenhoef G, Ades AE, Angelis A, Hillege HL, et al. Applying multiple criteria decision analysis to comparative benefit-risk assessment: choosing among statins in primary prevention. Med Decis Making. 2015;35(7):859–71. https://doi.org/10.1177/0272989X15587005.
US Food and Drug Administration. Benefit-risk assessment in drug regulatory decision-making. Draft PDUFA VI Implementation Plan (FY 2018-2022); 2018.
Egbrink MO, Ijerman M. The value of quantitative patient preferences in regulatory benefit-risk assessment. J Mark Access Health Policy. 2014. https://doi.org/10.3402/jmahp.v2.22761.
Ogden J, Ambrose L, Khadra A, Manthri S, Symons L, Vass A, et al. A questionnaire study of GPs’ and patients’ beliefs about the different components of patient centredness. Pat Educ Couns. 2002;47(3):223–7. https://doi.org/10.1016/s0738-3991(01)00200-2.
Devereaux PJ, Anderson DR, Gardner MJ, Putnam W, Flowerdew GJ, Brownell BF, et al. Differences between perspectives of physicians and patients on anticoagulation in patients with atrial fibrillation: observational study. BMJ. 2001;323(7323):1218–22. https://doi.org/10.1136/bmj.323.7323.1218.
Stafinski T, Menon D, Nardelli A, Bakal J, Ezekowitz J, Tymchak W, et al. Incorporating patient preferences into clinical trial design: results of the opinions of patients on treatment implications of new studies (OPTIONS) project. Am Heart J. 2015;169(1):122–3122. https://doi.org/10.1016/j.ahj.2014.10.002.
Johnson FR, Zhou M. Patient preferences in regulatory benefit-risk assessments: a US perspective. Value Health. 2016;19(6):741–5. https://doi.org/10.1016/j.jval.2016.04.008.
Muhlbacher AC, Juhnke C, Beyer AR, Garner S. Patient-focused benefit-risk analysis to inform regulatory decisions: the European union perspective. Value Health. 2016;19(6):734–40. https://doi.org/10.1016/j.jval.2016.04.006.
de Bekker-Grob EW, Berlin C, Levitan B, Raza K, Christoforidi K, Cleemput I, et al. Giving patients’ preferences a voice in medical treatment life cycle: the PREFER public-private project. Patient. 2017;10(3):263–6. https://doi.org/10.1007/s40271-017-0222-3.
Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–77. https://doi.org/10.1093/eurheartj/ehx393.
Everett B, Salamonson Y, Rolley JX, Davidson PM. Underestimation of risk perception in patients at risk of heart disease. Eur J Cardiovasc Nurs. 2016;15(3):e2-9. https://doi.org/10.1177/1474515114556712.
Broadbent E, Petrie KJ, Ellis CJ, Anderson J, Gamble G, Anderson D, et al. Patients with acute myocardial infarction have an inaccurate understanding of their risk of a future cardiac event. Intern Med J. 2006;36(10):643–7. https://doi.org/10.1111/j.1445-5994.2006.01150.x.
Samsa GP, Cohen SJ, Goldstein LB, Bonito AJ, Duncan PW, Enarson C, et al. Knowledge of risk among patients at increased risk for stroke. Stroke. 1997;28(5):916–21. https://doi.org/10.1161/01.str.28.5.916.
Dracup K, McKinley S, Doering LV, Riegel B, Meischke H, Moser DK, et al. Acute coronary syndrome: what do patients know? Arch Intern Med. 2008;168(10):1049–54. https://doi.org/10.1001/archinte.168.10.1049.
Webster R, Heeley E. Perceptions of risk: understanding cardiovascular disease. Risk Manag Healthc Policy. 2010;3:49–60. https://doi.org/10.2147/RMHP.S8288.
MacLean S, Mulla S, Akl EA, Jankowski M, Vandvik PO, Ebrahim S, et al. Patient values and preferences in decision making for antithrombotic therapy: a systematic review: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e1S-e23S. https://doi.org/10.1378/chest.11-2290.
