Abstract
Background
Sub-optimally controlled diabetes increases risks for adverse and costly complications. Self-management including glucose monitoring, medication adherence, and exercise are key for optimal glycemic control, yet, poor self-management remains common.
Objective
The main objective of the Trial to Incentivize Adherence for Diabetes (TRIAD) study was to determine the effectiveness of financial incentives in improving glycemic control among type 2 diabetes patients in Singapore, and to test whether process-based incentives tied to glucose monitoring, medication adherence, and physical activity are more effective than outcome-based incentives tied to achieving normal glucose readings.
Methods
TRIAD is a randomized, controlled, multi-center superiority trial. A total of 240 participants who had at least one recent glycated hemoglobin (HbA1c) being 8.0% or more and on oral diabetes medication were recruited from two polyclinics. They were block-randomized (blocking factor: current vs. new glucometer users) into the usual care plus (UC +) arm, process-based incentive arm, and outcome-based incentive arm in a 2:3:3 ratio. The primary outcome was the mean change in HbA1c at month 6 and was linearly regressed on binary variables indicating the intervention arms, baseline HbA1c levels, a binary variable indicating titration change, and other baseline characteristics.
Results
Our findings show that the combined incentive arms trended toward better HbA1c than UC + , but the difference is estimated with great uncertainty (difference − 0.31; 95% confidence interval [CI] − 0.67 to 0.06). Lending credibility to this result, the proportion of participants who reduced their HbA1c is higher in the combined incentive arms relative to UC + (0.18; 95% CI 0.04, 0.31). We found a small improvement in process- relative to outcome-based incentives, but this was again estimated with great uncertainty (difference − 0.05; 95% CI − 0.42 to 0.31). Consistent with this improvement, process-based incentives were more effective at improving weekly medication adherent days (0.64; 95% CI − 0.04 to 1.32), weekly physically active days (1.37; 95% CI 0.60–2.13), and quality of life (0.04; 95% CI 0.0–0.07) than outcome-based incentives.
Conclusion
This study suggests that both incentive types may be part of a successful self-management strategy. Process-based incentives can improve adherence to intermediary outcomes, while outcome-based incentives focus on glycemic control and are simpler to administer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A lack of diabetes self-management practices remains common. |
This study highlights the challenges in achieving improvements in glycemic control and provides only limited evidence that incentives may be part of a successful multicomponent intervention to improve glycemic control and self-management. |
More research is required to determine the strategy, size, and type of incentives that may cost-effectively improve self-monitoring and glycemic control among those with type 2 diabetes. |
1 Introduction
Sub-optimally controlled diabetes increases risks for a host of adverse and costly complications, including heart attacks, strokes, kidney failure, lower limb amputation(s), blindness, and nerve damage [1]. There is a strong consensus that optimal glycemic control is essential to minimize risks for these complications [2]. Many studies have shown that careful self-management, including regular glucose monitoring, medication adherence, regular exercise, and weight control, supports optimal glycemic control [3,4,5,6], resulting in improved health outcomes and lower healthcare utilization and costs, including a reduction in emergency department visits and hospitalizations [7, 8]. Yet, poor self-management remains common [9, 10].
From a behavioral economics perspective, poor self-management is thought to partly arise from people’s bias towards the present. Indeed, the cost of self-management, primarily in terms of time and effort, is immediate and certain, whereas the health benefits are distant and uncertain [11, 12]. One strategy to address present bias and improve short-term glycemic control and long-term health outcomes is to provide people with a near-term benefit, such as a financial incentive. Financial incentives, implemented either alone or as part of multi-component interventions, have been used in many health-related interventions in efforts to overcome present bias [13,14,15,16,17,18,19,20,21,22,23]. However, the literature provides little guidance on what objective one should tie the incentives to. In this study, we explored whether it is more effective to tie the incentives to intermediary processes supporting the desired outcome, or whether it is more effective to incentivize the outcome directly.
