Abstract
Background and Objective
Juvenile idiopathic arthritis (JIA) is a chronic autoimmune disorder that primarily affects the joints in children. Notably, it is known to co-occur with uveitis. Adalimumab, a monoclonal anti-TNF antibody, is effective in treating both conditions. A deeper understanding of the pharmacokinetics (PK) of adalimumab in JIA is crucial to advance in more personalized treatment approaches. The objective of this study is to evaluate the population PK profile of adalimumab in JIA and to explain causes for its variability.
Materials and Methods
Adalimumab and antidrug antibody concentrations were retrospectively retrieved from the charts of patients with JIA. Initially, five literature-based population PK models of adalimumab were evaluated to assess their ability to describe the observed concentration–time profiles in the JIA cohort. These models included one specifically for the pediatric Crohn’s disease population and four derived from studies in adult populations in healthy subjects and rheumatoid arthritis patients. Subsequently, a novel population PK model tailored to the JIA population was developed using NONMEM software. Monte Carlo simulations were then conducted utilizing the final PK model to visualize the concentration–time profile of adalimumab in patients with JIA and the impact of covariates.
Results
A cohort of 50 patients with JIA with 78 available adalimumab samples was assessed. The mean age was 11.8 ± 3.9 years, with a median body weight of 49 kg (interquartile range 29.4–59.8 kg). All literature models adequately described the concentration–time profiles in JIA. The best model, which was developed in patients with rheumatoid arthritis during the maintenance phase of treatment, served as a basis for estimating clearance in JIA, resulting in a value of 0.37 L per day per 70 kg. Patient body weight, antidrug antibodies, methotrexate use, CRP level, and comorbidity of uveitis were found to have a significant impact on adalimumab clearance, and these reduced the inter-patient variability from 58.6 to 28.0%. On steady state in the simulated patient population, the mean trough level was 7.4 ± 5.5 mg/L. The two dosing regimens of 20 and 40 mg every other week, based on patients’ body weight, resulted in comparable simulated overall drug exposure.
Conclusions
Five literature models effectively described adalimumab PK in this pediatric cohort, highlighting the potential for extrapolating existing models to the pediatric population. The new JIA model confirmed the effect of several known covariates and found a novel association for drug clearance with methotrexate use (lower) and uveitis (higher), which might have clinical relevance for personalized dosing in JIA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Adalimumab is a widely used treatment for juvenile idiopathic arthritis and its comorbid concomitant extra-articular manifestation, uveitis, but standard dosages result in varying drug levels. |
This study reveals that existing adalimumab pharmacokinetic models effectively capture drug levels in juvenile idiopathic arthritis patients, which was also reflected by the estimated adalimumab clearance in this population and notable covariate associations. |
By establishing a tailored pharmacokinetic model for juvenile idiopathic arthritis, this research sets the stage for personalized dosing approaches, with the potential to enhance treatment efficacy and cost effectiveness, although further comprehensive pharmacokinetic studies incorporating intensified sampling strategies are warranted to refine the model. |
1 Introduction
Juvenile idiopathic arthritis (JIA) is the most common rheumatic disease in children, characterized by chronic joint inflammation, persisting for at least 6 weeks without any identifiable cause in children younger than 16 years [1]. The most common extra-articular manifestation is uveitis, with an estimated prevalence of 10–20% in patients with JIA [2]. Prompt recognition and treatment is important to prevent ocular complications that could lead to permanent vision loss [3]. The introduction of monoclonal antibodies (mAbs) targeting various cytokines, such as tumor necrosis factor (TNF), has significantly expanded the number of treatment options and has substantially improved the prognosis of JIA and JIA associated uveitis [4, 5]. However, not all patients respond and about a quarter of the patients lose response over time [6, 7]. This can partially be attributed to variations in the pharmacokinetics (PK) of mAbs between individuals, which encompasses variations in how a drug is absorbed, distributed, metabolized, or eliminated, thereby impacting the drug’s concentration within the body and, thus, clinical effect and toxicity [8, 9]. By better understanding these processes, dosing could be personalized, potentially leading to the optimization of patient responses and reducing excessive drug exposure.
Adalimumab is a mAb that blocks TNF and has been approved for use in patients with JIA. In Europe, the recommended dosage for JIA and JIA associated uveitis is 20 mg every other week for patients weighing between 10 and 30 kg and 40 mg every other week for those weighing over 30 kg [10, 11]. This approach results in a wide range of drug levels and as a result some patients may experience inadequate drug exposure, which can result in suboptimal treatment outcomes, while others have possibly unnecessary high(er) drug concentrations [12]. To improve the effectiveness and safety of treatment in pediatric patients, there is a growing need to develop personalized dosing strategies for adalimumab in patients with JIA.
