Abstract
Background
Poor adherence to medications is a global public health concern with substantial health and cost implications, especially for chronic conditions. In the USA, poor adherence is estimated to cause 125,000 deaths and cost $US100 billion annually. The most successful adherence-promoting strategies that have been identified so far have moderate effect, are relatively costly, and raise availability, feasibility, and/or scalability issues.
Objective
The main objective of SIGMA (Study on Incentives for Glaucoma Medication Adherence) was to measure the effectiveness on medication adherence of a novel incentive strategy based on behavioral economics that we refer to as adherence-contingent rebates. These rebates offered patients a near-term benefit while leveraging loss aversion and regret and increasing the salience of adherence.
Methods
SIGMA is a 6-month randomized, controlled, open-label, single-center superiority trial with two parallel arms. A total of 100 non-adherent glaucoma patients from the Singapore National Eye Centre were randomized into intervention (adherence-contingent rebates) and usual care (no rebates) arms in a 1:1 ratio. The primary outcome was the mean change from baseline in percentage of adherent days at Month 6. The trial registration number is NCT02271269 and a detailed study protocol has been published elsewhere.
Findings
We found that participants who were offered adherence-contingent rebates were adherent to all their medications on 73.1% of the days after 6 months, which is 12.2 percentage points (p = 0.027) higher than in those not receiving the rebates after controlling for baseline differences. This better behavioral outcome was achieved by rebates averaging 8.07 Singapore dollars ($US5.94 as of 2 November 2017) per month during the intervention period.
Conclusion
This study shows that simultaneously leveraging several insights from behavioral economics can significantly improve medication adherence rates. The relatively low cost of the rebates and significant health and cost implications of medication non-adherence suggest that this strategy has the potential to cost-effectively improve health outcomes for many conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Even when treatment costs are reduced by generous public subsidies and/or insurance coverage, many patients are not adherent to their medication regimens and therefore do not receive the full benefits of treatment. |
This study shows that offering rebates on treatment costs to reward patients who are adherent to their medications can significantly increase adherence rates. |
The novel incentive strategy can be deployed in a wide range of clinical settings and has a strong potential to be cost effective as it directs subsidies/reimbursements at those more likely to benefit from their treatment. |
1 Introduction
In 2003, the World Health Organization reported that, in rich countries, only 50% of patients suffering from chronic disease were adherent to their medications [1]. The report concluded that increasing the effectiveness of adherence interventions may have a far greater impact on population health than any improvement in specific medical treatment [1]. Non-adherence to disease management has health and economic considerations. In the USA alone, poor adherence is estimated to cause 125,000 deaths and cost $US100 billion annually, with a considerable percentage coming from chronic diseases [2]. A large number of interventional studies have since then been conducted but adherence rates have not noticeably improved [3,4,5].
A systematic literature review of randomized controlled trials (RCTs) testing interventions to improve medication adherence over a comprehensive range of medical disorders (excluding addictions) concluded that the few interventions that managed to improve both adherence and clinical outcome were complex and involved frequent interaction with patients [6]. However, the review also concluded that these interventions were not very effective despite the effort and resources consumed. A recent review of RCTs testing interventions to improve medication adherence in the US context classified the interventions into the following categories: patient education, medication regimen management, clinical pharmacist consultation, cognitive behavioral therapy, medication-taking reminders, and incentives to promote adherence [5]. While the review found some evidence of effective trials within each strategy, including three of the five trials that tested economic incentives, no clear recommendations emerged. Instead, the authors recommended choosing the intervention(s) based on availability and feasibility within a given practice or health system.
In this study we followed the recommendation from a review by Loewenstein et al. [7] in deploying insights from behavioral economics in efforts to increase the effectiveness of medication adherence in a cost-effective and scalable manner. We operationalized this recommendation by designing an incentive strategy that we refer to as adherence-contingent rebates. The main component of this strategy is a monetary incentive that addresses a key challenge that all chronic disease patients face; they incur adherence costs in the present but reap the benefits far in the future. In such situations, decision makers have been shown to be biased towards the present and make suboptimal choices that their future self will regret [8]. By providing a near-term benefit for adherence, the monetary incentive aims at nudging patients towards greater adherence. Further, individuals have been shown to dislike losses more than they like gains of the same amount—a phenomenon known as loss aversion [9]. For this reason, unlike a price discount or monetary reward, which the prior incentive studies tested, our incentive is designed as a rebate to invoke the loss aversion frame. With rebates, the patient has to pay for the medications upfront only to have the amount refunded if adherence is satisfactory. Our incentive strategy also leverages regret [10] by informing patients who did not meet their adherence goal of the rebate amount they could have earned. Lastly, our strategy promotes the salience of the economic incentive by sending monthly messages reminding patients what is at stake. Leveraging loss aversion, regret, and salience is expected to have a larger impact than if the design solely offered a reward for meeting an adherence goal.