Process-based incentives focus on motivating near-term behaviors associated with optimal glycemic control, such as self-monitoring of blood glucose, with the underlying idea that incentivizing these behaviors can overcome people’s present bias [24, 25]. However, the challenge with this approach is that individuals could engage in only a subset of behaviors such that overall glycemic control is not effectively improved [26]. An alternative approach is to focus on near-term health outcomes, such as having glucose readings within the acceptable range. This approach also addresses present bias, while providing flexibility on how to attain the objective. Tying incentives to health outcomes is expected to be a more cost-effective strategy than tying it to self-management processes, as incentives are only paid out upon success. However, outcome-based incentives might be less effective than process-based incentives if many people find the specified goals too challenging to make an effort to reach them and/or do not see enough of a direct link between changes in behavior and changes in the target of the incentives (i.e., overall glycemic control) [27].
The aim of the Trial to Incentivize Adherence for Diabetes (TRIAD) study was to test these competing approaches to incentives via a three-arm randomized controlled trial. The first arm, usual care plus (UC +), merely added monitoring devices to diabetes usual care (glucometer, medication tracker, and pedometer) to create a control group. The second arm applied a process-based strategy that tied incentives to regular glucose monitoring, medication adherence, and physical activity. The third arm was outcome based and tied incentives solely to having self-monitored blood glucose readings in the acceptable range. We hypothesized that providing either form of incentive would be more effective than UC + in improving glycemic control. We did not hypothesize which incentive strategy would have the largest effect on glycemic control, as competing arguments can be made supporting each strategy.
2 Methods
2.1 Study Design
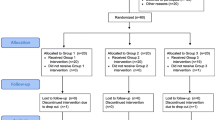
A detailed description of the TRIAD study protocol has been published previously [28]. In brief, 240 participants were block-randomized (blocking factor: current vs. new glucometer users) into the UC + , process-based incentives, and outcome-based incentives arms in a 2:3:3 ratio. Our sample size was calculated to detect an effect size of 1.0% in glycated hemoglobin (HbA1c) at a 5% significance level. The study was registered on ClinicalTrials.gov (ID: NCT02224417). Written informed consent was obtained from all participants.
2.2 Participants
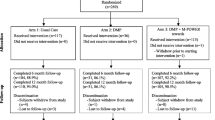
Participants were recruited from two polyclinics in Geylang and Bedok estates in Singapore between February 2015 and December 2017. The participant timeline is described in detail in Fig. 1. Recruited participants had sub-optimally controlled diabetes—defined as having at least one HbA1c within the last 6 months of 8.0% or more. Participants were on at least one oral diabetes medication (had been for at least 3 months), were aged 21–70 years, were Singapore citizens or permanent residents, and were able to converse in English or Mandarin. Patients who were on insulin, were unable to take their oral medication independently, were pregnant, or had a physical condition that could be made worse with exercise were excluded from the study.
2.3 Randomization
Prior to recruitment, randomization numbers were generated by the principal investigator and the project coordinator (PC) using Stata 13.2. Participants were block-randomized into one of the three study arms in a ratio of 2:3:3 based on whether they were current glucometer users or not. The PC and a witness external to the study enclosed the assignments in sequentially numbered, opaque, sealed randomization envelopes, which were handed over to the Clinical Research Coordinators (CRCs) at the polyclinics. Upon informed consent, the CRCs drew the next sequential envelope based on the participant’s glucometer use status. The allocation was then revealed to both the CRC and participant. The study arm assignment was not revealed to the laboratory staff assessing the primary outcome (i.e., HbA1c).
2.4 Intervention
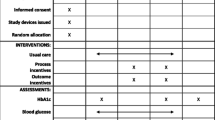
All participants received usual care, as per the SingHealth Polyclinics structured framework for diabetes patients, including information on the recommended guidelines on blood glucose monitoring, acceptable blood glucose range, medication adherence, and physical activity (see Fig. 2).
To tease out the independent effect of incentives, each participant, regardless of which arm they were assigned, received a TRUEresult™ glucometer to measure their blood glucose if they did not already have one, an eCAP™ to track their medication adherence, and a Fitbit Zip™ to monitor their physical activity. Participants also received regular arm-specific text messages (7, 28 and 56 days after the baseline visit and 28 and 56 days after the month 3 visit) to remind them to exercise regularly, take their medications as prescribed, and monitor their blood glucose regularly (see Fig. 3). Because of these additions to usual care, we called the control arm UC + .