PK studies performed in the adult rheumatoid arthritis population have shown that the patient-related variables body weight, immunogenicity, and use of concomitant medication are the most important factors to affect adalimumab serum levels [13, 14]. However, this has, to our knowledge, not been studied in patients with JIA. The same holds true for the optimal therapeutic drug range in this population [12]. To reach adequate clinical response in adults with rheumatoid arthritis, an adalimumab trough level of 5 mg/L has been described to be sufficient to reach clinical response [15]. However, it is unknown if this lower limit of therapeutic drug level applies to patients with JIA as well. For example, clinical observations suggest that children undergoing adalimumab treatment for JIA with concomitant uveitis may require higher adalimumab doses, potentially up to double the standard dosage, to achieve low disease activity or remission [16,17,18]. However, pediatric PK studies are lacking [19].
Understanding the PK of adalimumab in patients with JIA is fundamental to introduce personalized medicine in these children. This study, therefore, aims to describe the PK of adalimumab in patients with JIA. First, we compared previously established PK models of adalimumab in other populations with data from our pediatric JIA cohort. Second, PK modeling techniques were used to estimate relevant PK parameters and explain interindividual variability in drug exposure.
2 Patients and Methods
2.1 Patients and Study Design
Data of patients with JIA (< 18 years), who have been treated with subcutaneous (sc) adalimumab injections at the Emma Children’s Hospital from Amsterdam University Medical Center (AUMC) in the Netherlands, were retrospectively screened between January 2013 and August 2023. Patients were included if at least one adalimumab serum trough concentration was performed based on clinical indication. According to local protocol, when drug levels were measured, the treating physician noted the date and time of last administration of the drug. Patients’ demographics and characteristics were collected from medical charts and contained patient sex, age, body weight, diagnosis, adalimumab treatment characteristics (dose, interval injection, and specific date/time of last injection), adalimumab serum (trough) concentration, and, if measured, antidrug antibodies (ADA) and use of concomitant immunomodulators. Active joint inflammation was determined based on clinical assessment of the joints by a pediatric rheumatologist: presence of swelling, pain on motion/tenderness, and/or limited range of motion. Data entry and management was carried out anonymously; hence, informed consent was waved by the medical ethical committee of the AUMC.
2.2 Bioanalysis
Serum adalimumab concentrations were measured using an enzyme-linked immunosorbent assay (ELISA), with a lower limit of quantification (LLOQ) of 0.01 mg/L [20]. ADA were measured when the concentration of adalimumab was below 5 mg/L, utilizing a drug-sensitive antigen binding test. The 5 mg/L cutoff is determined by previous research, indicating that the employed test no longer detects clinically relevant antibodies against adalimumab when the adalimumab serum level exceeds 5 mg/L [21]. Both assays were developed by Sanquin Laboratories (Amsterdam, The Netherlands) [20, 22, 23].
2.3 Population Pharmacokinetic Analyses
NONMEM version 7.5 was used to perform the PK analyses in conjunction with Pirana version 21.11.1, PsN version 5.3.0, R version 4.3.1, and RStudio version 2023.03.0+386. In April 2023, a literature search was conducted on PubMed using MeSH terms related to adalimumab PK and inflammatory diseases. From the identified publications, 9 deemed relevant, presenting a total of 11 different adalimumab PK models (Supplementary Table 1). Out of these, five were selected for further analysis. One model was specifically chosen for its description of the PK of adalimumab in pediatric patients [25], while another was selected based on prior application in a TDM study in our group involving adult rheumatoid arthritis patients [13] (clinical trial number 2019-001793-28). Additionally, three of the most recent and largest adalimumab PK models for rheumatoid arthritis patients, as detailed in a single article, were also incorporated [14]. Model fit was evaluated using prediction-corrected visual predictive checks (VPC’s) [24], with 1000 simulations. An overview of the evaluated PK models is provided in Supplementary Table 2. In short, Sharma et al. described the PK of adalimumab in a cohort of pediatric patients with Crohn’s disease with a one-compartment model and the covariates body weight and ADA on clearance and body weight on volume of distribution [25]. Next, Ternant et al. described the PK of adalimumab in adult rheumatoid arthritis patients, with one compartment and the covariates body weight and sex on clearance [13]. Finally, Kang et al. developed three PK models in different populations consisting of healthy volunteers (model 1), adult rheumatoid arthritis patients in their first 48 weeks of adalimumab treatment (model 2), and these same patients in the maintenance phase after 48 weeks of treatment (model 3) [14]. These models consisted of two compartments and contained the clinically relevant covariates body weight and ADA.