Myles PS, Thompson G, Fedorow C, Farrington C, Sheridan N. Evaluation of differences in patient and physician perception of benefit and risks of aspirin and antifibrinolytic therapy in cardiac surgery. Anaesth Intensive Care. 2014;42(5):592–8. https://doi.org/10.1177/0310057X1404200508.
Najafzadeh M, Gagne JJ, Choudhry NK, Poliniski J, Avorn JL. Patient versus general population preferences in anticoagulant therapy. Value Health. 2015;18(3):9–10. https://doi.org/10.1016/j.jval.2015.03.064.
Najafzadeh M, Gagne JJ, Choudhry NK, Polinski JM, Avorn J, Schneeweiss SS. Patients’ preferences in anticoagulant therapy: discrete choice experiment. Circ Cardiovasc Qual Outcomes. 2014;7(6):912–9. https://doi.org/10.1161/CIRCOUTCOMES.114.001013.
Wanishayakorn T, Sornlertlumvanich K, Ngorsuraches S. Benefit-risk assessment of HMG-CoA reductase inhibitors (statins): a discrete choice experiment. BMJ Open. 2016;6(2):e009387. https://doi.org/10.1136/bmjopen-2015-009387.
Yuan Z, Levitan B, Burton P, Poulos C, Brett Hauber A, Berlin JA. Relative importance of benefits and risks associated with antithrombotic therapies for acute coronary syndrome: patient and physician perspectives. Curr Med Res Opin. 2014;30(9):1733–41. https://doi.org/10.1185/03007995.2014.921611.
Prefer. Patient preferences. 2021. https://www.imi-prefer.eu/. Accessed 18 Aug 2021.
Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, et al. Conjoint analysis applications in health–a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14(4):403–13. https://doi.org/10.1016/j.jval.2010.11.013.
Johnson FR, Lancsar E, Marshall D, Kilambi V, Muhlbacher A, Regier DA, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task force. Value Health. 2013;16(1):3–13. https://doi.org/10.1016/j.jval.2012.08.2223.
Gonzalez JM, Poulos C, Mollon P. Understanding medication adherence using stated-preference data. Value Health. 2014;17(7):A492–3. https://doi.org/10.1016/j.jval.2014.08.1460.
Mühlbacher AC, Bethge S, Kaczynski A, Juhnke C. Patient preferences for long-term treatment after acute coronary syndrome: a discrete choice experiment and analytic hierarchy process. Value Health. 2013;16(7):A534. https://doi.org/10.1016/j.jval.2013.08.1329.
Gusto Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329(10):673–82. https://doi.org/10.1056/NEJM199309023291001.
Scirica BM, Bonaca MP, Braunwald E, De Ferrari GM, Isaza D, Lewis BS, et al. Vorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: a prespecified subgroup analysis of the TRA 2 degrees P-TIMI 50 trial. Lancet. 2012;380(9850):1317–24. https://doi.org/10.1016/S0140-6736(12)61269-0.
Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36(8):588–94.
Peters E, Vastfjall D, Slovic P, Mertz CK, Mazzocco K, Dickert S. Numeracy and decision making. Psychol Sci. 2006;17(5):407–13. https://doi.org/10.1111/j.1467-9280.2006.01720.x.
Heidenreich S, Phillips-Beyer A, Flamion B, Ross M, Seo J, Marsh K. Benefit-risk or risk-benefit trade-offs? Another look at attribute ordering effects in a pilot choice experiment. Patient. 2021;14(1):65–74. https://doi.org/10.1007/s40271-020-00475-y.
Manski CF. The structure of random utility models. Theor Decis. 1977;8(3):229–54. https://doi.org/10.1007/bf00133443.
Marschak J. Binary choice constraints on random utility indicators. In: Mathematical methods in the social sciences. Palo Alto: Stanford University Press; 1959. p. 312–29.
Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366(1):9–19. https://doi.org/10.1056/NEJMoa1112277.