The process-based incentive participants earned financial incentives contingent on meeting specified intermediary health behaviors: 3.50 Singapore dollars (S$) (S$1 = US$0.74, as at 11 November 2020) weekly if they measured their blood glucose on three non-consecutive days each week, S$0.50 daily if they took their medication as prescribed, and S$1 daily if they completed 8000 steps as recorded by the Fitbit Zip™. The outcome-based incentive participants earned S$2 weekly if they had one pre-meal glucose reading within the acceptable range of 4–7 mmol/L (72–126 mg/dL), S$7 if they had two pre-meal glucose readings within the acceptable range, or S$14 if they had three pre-meal glucose readings within the acceptable range in a week. The maximum amount of incentives that participants in the process and outcome arms could earn per week was S$14 (US$10.36). This is a relatively small amount but more than enough to offset the additional cost of buying test strips and lancets for three glucose measurements per week, which ranges from S$2.25 to S$5.52 (prices as of 11 November 2020) depending on the glucometer brand and model. Participants in the control arm did not receive any financial incentive but received a S$75 non-contingent payment at the end of month 6 for participating in the study. Incentives were paid out in the form of supermarket vouchers at month 3 and month 6. The intervention lasted for 6 months, from March 2015 to June 2018. Although a longer post-incentive follow-up period was originally planned, due to the enactment of a new regulation on human biomedical research, only a 6-month evaluation was possible.
2.5 Data Collection
Participants were assessed at baseline, month 3, and month 6. At each assessment, information from their pedometer, medication tracker and glucometer were uploaded to the study website and participants’ HbA1c was recorded. Paper-based survey questionnaires were also administered at baseline and month 6. The baseline questionnaire assessed the socio-demographic characteristics of the participants. Both baseline and month 6 questionnaires included the European Quality of Life–5 Dimensions–5 Levels (EQ-5D-5L), Brief Illness Perception Questionnaire (BIPQ), Self-Monitoring of Blood Glucose (SMBG), and Beliefs about Medication Questionnaire (BMQ).
2.6 Outcomes
The primary outcome was the mean change in HbA1c at month 6 from baseline. Secondary outcomes included mean number of glucose readings within the acceptable range (4–7 mmol/L), mean number of glucose readings (i.e., with a minimum of three non-consecutive testing days in a week as per SingHealth Polyclinics recommendation), mean number of medication adherent days (i.e., taking all the medication doses in a day within the pre-defined time windows, as verified by the eCAP™ medication tracker), and mean number of physically active days (i.e., logging at least 8000 steps per day on the Fitbit Zip™) on the last week of intervention.
Exploratory outcomes included the proportion of participants whose oral medication was titrated up and/or who switched to another medication, including insulin, during the intervention, and mean changes from baseline in EQ-5D-5L, BIPQ, SMBG, and BMQ scores.
2.7 Statistical Analysis
All analyses were carried out as pre-specified in the study protocol and followed an intention-to-treat approach. As such, all missing data in both independent and dependent variables were filled using Markov chain Monte Carlo multiple imputation.
2.7.1 Primary Analyses
We first tested the effectiveness of financial incentives, either process or outcome based, in reducing HbA1c levels. Change in HbA1c readings from baseline to month 6 was linearly regressed on a binary variable indicating participation in the incentive arms, with UC as the reference category, baseline HbA1c readings, the interval (in days) between baseline and month 6 HbA1c test date, and baseline characteristics, including gender, age, education level, employment status, income, EQ-5D-5L, BIPQ, SMBG, and BMQ scores, and number of comorbidities. The analysis was performed using an intention-to-treat approach.
To measure the difference in effectiveness between incentive strategies, we used the same model as above but restricted the sample to the participants in the incentive arms and changed the reference category of the binary variable to the process-incentive arm.
As sensitivity analyses, we ran a regression analysis carrying forward the last measured HbA1c level (baseline or month 3), and a regression with no imputation of the missing HbA1c observations.