Next, the literature model with the best fit to the JIA data was selected, and the dataset was used to estimate relevant PK parameters using the first-order conditional estimation method with interaction (FOCE+I). Inter- and intraindividual variability were explained by adding covariates to the model using a forward backward stepwise regression method. Variables were retained in the model if they improved the objective function value (OFV) by 3.84 points, which compares to a p-value of 0.05 [26]. The covariates sex, body weight, body surface area, ever detection of ADA (yes/no), use of concomitant immunosuppressive medication (yes/no), use of methotrexate (yes/no), C-reactive protein (CRP) level, and uveitis during treatment with adalimumab (yes/no) were evaluated. All variables were evaluated using a power model, and continuous covariates were centered respective to the median in the population. This is shown in Eqs. 1 and 2:
In Eq. 1, typical value parameter (TVP), corresponds to \({\theta }_{{\text{p}}}\) for the reference covariate value, which is ADA negative. The effect of the presence of ADA is quantified by \({\theta }_{{\text{ADA}}}\). In Eq. 2, the population central tendency (\({\theta }_{{\text{P}}}\)) tendency is equal to the TVP when CRP equals the reference CRP (rCRP). The reference value is the median of the population. The scaling factor \({\theta }_{{\text{CRP}}}\) quantifies the effect of the covariate on the parameter.
Besides the OFV value, precision of the parameter estimates and visual inspection of the goodness of fit plots also contributed to model evaluation. A bootstrap of 1000 samples was performed to evaluate robustness of the final model. Subsequently, a sensitivity analysis was performed to investigate the importance of incorporating CRP as a covariate on clearance in the model, given the predominantly low measured CRP values in patients with JIA in this study cohort and clinical practice.
2.4 Simulations
To visualize the concentration–time profiles of adalimumab in patients with JIA and the covariate effects, Monte Carlo simulations were performed using the final PK model. For these analyses, a simulated dataset of 500 patients with JIA was used, and simulations were replicated 25 times. The median values and distribution of covariates in the simulated dataset was based on the original JIA study population. The standard dose was simulated for a period of 10 weeks, after which steady state was reached, and patients were stratified on adalimumab dose and the model covariates. NONMEM version 7.5, R version 4.3.1 and RStudio version 2023.03.0+386 were used for the analyses.
2.5 Statistical Analysis
Patient characteristics are presented as means and standard deviations for continuous variables with a normal distribution, medians, and interquartile ranges for continuous variables with a skewed distribution and counts and percentages for categorical variables. Regarding missing data, the last observation carried forward was applied if it concerned a time-varying covariate. In other cases, the median of the population was imputed. Data analyses were performed using R version 4.3.1 and RStudio version 2023.03.0+386.
3 Results
3.1 Patient Characteristics
A total of 78 adalimumab serum concentrations were available from 50 patients with JIA, with 33 individuals having only sample available. Baseline patient characteristics are provided in Table 1. The mean age of the study population was 11.8 ± 3.9 years, consisted mostly of females (72%), and had a median body weight of 49 kg (IQR 29.4–59.8 kg). Adalimumab levels below 5 mg/L were observed 16 times, with ADA testing conducted in 12 of these instances, resulting in the detection of ADA ten times (in nine different patients). In 34 patients (68%), there were signs of active joint inflammation the moment of drug measurement. Besides arthritis, there was active uveitis in eight patients (16%) during treatment with adalimumab. The median adalimumab serum level was 12.0 (IQR 6.12–15.8) mg/L, with samples collected at random time points in the treatment interval. Most patients in the cohort received 40 mg adalimumab every other week. In seven samples (9.0%), adalimumab drug levels were below the detection limit of the assay. These were fixed to 0.005 mg/L, slightly below the assay’s lower limit of quantification.
3.2 Fit of Literature Models
Supplementary Fig. 1 demonstrates the fit of the selected literature models to the concentration–time data from the JIA cohort using prediction-corrected visual predictive checks [24]. The middle solid line, which represents the median adalimumab concentration from the JIA population, is mostly situated within the associated confidence interval represented by the red shaded area, representing a good fit. Overall, model 3 from Kang et al. [14] had the best fit to the JIA data, particularly after consideration of the models’ OFV values (data not shown). This model was developed using data from a phase 3 study and included adult rheumatoid arthritis patients during the maintenance phase after 48 weeks of treatment [14].
3.3 New PK Model
Commencing with the structural model from Kang et al. [14], parameters were newly estimated using the JIA data to refine model precision. In addition, this approach also aimed to further discern potential differences between the examined literature models and the new estimates, to better understand the PK of adalimumab in this pediatric population. Limited by the sparseness of the dataset, only clearance and its association with certain covariates, along with interpatient variability on clearance and residual variability, could be estimated. All other parameters were fixed to their values from the original model.