Man-Son-Hing M, Laupacis A, O’Connor AM, Biggs J, Drake E, Yetisir E, et al. A patient decision aid regarding antithrombotic therapy for stroke prevention in atrial fibrillation: a randomized controlled trial. JAMA. 1999;282(8):737–43. https://doi.org/10.1001/jama.282.8.737.
Manoning M, Oonnor AM, Drake E, Biggs J, Hum V, Laupacis A. The effect of qualitative vs. quantitative presentation of probability estimates on patient decision-making: a randomized trial. Health Expect. 2002;5(3):246–55. https://doi.org/10.1046/j.1369-6513.2002.00188.x.
Veldwijk J, Essers BA, Lambooij MS, Dirksen CD, Smit HA, de Wit GA. Survival or mortality: does risk attribute framing influence decision-making behavior in a discrete choice experiment? Value Health. 2016;19(2):202–9. https://doi.org/10.1016/j.jval.2015.11.004.
Tversky A, Kahneman D. Loss aversion in riskless choice: a reference-dependent model. Q J Econ. 1991;106(4):1039–61. https://doi.org/10.2307/2937956.
Ho MP, Gonzalez JM, Lerner HP, Neuland CY, Whang JM, McMurry-Heath M, et al. Incorporating patient-preference evidence into regulatory decision making. Surg Endosc. 2015;29(10):2984–93. https://doi.org/10.1007/s00464-014-4044-2.
Coast J, Al-Janabi H, Sutton EJ, Horrocks SA, Vosper AJ, Swancutt DR, et al. Using qualitative methods for attribute development for discrete choice experiments: issues and recommendations. Health Econ. 2012;21(6):730–41. https://doi.org/10.1002/hec.1739.
Patient Preferences in Benefit-Risk Assessments during the Drug Life Cycle (PREFER). The patient perspective. 2020. https://www.imi-prefer.eu/. Accessed 17 Nov 2020.
Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21(1):37–44. https://doi.org/10.1177/0272989X0102100105.
Acknowledgements
The authors thank Andrew Tershakovec (formerly Merck & Co., Inc., Kenilworth, NJ, USA) and the IMI-PREFER consortium for their valuable advice on the study design. The authors also thank Kevin Marsh and Heather Gelhorn (Evidera), Tarek Hammad (formerly Merck & Co., Inc., Kenilworth, NJ, USA), and Natalia Hawken (formerly Evidera) for their involvement in earlier phases of study implementation. Medical writing was provided by Phillip S. Leventhal and Rangan Gupta (Evidera) and was paid for by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Conflict of interest
Cathy Anne Pinto and Johanna Hyacinthe are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Gin Nie Chua, Ella Brookes, and Tommi Tervonen are employees of Evidera, which was paid by Merck & Co for work related to this study. John F.P. Bridges received consulting fees by Evidera and has previously received grant, travel, and consultation fees from Merck & Co.
Ethics approval
The study was approved by the Bloomsbury Research Ethics Committee and Health Research Authority and was conducted in accordance with the General Data Protection Regulation.
Consent to participate
Patients had to provide electronic consent before taking part in the study.
Consent for publication
Not applicable.
Availability of data and material
The datasets generated during and/or analyzed during the current study are not publicly available as no consent was sought from participants to allow sharing of data with third parties.
Code availability
Not applicable.
Author contributions
CAP contributed to conception, design, interpretation of results, and revision of the manuscript. GNC contributed to the implementation of the work and analysis, interpretation of data for the work, and drafting and revision of the manuscript. JFPB contributed to design, interpretation of results, and revision of the manuscript. EB contributed to the implementation of the work and revision of the manuscript. JH contributed to the implementation of the work and revision of the manuscript. TT contributed to the conception, design, and implementation of the work and analysis, interpretation of data for the work, and drafting and revision of the manuscript. All authors gave final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Pinto, C.A., Chua, G.N., Bridges, J.F.P. et al. Comparing Patient Preferences for Antithrombotic Treatment During the Acute and Chronic Phases of Myocardial Infarction: A Discrete-Choice Experiment. Patient 15, 255–266 (2022). https://doi.org/10.1007/s40271-021-00548-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-021-00548-6