2.7.2 Secondary Analyses
Using the same approach as for the primary analyses, we tested the effectiveness of financial incentives in improving treatment adherence as measured by the mean number of glucose readings within the acceptable range, mean number of glucose readings, mean number of medication-adherent days, and mean number of physically active days on the last week of the intervention. These were separately regressed against the same set of independent variables used in the primary analyses.
2.7.3 Exploratory Analyses
To test whether the intervention encouraged changes in medication adherence, we estimated separate logistic regressions of binary variables indicating whether the participants had a titration/change of their oral medicine or whether they switched to insulin therapy during the intervention. We also ran separate linear regressions on the changes from baseline in EQ-5D-5L, BIPQ, SMBG, and BMQ scores at month 6. For all models, the covariates were the binary variables indicating the incentive arms and the same baseline characteristics used in the primary analyses.
We also analyzed the effect of potential moderators, including gender, age, language spoken, education, occupation, income, EQ-5D-5L, BIPQ, SMBG, and BMQ scores, and number of comorbidities on the change in HbA1c at month 6. To do so, the model used in the primary analysis was extended by adding interaction terms (in separate regressions) between each potential moderator and the binary variables indicating study arms.
3 Results
A total of 1387 patients were assessed for eligibility (Fig. 4). Eight hundred and eighty-four (63.7%) were found eligible to participate. Among the eligible, 240 (27%) responded to the invitation to participate and were consented and randomized into the three arms, 61 into UC + , 87 into the process-based incentive arm, and 92 into the outcome-based incentive arm. Twenty-eight participants (11.7%) withdrew from the study before the month 6 assessment, while 14 (5.8%) were discontinued due to insulin initiation. The proportion of participants who were lost to follow-up, either due to insulin initiation or other reasons, is not statistically different across study arms (p value 0.71). A summary of missing observations is reported in Fig. 5.
Baseline participant characteristics are shown in Table 1. On average, participants were 55 years old, had two children, and had a baseline HbA1c of 9.1%. Only 19% reported that their health was excellent or very good. Thirty-four percent reported that they were self-monitoring their blood glucose. There were no observed statistical differences in socio-demographic characteristics between the incentive and control arms.
Table 2 shows the estimated incremental effects of incentives, either outcome or process based. The mean difference is in the hypothesized direction, a greater reduction in HbA1c (difference − 0.31; 95% confidence interval [CI] − 0.67 to 0.06) compared to UC + , but the difference is estimated with great uncertainty (p value 0.11). Consistent with incentives having an effect, the proportion of participants who had any improvement in HbA1c is higher in the incentive arms by 18% (95% CI 4–31) than in UC + . Incentive arm participants, on average, also had 0.32 more glucose readings within the acceptable range (out of three tests per week) (95% CI 0.07–0.57), had 0.40 more glucose readings (out of three tests per week) (95% CI 0.04–0.76), were more adherent to their medication by 0.72 days (95% CI 0.05–1.38), and were more physically active by 1.12 days (95% CI 0.38–1.86). Participants in the incentive arms also had higher treatment control scores by 0.62 (95% CI − 0.02 to 1.26) compared to control group participants, but this result was also estimated with great uncertainty. Other outcome and process measures showed no consistent trends and were associated with large CIs.
The incremental effects of outcome- compared to process-based incentives are reported in Table 3. The difference in HbA1c between the process and outcome arms was small and estimated with great uncertainty (difference − 0.05; 95% CI − 0.42 to 0.31). This conclusion holds for the secondary outcomes considered: the proportion of participants who had any improvement in HbA1c (difference − 0.02; 95% CI − 0.14 to 0.11), the mean number of glucose readings within the acceptable range (out of three tests per week) (difference 0.07; 95% CI − 0.21 to 0.34), and the mean number of glucose readings (out of three tests per week) (difference − 0.06; 95% CI − 0.45 to 0.32). The mean incentive payout on the last week of the intervention was also similar between the two arms (process = S$5.43 [95% CI 4.22–6.62] vs. outcome = S$6.21 [95% CI 4.47–7.95]).