The final PK model estimates are displayed in Table 2. JIA population clearance (CL) was estimated at 0.37 L per day per 70 kg. Standard allometric scaling was used for the effect of body weight on CL, central volume of distribution (V1), intercompartmental clearance (Q), and peripheral volume of distribution (V2), with a reference weight of 70 kg. The final model also incorporated associations between CL and the covariates ADA, concomitant use of methotrexate, CRP level, and comorbidity of uveitis during adalimumab treatment. Due to limited statistical power to stably identify the covariate effect of ADA, it was fixed at a value of 2.08, indicating a 108% increase in CL in the presence of ADA. This value was derived from the Kang model, which utilized patients that are ADA-positive as the reference group. According to the Kang model, patients that are ADA-negative exhibited approximately one-third lower clearance, at 65%, compared with patients that are ADA-positive. Since Kang’s model also incorporated ADA titer as a distinct covariate, the covariate effect of ADA was effectively doubled, resulting in a value of 2.08. Next, concomitant use of methotrexate was associated with a decreased CL of 28% and an association was found between CL and concomitant uveitis: patients with concomitant uveitis had 44% higher clearance compared with patients without concomitant uveitis. Another positive association was found between CL and CRP level; in patients with a CRP level of 10 mg/L, CL increased by 59% when compared with the reference value of 0.6 mg/L.
Interindividual variability (IIV) was estimated for CL while keeping the full omega variance–covariance matrix for the other parameters as described previously [14]. IIV for CL, expressed in terms of % coefficient of variation (CV), decreased in the final model from 58.6 to 28.0% compared with the structural model without any covariates. Finally, residual variability was modeled using an additive error model.
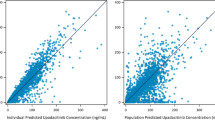
Figure 1 and Supplementary Figs. 2 and 3 demonstrate the prediction corrected VPC, the original VPC, and goodness of fit plots of the final adalimumab PK model in patients with JIA, respectively, and all indicate a good model fit. Regarding the prediction-corrected VPC in Fig. 1, the estimated median and upper and lower bound of the confidence interval are all well within the shaded areas, the associated confidence intervals. A bootstrap of 1000 samples was performed in which 885 (88.5%) of the runs completed successfully. The results of this bootstrap procedure are shown next to the final parameter estimates in Table 2. The ƞ-shrinkage for CL was 27%, which is reasonable. The sensitivity analysis, which involved excluding CRP from the final model, had virtually no impact on the estimated clearance and covariate effects (Supplementary Table 3). However, it did result in a somewhat higher OFV value with 10.5 points and there was a slight increase in interindividual variability.
Visual predictive check of final model. This figure shows the prediction-corrected visual predictive check of the final model. The black dots represent individual prediction-corrected adalimumab observations, and the red solid line demonstrates the median of these values. The solid blue lines depict the observed 5th and 95th percentiles. The shaded areas represent the simulated data, in red the 95% confidence interval of the median and in blue the 95% confidence intervals of the 5th and 95th percentiles
3.4 Simulations
The final PK model was used to calculate adalimumab concentrations over time in a dataset with 500 simulated patients to give additional insights into the average concentration-time profiles of patients with JIA and the effect of the identified covariates. The simulated patients with JIA had a median body weight of 45.3 kg (IQR 37.5–53.9 kg), 13.2% had detectable ADA, 77.4% used methotrexate, and 15.4% experienced uveitis during adalimumab treatment. The median CRP level was 4.0 (IQR 0.3–10.6) mg/L. After 10 weeks on the standard dose of adalimumab [10], the mean trough level in the simulated dataset was 7.4 ± 5.5 mg/L. The first five treatment intervals, until steady state is reached, are displayed in Supplementary Fig. 4A, which also demonstrates the large interpatient variability in adalimumab concentrations in the simulated population. The two dosing regimens of 20 mg and 40 mg every other week, based on patients’ weight, resulted in comparable simulated overall drug exposure (Supplementary Fig. 4B).
Figure 2 demonstrates the covariate effect of patient body weight, uveitis, methotrexate use, and detection of ADA on simulated adalimumab serum levels. Stratification based on patients’ body weight into five different categories revealed that, as anticipated, individuals closer to the 30 kg threshold exhibited higher adalimumab concentrations compared with those in heavier weight categories when following the standard approved dose (20 mg every other week for patients < 30 kg and 40 mg every other week for patients ≥ 30 kg), as depicted in Fig. 2A. For example, patients weighing between 30–40 kg exhibited drug levels nearly twice as high compared with those in patients weighing over 60 kg. Finally, drug concentrations were highest in methotrexate users without detectable ADA, without uveitis during adalimumab treatment and with CRP levels below 1 mg/L (Fig. 2B–E).
