Abstract
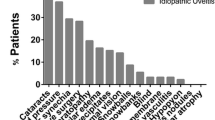
Weekly adalimumab dosing is used to treat juvenile idiopathic arthritis (JIA), uveitis, and other pediatric rheumatic diseases, but the safety of such dosing has not previously been studied. A retrospective chart review was conducted to assess the safety of weekly adalimumab. Demographic and clinical data were collected. Basic descriptive analysis was performed to assess for adverse events from weekly adalimumab. Sixty-nine patients at the University of Minnesota or Gillette Children’s Hospital were identified as treated with weekly adalimumab. Sixty (87%) were eligible for the chart review. Weekly adalimumab was used most commonly to treat uveitis (28%, 17/60) and rheumatoid factor-negative polyarticular JIA (25%, 15/60). Mean age at the start of weekly dosing was 13.9 years. The majority of patients were concurrently treated with a non-steroidal anti-inflammatory drug and methotrexate. Fifty-three (90%) patients continued weekly dosing for greater than 3 months. The mean duration of weekly adalimumab was 2 years. Throughout the duration of weekly dosing, 24/60 (40%) patients had documented minor infections not requiring antimicrobials and 24/60 (40%) had documented infections requiring antimicrobial treatment. Only three patients (5%) had an infection requiring hospitalization. Two patients (3%) developed autoimmune disease. Laboratory abnormalities and injection site reactions were rare. Weekly adalimumab was used most commonly to treat uveitis and rheumatoid factor-negative polyarticular JIA, and mean duration of weekly dosing was 2 years. Serious adverse events were rare.
Similar content being viewed by others
References
Humira Package Insert (2016). http://www.rxabbvie.com/pdf/humira.pdf
Lovell DJ, Ruperto N, Goodman S et al (2008) Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med 359:810–820. https://doi.org/10.1056/NEJMoa0706290
Beukelman T, Ringold S, Davis TE et al (2012) Disease-modifying antirheumatic drug use in the treatment of juvenile idiopathic arthritis: a cross-sectional analysis of the CARRA Registry. J Rheumatol 39:1867–1874. https://doi.org/10.3899/jrheum.120110
Burgos-Vargas R, Tse SM, Horneff G et al (2015) A randomized, double-blind, placebo-controlled multicenter study of adalimumab in pediatric patients with enthesitis-related arthritis. Arthritis Care Res (Hoboken) 67:1503–1512. https://doi.org/10.1002/acr.22657
Zannin ME, Birolo C, Gerloni VM et al (2013) Safety and efficacy of infliximab and adalimumab for refractory uveitis in juvenile idiopathic arthritis: 1-year followup data from the Italian Registry. J Rheumatol 40:74–79. https://doi.org/10.3899/jrheum.120583
van de Putte LB, Atkins C, Malaise M et al (2004) Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rheum Dis 63:508–516. https://doi.org/10.1136/ard.2003.013052
Sandborn WJ, Hanauer SB, Rutgeerts P et al (2007) Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut 56:1232–1239
Kimball AB, Kerdel F, Adams D et al (2012) Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med 157:846–855. https://doi.org/10.7326/0003-4819-157-12-201212180-00004
Leonardi C, Sobell JM, Crowley JJ et al (2012) Efficacy, safety and medication cost implications of adalimumab 40 mg weekly dosing in patients with psoriasis with suboptimal response to 40 mg every other week dosing: results from an open-label study. Br J Dermatol 167:658–667. https://doi.org/10.1111/j.1365-2133.2012.11041.x
Wolf D, D’Haens G, Sandborn WJ et al (2014) Escalation to weekly dosing recaptures response in adalimumab-treated patients with moderately to severely active ulcerative colitis. Aliment Pharmacol Ther 40:486–497. https://doi.org/10.1111/apt.12863
Dubinsky MC, Rosh J, Faubion WA Jr et al (2016) Efficacy and safety of escalation of adalimumab therapy to weekly dosing in pediatric patients with Crohn’s disease. Inflamm Bowel Dis 22:886–893. https://doi.org/10.1097/MIB.0000000000000715
Aljebab F, Choonara I, Conroy S (2017) Systematic review of the toxicity of long-course oral corticosteroids in children. PLoS One 12:e0170259. https://doi.org/10.1371/journal.pone.0170259
Ghiti Moghadam M, Vonkeman HE, ten Klooster PM et al (2016) Stopping tumor necrosis factor inhibitor treatment in patients with established rheumatoid arthritis in remission or with stable low disease activity: a pragmatic multicenter, open-label randomized controlled trial. Arthritis Rheumatol 68:1810–1817. https://doi.org/10.1002/art.39626
A LOOK AT TARGETED IMMUNE MODULATORS. WWW.ICER-REVIEW.ORG 1. Accessed 9/26/2017
Ungar WJ, Costa V, Hancock-Howard R et al (2011) Cost-effectiveness of biologics in polyarticular-course juvenile idiopathic arthritis patients unresponsive to disease-modifying antirheumatic drugs. Arthritis Care Res (Hoboken) 63:111–119. https://doi.org/10.1002/acr.20337
Ramos-Casals M, Brito-Zerón P, Soto MJ et al (2008) Autoimmune diseases induced by TNF-targeted therapies. Best Pract Res Clin Rheumatol 22:847–861. https://doi.org/10.1016/j.berh.2008.09.008
Zhu TH, Nakamura M, Abrouk M et al (2016) Demyelinating disorders secondary to TNF-inhibitor therapy for the treatment of psoriasis: a review. J Dermatolog Treat 27:406–413. https://doi.org/10.3109/09546634.2015.1136385
Mannion ML, Beukelman T (2013) What is the background incidence of malignancy in children with rheumatic disease? Curr Rheumatol Rep 15:310–312. https://doi.org/10.1007/s11926-012-0310-2
Nash P, Vanhoof J, Hall S et al (2016) Randomized crossover comparison of injection site pain with 40 mg/0.4 or 0.8 mL formulations of adalimumab in patients with rheumatoid arthritis. Rheumatol Ther 3:257–270. https://doi.org/10.1007/s40744-016-0041-3
Observational study of pediatric rheumatic diseases: the CARRA Registry. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02418442?term=carra+registry&rank=2. Accessed 1 Feb 2017
Acknowledgements
The authors would like to thank Betty Bishop, Registered Nurse, for proposing this research idea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All human studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. This study was approved by the University of Minnesota Institutional Review Board (IRB Code Number: 1511M80622).
Disclosures
None.
Rights and permissions
About this article
Cite this article
Correll, C.K., Bullock, D.R., Cafferty, R.M. et al. Safety of weekly adalimumab in the treatment of juvenile idiopathic arthritis and pediatric chronic uveitis. Clin Rheumatol 37, 549–553 (2018). https://doi.org/10.1007/s10067-017-3890-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-017-3890-4