Lending some support to the hypothesis that process-based incentives improve intermediate outcomes, outcome-arm participants had fewer medication adherent days (difference − 0.64; 95% CI − 1.32 to 0.04) and were less physically active during the last week of the intervention (difference − 1.37 days; 95% CI − 2.13 to − 0.60), although these results were again estimated with great uncertainty. Further, self-reported EQ-5D-5L scores worsened among outcome-arm participants compared to process-arm participants (difference − 0.04; 95% CI − 0.07 to − 0.004). There were no clear differences between groups in the treatment control domain (“How much do you think your treatment can help your diabetes?”) or in the other exploratory outcomes considered.
We tested the robustness of our primary and secondary outcome estimates using [1] regressions not controlling for confounders, [2] last observation carried forward and [3] using a per protocol approach. Results from these estimation methods were similar to the reported estimates in Tables 2 and 3. Lastly, we tested for a range of moderators for the incentive effect and found no clear signs of effect for any of those tested, although this may be due to a lack of statistical power for testing such hypotheses (please refer to Supplementary Information Tables 1.1–1.3 and 2.1–2.3 in the supplementary electronic material).
4 Discussion
The first objective of this study was to quantify the effect of financial incentives on glycemic control among polyclinic patients with sub-optimally controlled diabetes. We found that financial incentives reduced HbA1c by 0.31 compared to a control group receiving usual care and monitoring devices. Even though this result was associated with a fairly large p value (p value 0.11), there are several reasons to suggest that incentives had an effect. First, we calculated an additional outcome for the proportion of participants who improved their baseline glycemic control at month 6 and found that 18% more participants improved their HbA1c in the incentive groups (p value 0.013). Moreover, we also found evidence consistent with the effectiveness of incentives for the number of glucose readings within the acceptable range (0.32), number of glucose readings (0.40), weekly medication adherent days (0.72), and weekly physically active days (1.12).
Our results are consistent with other studies that aimed to achieve a reduction in HbA1c through financial incentives. These studies showed significant behavior changes, i.e., improvements in medication adherence, regular physical activity, and/or glucose monitoring but did not show clinically significant improvements in glycemic control [18, 29,30,31], or had clinically significant improvements that were not statistically significant based on conventional p values [32, 33]. Overall, evidence suggests that financial incentives alone have a small to moderate effect on glucose control and, consequently, that a larger sample size than that used in our study is needed to confirm this effect.
A notable finding is that only 34% of the participants in our sample engaged in self-monitoring of blood glucose at baseline. The low rate of monitoring and lack of regular monitoring may be due to patients’ negative perception and attitude towards glucose self-monitoring. The discomfort associated with testing and potential frustration with high glucose readings could be some of the barriers to regular self-monitoring [34]. Cost could also be an additional barrier as the approximate cost of glucose monitoring three times a week incurred by a patient ranges from S$2.25 to S$5.52 (prices as of 11 November 2020) depending on the glucometer brand and model. Despite the low level of glucose monitoring at baseline, financial incentives were effective in increasing glucose monitoring. However, the glucose monitoring incentive may have inadvertently encouraged process-arm participants to overemphasize testing in lieu of behavior change. Several authors have noted that it is not uncommon for people who are testing their blood glucose to be proficient at and feel confident in their ability to perform tests, but to not use the results to influence their diet and exercise choices [35,36,37,38].
Our results do not show any significant difference in effectiveness between process- and outcome-based incentives on glycemic control. However, process-based incentives were statistically significantly more effective at improving medication adherence and physical activity at the 10% and 5% level, respectively, behaviors which are necessary for good glycemic control. Furthermore, process-based incentives participants reported an increase in their quality of life compared to UC + and outcome-based incentives participants. This increase in perceived well-being could possibly be due to the reinforcing effect of incentives on healthy self-management behaviors such as increased physical activity [39]. While there was no difference in incentive payouts between incentive groups, process-group incentives took more time to administer in the context of this trial and required more monitoring devices.
Although our goal was to tease out the independent effectiveness of process versus outcome incentives, it is likely that neither strategy is sufficient as a stand-alone intervention. Our study coupled the incentives with technology (i.e., monitoring devices), reminders, and other program features. However, since those in the control group received these intervention components as an addition to usual care, we cannot state whether the combined intervention would have been more effective relative to usual care alone. This should be an area of future research. Future studies could also test incentivizing both processes and outcomes to see whether or not this approach is more effective than incentivizing just one or the other [18, 19, 40].