Simulated covariate effects. This figure demonstrates the simulated median adalimumab concentrations (in mg/L) of one adalimumab treatment interval of 14 days at steady state for the virtual patient dataset. Results are stratified for patient body weight (A), diagnosis of uveitis during adalimumab treatment (B), concomitant methotrexate use (C), detection of ADA (D), and CRP level (E). ADL conc. adalimumab concentration, MTX methotrexate, ADA antidrug antibodies, CRP C-reactive protein
4 Discussion
This is the first study describing the population PK of adalimumab in patients with JIA. We found that five adalimumab literature models adequately described the concentration-time data from our JIA cohort. However, some overestimation of the observed data in the lower concentration ranges and underestimation of adalimumab concentrations in the higher concentration ranges by the models from Sharma et al. [25] and Ternant et al. [13] reflect the greater variability in adalimumab levels among patients with JIA compared with populations included in prior studies. To further characterize the PK of adalimumab specifically in patients with JIA, its clearance and association with several potential covariates was examined. In these analyses, clearance was estimated at 0.37 L per day per 70 kg, and associations were found with patient body weight, ADA, concomitant use of methotrexate, CRP level, and comorbidity of uveitis during adalimumab treatment.
Our study illustrates a significant resemblance in adalimumab PK among patients with JIA and various cohorts, including healthy adults, adult rheumatoid arthritis patients, and pediatric Crohn’s disease patients. Despite the potential challenges associated with extrapolating PK data from adults to the pediatric population [27, 28], these results suggest the feasibility of such extrapolation for adalimumab, particularly when utilizing standard allometric scaling based on body weight for CL, V1, Q, and V2 [29]. This approach effectively addressed potential differences between adults and children, such as the higher body water content and increased tissue perfusion rate in the latter [28], as evidenced by a significantly improved population PK estimate. In contrast, the disease-related PK differences between pediatric Crohn’s disease and JIA, particularly the leakage of protein (including adalimumab) from an inflamed colon in patients with Crohn’s disease [30], probably introduced complexity in extrapolating this model to the JIA population, demonstrated by a worse fit to the data.
Adalimumab is a known immunogenic protein that can trigger an immune response, and binding of antibodies to adalimumab is known to increase the drug’s clearance [31]. Although the PK model had difficulty to stably identify the covariate effect of ADA in the bootstrap procedure, this study found the same association. However, imputing this covariate effect based on another PK model has several potential drawbacks. Primarily, the variability in ADA assays across different laboratories raises concerns about the accuracy of the imputed ADA estimate, potentially leading to a misspecification of ADA’s true impact on clearance. This concern is particularly pertinent considering the use of a drug-sensitive assay in this study and the one published by Kang et al. [14], which underestimates the prevalence of patients that are ADA-positive [31]. Consequently, correcting too little for the presence of ADA could have resulted in an overestimation of clearance, as this parameter would correct for a potentially inaccurately assumed ADA effect.
As a counterpart to ADA, when adalimumab is coadministered with methotrexate, which most patients in rheumatology take as a concomitant drug, adalimumab clearance was reduced by 28% to 0.27 L per day per 70 kg. This effect of methotrexate aligns with previous studies, as it is a known inhibitor of ADA formation, consequently reducing adalimumab clearance [32, 33]. Furthermore, this diminished clearance is consistent with literature models developed for other indications where methotrexate is concurrently administered [13, 14, 25, 34]. Unfortunately, our JIA dataset did not allow for the determination of a specific dose-effect relationship, which was described previously for the adult rheumatoid arthritis population, were it is recommended to use a methotrexate dose of at least 10 mg/week to mitigate the development of ADA [35, 36].
Clearance also increased with higher CRP level, an association that has been described before [14, 37]. Theoretically, elevated CRP levels, indicative of heightened disease activity and inflammation, may impact antibody breakdown through increased TNF levels and heightened target-mediated drug disposition (TMDD). However, a recent study discredits this notion, revealing comparable TNF levels during TNFi treatment in healthy controls and adult rheumatoid arthritis patients, suggesting that there is no association between CRP and TNF levels, as measured in the circulation, has been demonstrated [38]. In addition, TNF may not even effectively contribute to TMDD, and thus clearance, of TNFi [39]. Therefore, the pathophysiology between CRP level and increased adalimumab clearance remains unknown. The clinical relevance of this association also remains unclear, especially because CRP levels are seldom increased in patients with JIA who usually have low-grade inflammatory disease [40]. Our conducted sensitivity analysis further supports these observations, demonstrating that the parameter estimate of clearance and other covariate estimates remained consistent even when CRP was not included in the PK model.
Finally, a novel association with uveitis was found, and PK analyses concluded an increase in clearance of 44% in these patients. Experience from clinical practice already informed us that patients with JIA with uveitis need more frequent or higher mAbs dosage compared with patients with JIA without uveitis, to control inflammation of the eye (i.e., escalation to weekly adalimumab dosing) [16, 41]. Also, in nonresponding patients with uveitis without JIA, it was already demonstrated that patients benefited from weekly adalimumab injections [42]. This might be explained by the fact that drug exposure resulting from the standard approved dose of adalimumab is inadequate to achieve sufficiently high serum concentrations at the disease site, the eye.
The median (IQR) adalimumab serum level in our cohort was 12.0 (6.2–15.8) mg/L, which is comparable with two earlier publications who reported the adalimumab concentrations in their JIA cohorts [43, 44]. Imagawa et al. described adalimumab concentrations in the range of 0–24.6 mg/L for patients who received either 20 or 40 mg of adalimumab every other week, while Kingsbury et al. [44] reported a mean steady-state adalimumab trough concentration of 7–8 mg/L in young patients with a mean age of 3 years. A comparable mean concentration was found in the simulated dataset at trough after 10 weeks on the standard dose, which was 7.4 ± 5.5 mg/L. The data also revealed a significant variability in adalimumab serum levels among patients with JIA, mirroring observations in the adult population. These findings indicate that the identified covariates may be instrumental in dose optimization for patients with JIA.
Our study has several limitations, primarily attributed to the small sample size and its retrospective nature. Also, concentration measurement was not done in a standardized manner and mainly ordered in case of clinical ineffectiveness. The latter may have led to some bias in data collection, especially with regards to sampling patients with concomitant uveitis. These results should therefore be interpreted with caution. Also, the limited number of available serum measurements made it infeasible to estimate all relevant PK parameters within the JIA population and prospective studies with several PK samples per patient with JIA are warranted. However, using a large literature PK model as a starting point for the analyses, with a previously published structure and fixed key model parameters and focusing on the cohort’s clearance and covariates affecting clearance, did result in the development of a robust model. Not fitting all parameters to this study’s population offers the benefit of enhancing the model’s overall generalizability. However, the downside is that it resulted in a model with a noticeable residual error, complicating its direct application for TDM. This limitation could also be addressed by conducting future PK studies specifically targeted toward the pediatric population and tailored for this particular objective.
5 Conclusions
Five literature PK models for adalimumab were successfully extrapolated to the JIA population. A new PK model for patients with JIA was developed and, among others, methotrexate use and concomitant uveitis were identified as covariates associated with lower and higher clearance, respectively. Simulations were performed to visualize the covariate effects on adalimumab drug levels. Overall, this study aims to contribute to personalized treatment decisions in pediatric rheumatology by identifying covariates that impact the PK of adalimumab in patients with JIA. This knowledge enables healthcare providers to make more informed and tailored treatment decisions for each pediatric patient, ensuring that they receive the most effective and safe dose based on their unique characteristics. Furthermore, the developed PK model can serve as a foundation for investigating serum drug-level guided dosing strategies in the JIA population.
References
Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet. 2011;377(9783):2138–49. https://doi.org/10.1016/S0140-6736(11)60244-4.
Angeles-Han ST, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the screening, monitoring, and treatment of juvenile idiopathic arthritis-associated uveitis. Arthritis Care Res (Hoboken). 2019;71(6):703–16. https://doi.org/10.1002/acr.23871.
Gregory AC 2nd, et al. Risk factors for loss of visual acuity among patients with uveitis associated with juvenile idiopathic arthritis: the SITE study. Ophthalmology. 2013;120(1):186–92. https://doi.org/10.1016/j.ophtha.2012.07.052.
Giancane G, Ruperto N, O. Paediatric Rheumatology International Trials. Treatment of juvenile idiopathic arthritis: what’s new? Curr Opin Rheumatol. 2019;31(5):428–35. https://doi.org/10.1097/BOR.0000000000000632.
Giancane G, et al. Disease activity and damage in juvenile idiopathic arthritis: methotrexate era versus biologic era. Arthritis Res Ther. 2019;21(1):168. https://doi.org/10.1186/s13075-019-1950-7.
Otten MH, et al. Effectiveness and safety of a second and third biological agent after failing etanercept in juvenile idiopathic arthritis: results from the Dutch National ABC Register. Ann Rheum Dis. 2013;72(5):721–7. https://doi.org/10.1136/annrheumdis-2011-201060.
Skrabl-Baumgartner A, Erwa W, Muntean W, Jahnel J. Anti-adalimumab antibodies in juvenile idiopathic arthritis: frequent association with loss of response. Scand J Rheumatol. 2015;44(5):359–62. https://doi.org/10.3109/03009742.2015.1022213.
Ryman JT, Meibohm B. Pharmacokinetics of monoclonal antibodies. CPT Pharmacomet Syst Pharmacol. 2017;6(9):576–88. https://doi.org/10.1002/psp4.12224.
Roberts JA, Pea F, Lipman J. The clinical relevance of plasma protein binding changes. Clin Pharmacokinet. 2013;52(1):1–8. https://doi.org/10.1007/s40262-012-0018-5.
Adalimumab: Summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000481/WC500050870.pdf. Accessed 03 July 2023.
Kinderformularium adalimumab. https://www.kinderformularium.nl/geneesmiddel/598/adalimumab. Accessed 20 Oct 2023.