Our study has many strengths, including the rigorous trial design, but also several weaknesses. Our statistically insignificant HbA1c results may be due to the lack of power given by our sample size calculation. Our results may have been stronger if we recruited participants who had higher HbA1c values at baseline, as they have more ability to improve. However, that would have limited our ability to recruit the required number of participants in the time available. Another challenge was in using blood glucose as an outcome to be incentivized. The advantage of blood glucose, over HbA1c, is that it is modifiable and measurable in the near-term. However, intraday fluctuations in glucose readings suggest that an incentive scheme integrating blood glucose outcomes with HbA1c outcomes may better translate into long-term glycemic control. Our results are also likely driven by the size and type of incentives offered [20, 41]. This is a problem with all incentive-based studies. It is also important to consider in interpreting our results that our study participation rate is low. Only 27% of the eligible participants joined the study, thus our results may not be generalizable. Finally, we deviated from the protocol by not analyzing the changes from baseline in HbA1c at month 12 and month 18 due to recent changes in the regulations on human biomedical research in Singapore that made it infeasible to collect information beyond the month 6 assessment. A longer duration would have enabled us to observe the intervention’s full effect on HbA1c [42, 43].
5 Conclusion
Our results highlight the challenges in achieving improvements in glycemic control, yet provide suggestive evidence that incentives may be part of a successful multicomponent intervention strategy [18, 20]. While there was no significant difference in effectiveness between process- and outcome-based incentives, process-based incentives improved medication adherence, physical activity level, and quality of life. On the other hand, process-based incentives are potentially more costly to administer due to the number of devices they require. More research is needed to determine the most cost-effective strategies for implementing incentives for management of diabetes and other chronic diseases.
References
WHO. Global Report on Diabetes. World Health Organisation. 2016.
ADA. American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes. Diabetes Care. 2004;27(2):596.
Thomas D, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006(3).
Cohen ND, Dunstan DW, Robinson C, Vulikh E, Zimmet PZ, Shaw JE. Improved endothelial function following a 14-month resistance exercise training program in adults with type 2 diabetes. Diabetes Res Clin Pract. 2008;79(3):405–11.
Umpierre D, Ribeiro PAB, Kramer CK, Leitão CB, Zucatti ATN, Azevedo MJ, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes a systematic review and meta-analysis. J Am Med Assoc. 2011;305(17):1790–9.
Sjöström CD, Lissner L, Wedel H, Sjöström L. Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res. 2012;7(5):477–84.
Encinosa WE, Bernard D, Dor A. Does prescription drug adherence reduce hospitalizations and costs? The case of diabetes. Adv Health Econ Health Serv Res. 2010;22:151–73.
Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521.
Xu Y, Lim JH, Henry R, Lee Y-cJ. The impact of provision of self-monitoring of blood glucose supplies on self-care activities among patients with uncontrolled Type 2 diabetes mellitus: a prospective study. Diabetes Res Clin Pract. 2019;157:107873.
Zhang X, Foo S, Majid S, Chang Y-K, Dumaual HTJ, Suri VR. Self-care and health-information-seeking behaviours of diabetic patients in Singapore. Health Commun. 2019:1–10.
Chapman GB, Brewer NT, Coups EJ, Brownlee S, Leventhal H, Levanthal EA. Value for the future and preventive health behavior. J Exp Psychol Appl. 2001;7(3):235.
Elliott RA, Shinogle JA, Peele P, Bhosle M, Hughes DA. Understanding medication compliance and persistence from an economics perspective. Value Health. 2008;11(4):600–10.
DeFulio A, Silverman K. The use of incentives to reinforce medication adherence. Prevent Med. 2012.
Giuffrida A, Torgerson DJ. Should we pay the patient? Review of financial incentives to enhance patient compliance. BMJ. 1997;315:703–7.
Johnston M, Sniehotta F. Financial incentives to change patient behaviour. J Health Serv Res Policy. 2010;15(3):131–2.
Volpp KG, John LK, Troxel AB, Norton L, Fassbender J, Loewenstein G. Financial incentive–based approaches for weight loss. JAMA. 2008;300(22):2631–7.
Bilger M, Wong TT, Lee JY, Howard KL, Bundoc FG, Lamoureux EL, Finkelstein EA. Using adherence-contingent rebates on chronic disease treatment costs to promote medication adherence: results from a randomized controlled trial. Appl Health Econ Health Policy. 2019;17(6):841–55.
Wong CA, Miller VA, Murphy K, Small D, Ford CA, Willi SM, et al. Effect of financial incentives on glucose monitoring adherence and glycemic control among adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA Pediatr. 2017;171(12):1176–83.
Finkelstein EA, Tham KW, Haaland BA, Sahasranaman A. Applying economic incentives to increase effectiveness of an outpatient weight loss program (TRIO)—a randomized controlled trial. Soc Sci Med. 2017;185:63–70.
Finkelstein EA, Bilger M, Baid D. Effectiveness and cost-effectiveness of incentives as a tool for prevention of non-communicable diseases: a systematic review. Soc Sci Med. 2019;232:340–50.
Raiff BR, Jarvis BP, Dallery J. Text-message reminders plus incentives increase adherence to antidiabetic medication in adults with type 2 diabetes. J Appl Behav Anal. 2016;49(4):947–53.
Sen AP, Sewell TB, Riley EB, Stearman B, Bellamy SL, Hu MF, et al. Financial incentives for home-based health monitoring: a randomized controlled trial. J Gen Intern Med. 2014;29(5):770–7.
Misra-Hebert AD, Hu B, Taksler G, Zimmerman R, Rothberg MB. Financial incentives and diabetes disease control in employees: a retrospective cohort analysis. J Gen Intern Med. 2016;31(8):871–7.
Luoto J, Carmen KG. Behavioral economics guidelines with applications for health interventions. Inter-American Development Bank, 2014.
O’Donoghue T, Rabin M. Doing it now or later. Am Econ Rev. 1999;89(1):103–24.
Antonio JP, Sarmento RA, de Almeida JC. Diet quality and glycemic control in patients with type 2 diabetes. J Acad Nutr Diet. 2019;119(4):652–8.
Blondon KS. Patient attitudes about financial incentives for diabetes self-management: a survey. World J Diabetes. 2015;6(5):752–8.
Bilger M, Shah M, Tan NC, Howard KL, Xu HY, Lamoureux EL, et al. Trial to Incentivise Adherence for Diabetes (TRIAD): study protocol for a randomised controlled trial. Trials. 2017;18(1):551.
Kranker K. The efficacy of using financial incentives to change unhealthy behaviors among a rural chronically ill and uninsured population. Am J Health Promot. 2018;32(2):301–11.
VanEpps EM, Troxel AB, Villamil E, Saulsgiver KA, Zhu J, Chin J-Y, et al. Financial incentives for chronic disease management: results and limitations of 2 randomized clinical trials with New York Medicaid patients. Am J Health Promot. 2018;32(7):1537–43.
Fernandes R, Chinn CC, Li D, Frankland TB, Wang CM, Smith MD, et al. A randomized controlled trial of financial incentives for Medicaid beneficiaries with diabetes. Perm J. 2018;22:17–080.
Miranda JJ, Lazo-Porras M, Bernabe-Ortiz A, Pesantes MA, Diez-Canseco F, Cornejo SDP, et al. The effect of individual and mixed rewards on diabetes management: a feasibility randomized controlled trial. Wellcome Open Res. 2018;3:139.
Long JA, Jahnle EC, Richardson DM, Loewenstein G, Volpp KG. Peer mentoring and financial incentives to improve glucose control in African American veterans: a randomized trial. Ann Intern Med. 2012;156(6):416–24.
Chen CM, Hung LC, Chen YL, Yeh MC. Perspectives of patients with non-insulin-treated type 2 diabetes on self-monitoring of blood glucose: a qualitative study. J Clin Nurs. 2018;27(7–8):1673–83.
Grant RW, Huang ES, Wexler DJ, Laiteerapong N, Warton ME, Moffet HH, et al. Patients who self-monitor blood glucose and their unused testing results. Am J Manag Care. 2015;21(2):e119–29.