Nassar-Sheikh Rashid A, et al. Therapeutic drug monitoring of anti-TNF drugs: an overview of applicability in daily clinical practice in the era of treatment with biologics in juvenile idiopathic arthritis (JIA). Pediatr Rheumatol Online J. 2021;19(1):59. https://doi.org/10.1186/s12969-021-00545-x.
Ternant D, et al. Pharmacokinetics and concentration-effect relationship of adalimumab in rheumatoid arthritis. Br J Clin Pharmacol. 2015;79(2):286–97. https://doi.org/10.1111/bcp.12509.
Kang J, Eudy-Byrne RJ, Mondick J, Knebel W, Jayadeva G, Liesenfeld KH. Population pharmacokinetics of adalimumab biosimilar adalimumab-adbm and reference product in healthy subjects and patients with rheumatoid arthritis to assess pharmacokinetic similarity. Br J Clin Pharmacol. 2020;86(11):2274–85. https://doi.org/10.1111/bcp.14330.
Pouw MF, Krieckaert CL, Nurmohamed MT, van der Kleij D, Aarden L, Rispens T, Wolbink G. Key findings towards optimising adalimumab treatment: the concentration-effect curve. Ann Rheum Dis. 2015;74(3):513–8. https://doi.org/10.1136/annrheumdis-2013-204172.
Quartier P. Juvenile idiopathic arthritis-associated chronic uveitis: recent therapeutic approaches. J Clin Med. 2021. https://doi.org/10.3390/jcm10132934.
Roberts JE, Nigrovic PA, Lo MS, Chang MH. Weekly adalimumab, an effective alternative for refractory uveitis in children. J Clin Rheumatol. 2022;28(1):e301–4. https://doi.org/10.1097/rhu.0000000000001707.
Lee J, Koreishi AF, Zumpf KB, Minkus CL, Goldstein DA. Success of weekly adalimumab in refractory ocular inflammatory disease. Ophthalmology. 2020;127(10):1431–3. https://doi.org/10.1016/j.ophtha.2020.04.009.
Renton WD, Jung J, Palestine AG. Tumor necrosis factor (TNF) inhibitors for juvenile idiopathic arthritis-associated uveitis. Cochrane Database Syst Rev. 2022;10(10):Cd013818. https://doi.org/10.1002/14651858.CD013818.pub2.
Kneepkens EL, et al. Immunogenicity, adalimumab levels and clinical response in ankylosing spondylitis patients during 24 weeks of follow-up. Ann Rheum Dis. 2015;74(2):396–401. https://doi.org/10.1136/annrheumdis-2013-204185.
van Bezooijen JS, Koch BC, van Doorn MB, Prens EP, van Gelder T, Schreurs MW. Comparison of three assays to quantify infliximab, adalimumab, and etanercept serum concentrations. Ther Drug Monit. 2016;38(4):432–8. https://doi.org/10.1097/ftd.0000000000000310.
Bartelds GM, et al. Clinical response to adalimumab: relationship to anti-adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritis. Ann Rheum Dis. 2007;66(7):921–6. https://doi.org/10.1136/ard.2006.065615.
van Schouwenburg PA, Bartelds GM, Hart MH, Aarden L, Wolbink GJ, Wouters D. A novel method for the detection of antibodies to adalimumab in the presence of drug reveals “hidden” immunogenicity in rheumatoid arthritis patients. J Immunol Methods. 2010;362(1–2):82–8. https://doi.org/10.1016/j.jim.2010.09.005.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–51. https://doi.org/10.1208/s12248-011-9255-z.
Sharma S, et al. Pharmacokinetics and exposure-efficacy relationship of adalimumab in pediatric patients with moderate to severe Crohn’s disease: results from a randomized, multicenter, phase-3 study. Inflamm Bowel Dis. 2015;21(4):783–92. https://doi.org/10.1097/mib.0000000000000327.
P.L. Bonate, The art of modeling. In: Pharmacokinetic-pharmacodynamic modeling and simulation. 2nd ed. New York: Springer; 2011. p. 24.
Lovell DJ, et al. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis, (in English). N Engl J Med. 2008;359(8):810–20. https://doi.org/10.1056/NEJMoa0706290.
Temrikar ZH, Suryawanshi S, Meibohm B. Pharmacokinetics and clinical pharmacology of monoclonal antibodies in pediatric patients, (in English). Paediatr Drugs. 2020;22(2):199–216. [Online]. Available: <Go to ISI>://MEDLINE:32052309.
Holford NH. A size standard for pharmacokinetics. Clin Pharmacokinet. 1996;30(5):329–32. https://doi.org/10.2165/00003088-199630050-00001.
Brandse JF, et al. Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis, (in English). Gastroenterology. 2015;149(2):350–5.e2. [Online]. Available: <Go to ISI>://MEDLINE:25917786.
Atiqi S, Hooijberg F, Loeff FC, Rispens T, Wolbink GJ. Immunogenicity of TNF-inhibitors. Front Immunol. 2020;11:312. https://doi.org/10.3389/fimmu.2020.00312.
Jani M, Barton A, Warren RB, Griffiths CE, Chinoy H. The role of DMARDs in reducing the immunogenicity of TNF inhibitors in chronic inflammatory diseases. Rheumatology (Oxf). 2014;53(2):213–22. https://doi.org/10.1093/rheumatology/ket260.
Emi Aikawa N, de Carvalho JF, Artur Almeida Silva C, Bonfá E. Immunogenicity of anti-TNF-α agents in autoimmune diseases. Clin Rev Allergy Immunol. 2010;38(2):82–9. https://doi.org/10.1007/s12016-009-8140-3.
Berends SE, Strik AS, Van Selm JC, Löwenberg M, Ponsioen CY, DʼHaens GR, Mathôt RA. Explaining interpatient variability in adalimumab pharmacokinetics in patients with Crohn’s disease. Ther Drug Monit. 2018;40(2):202–11. https://doi.org/10.1097/ftd.0000000000000494.
Schiff MH, Simon LS, Dave KJ, Jaffe J, Freundlich B. Self-administered methotrexate using a medi-jet auto-injector improves bioavailability compared with oral methotrexate in adults with rheumatoid arthritis, (in English). Ann Rheum Dis. 2013;72:249–250. [Online]. Available: <Go to ISI>://WOS:000331587902168.
Burmester GR, et al. Efficacy and safety of ascending methotrexate dose in combination with adalimumab: the randomised CONCERTO trial, (in English). Ann Rheum Dis. 2015;74(6):1037–44. https://doi.org/10.1136/annrheumdis-2013-204769.
Nader A, Beck D, Noertersheuser P, Williams D, Mostafa N. Population pharmacokinetics and immunogenicity of adalimumab in adult patients with moderate-to-severe hidradenitis suppurativa. Clin Pharmacokinet. 2017;56(9):1091–102. https://doi.org/10.1007/s40262-016-0502-4.
Berkhout LC, et al. Dynamics of circulating TNF during adalimumab treatment using a drug-tolerant TNF assay. Sci Transl Med. 2019. https://doi.org/10.1126/scitranslmed.aat3356.
Berkhout LC, et al. Formation and clearance of TNF-TNF inhibitor complexes during TNF inhibitor treatment. Br J Pharmacol. 2023. https://doi.org/10.1111/bph.16269.
Alberdi-Saugstrup M, et al. High-sensitive CRP as a predictive marker of long-term outcome in juvenile idiopathic arthritis, (in English). Rheumatol Int. 2017;37(5):695–703. https://doi.org/10.1007/s00296-017-3657-x.
Correll CK, Bullock DR, Cafferty RM, Vehe RK. Safety of weekly adalimumab in the treatment of juvenile idiopathic arthritis and pediatric chronic uveitis. Clin Rheumatol. 2018;37(2):549–53. https://doi.org/10.1007/s10067-017-3890-4.
Sejournet L, Kerever S, Mathis T, Kodjikian L, Jamilloux Y, Seve P. Therapeutic drug monitoring guides the management of patients with chronic non-infectious uveitis treated with adalimumab: a retrospective study. Br J Ophthalmol. 2022;106(10):1380–6. https://doi.org/10.1136/bjophthalmol-2021-319072.
Imagawa T, et al. Efficacy, pharmacokinetics, and safety of adalimumab in pediatric patients with juvenile idiopathic arthritis in Japan. Clin Rheumatol. 2012;31(12):1713–21. https://doi.org/10.1007/s10067-012-2082-5.
Kingsbury DJ, Bader-Meunier B, Patel G, Arora V, Kalabic J, Kupper H. Safety, effectiveness, and pharmacokinetics of adalimumab in children with polyarticular juvenile idiopathic arthritis aged 2 to 4 years. Clin Rheumatol. 2014;33(10):1433–41. https://doi.org/10.1007/s10067-014-2498-1.
Acknowledgements
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this article.
Conflict of Interest
The authors declare no conflicts of interests.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Code
Aggregated data and code for reproducing the results of this analysis can be shared upon reasonable request.
Authors’ Contributions
All authors contributed significantly to the conception and design of the study, data analyses, provided critical revisions for important intellectual content, approved the final version for publication, and agreed to be accountable for all aspects of the work to ensure that any questions related to its accuracy or integrity are appropriately addressed and resolved.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Nassar-Sheikh Rashid, A., Hooijberg, F., Bergkamp, S.C. et al. Population Pharmacokinetics of Adalimumab in Juvenile Idiopathic Arthritis Patients: A Retrospective Cohort Study Using Clinical Care Data. Pediatr Drugs 26, 441–450 (2024). https://doi.org/10.1007/s40272-024-00629-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-024-00629-7