Parkin CG, Buskirk A, Hinnen DA, Axel-Schweitzer M. Results that matter: structured vs. unstructured self-monitoring of blood glucose in type 2 diabetes. Diabetes Res Clin Pract. 2012;97(1):6–15.
Austin MM. The two skill sets of self-monitoring of blood glucose education: the operational and the interpretive. Diabetes Spectrum. 2013;26(2):83–90.
Song M, Ratcliffe SJ, Tkacs NC, Riegel B. Self-care and health outcomes of diabetes mellitus. Clin Nurs Res. 2012;21(3):309–26.
Meredith SE, Jarvis BP, Raiff BR, Rojewski AM, Kurti A, Cassidy RN, et al. The ABCs of incentive-based treatment in health care: a behavior analytic framework to inform research and practice. Psychol Res Behav Manag. 2014;7:103–14.
VanEpps EM, Troxel AB, Villamil E, Saulsgiver KA, Zhu J, Chin J-Y, et al. Effect of process- and outcome-based financial incentives on weight loss among prediabetic New York Medicaid patients: a randomized clinical trial. Am J Health Promot. 2018;33(3):372–80.
Farooqui MA, Tan Y-T, Bilger M, Finkelstein EA. Effects of financial incentives on motivating physical activity among older adults: results from a discrete choice experiment. BMC Public Health. 2014;14(1):141.
Lorincz IS, Lawson BC, Long JA. Provider and patient directed financial incentives to improve care and outcomes for patients with diabetes. Curr Diab Rep. 2013;13(2):188–95.
Vlaev I, King D, Darzi A, Dolan P. Changing health behaviors using financial incentives: a review from behavioral economics. BMC Public Health. 2019;19(1):1059.
Acknowledgements
The authors would like to thank Kaye L. Howard for helping to design the study protocol, coordinate the study, and collect data, and Hui Yan Xu and Syarafina Hasnan for their recruitment and coordination efforts on site. We would also like to thank Dr. Gabriel Ding for his assistance with the study at Geylang Polyclinic and are grateful to doctors of SHP-Geylang and SHP-Bedok for their referral of patients.
Funding
Open Access funding provided by Vienna University of Economics and Business (WU).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the National University of Singapore (NUS) Initiative to Improve Health in Asia (NIHA-2013-1-005). The open-access fee for the publication of this article is funded by Duke-NUS Medical School.
Conflict of Interest
The authors do not have any conflicts of interest to declare.
Ethics Approval
The study was approved by the SingHealth Centralised Institutional Review Board E (Ref. Numbers 2013/830/E & 2018/2162) and conducted in accordance with the ethical standards of the Declaration of Helsinki.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
All authors have reviewed and approved the manuscript and consent to its publication.
Availability of data and material
Access to de-identified data will be granted to entities with an IRB approval for use that is consistent with Singapore’s data privacy laws and the study research protocol.
Code availability
The code can be made available upon request for non-commercial use.
Trial Registry
The ClinicalTrials.gov Identifier is NCT02224417. The trial was registered 22 August 2014.
Trial Protocol
The protocol is available from https://trialsjournal-biomedcentral-com.libproxy1.nus.edu.sg/articles/10.1186/s13063-017-2288-6.
Authors’ Contribution
MB and EAF conceived and designed the study, conducted the analysis and interpretation of the data, and led the manuscript preparation. MS and NCT contributed to the conception and design of the study and helped oversee recruitment, data acquisition, and implementation on site. CYLT assisted with overseeing recruitment and the implementation of the study on site. FGB contributed to the analysis and interpretation of data and helped draft and finalize the manuscript. JB helped amend the study protocol, coordinate the study, collect data, and draft and finalize the manuscript. All authors reviewed and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bilger, M., Shah, M., Tan, N.C. et al. Process- and Outcome-Based Financial Incentives to Improve Self-Management and Glycemic Control in People with Type 2 Diabetes in Singapore: A Randomized Controlled Trial. Patient 14, 555–567 (2021). https://doi.org/10.1007/s40271-020-00491-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-020-00491-y