We assessed the effect of our adherence-contingent rebate strategy by means of a RCT conducted among 100 non-adherent glaucoma patients at the Singapore National Eye Centre (SNEC). We focused on glaucoma because it is a condition where lack of adherence can lead to serious vision loss. Prior interventions targeting glaucoma patients and those with vision impairment more generally have largely focused on improving follow-up appointment adherence [11,12,13] and/or improving medication adherence through education and health communication interventions [14,15,16,17,18,19]. Despite these efforts, many patients remain non-adherent [20,21,22].
The main objective of SIGMA (Study on Incentives for Glaucoma Medication Adherence) was to provide evidence on whether adherence-contingent rebates can improve medication adherence among non-adherent glaucoma patients. We hypothesized that medication adherence would be higher among patients who can receive adherence-contingent rebates than in those who cannot. Secondary objectives were to determine whether intraocular pressure (IOP) and quality of life can also be improved, and whether the intervention represents a promising strategy to cost effectively improve glaucoma management. Finally, explanatory analysis aimed at uncovering factors that might moderate the intervention effect and explain medication adherence.
2 Methods
This section summarizes the most important features of the study. The detailed study protocol can be found elsewhere [23].
2.1 Study Design
The study was designed as an open-label, single-site RCT with two parallel arms. A total of 100 non-adherent glaucoma patients were block-randomized into the adherence-contingent rebates and control groups in a 1:1 ratio based on the number of glaucoma medications prescribed (one vs. multiple eye drops). The sample size calculation was based on the ability to detect differences of ten percentage points in average monthly adherence rates assuming a two-sided test, αof 0.05, power of 0.8, and 20% loss to follow-up at Month 6. Recruitment took place between November 2014 and April 2016, and the last follow-up assessment took place in January 2017.
2.2 Participants
All patients were recruited at the SNEC. The trial was registered before the recruitment of participants with the ClinicalTrials.gov registration number NCT02271269. Eligible participants were non-adherent (8-item Morisky Medication Adherence Scale™ [MMAS-8] score ≤ 6 [24,25,26]) glaucoma patients taking at least one eye drop medication aged between 21 and 85 years who were Singaporean citizens or permanent residents and conversant in English or Mandarin. The exclusion criteria included having Stage 4 (severe) or Stage 5 (end-stage) glaucoma according to the glaucoma staging system based on the Humphrey Visual Field [27], and having significant co-morbid conditions preventing application of medication without assistance.
2.3 Intervention
Both the intervention and control groups received usual care, which consists of routine check-ups with an ophthalmologist and counseling sessions with a nurse on effective glaucoma treatment, underlining the health risks raised by non-adherence to the medication regimen. The nurse also determines a dosing schedule for each medication that accommodates the patients’ lifestyle taking into account working hours.
The intervention group was given the opportunity to earn rebates on their treatment costs (glaucoma-related medication and check-up costs) contingent on meeting the target adherence, while the control group was given a non-contingent payment at Months 3 and 6. Rebates were earned based on the percentage of adherent days. Participants achieving a medication adherence ≥ 90% of days received a 50% rebate on their treatment costs, while those who had adherence between 75% and 90% received a 25% rebate.
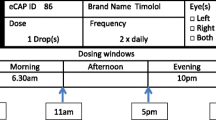
We defined medication adherence as taking all prescribed daily glaucoma medications within their appropriate dosing windows. Type and number of glaucoma medications as well as dosing windows were tailored to the specific needs of each patient. We defined up to three windows per day (morning, afternoon, and evening) of a duration of 6–8 h each [23]. When a patient fails to take any dose on time, the whole day was counted as non-adherent. We chose this rather stringent definition of medication adherence to account for the fact that not only elevated IOP but also variations in IOP are important risk factors of disease progression [28,29,30,31]. For the same reason, we decided to incentivize near-perfect adherence by setting a demanding goal (90% adherence) while setting a less demanding intermediary goal (75% adherence) in our efforts to encourage those with low adherence levels at baseline and who might initially see the higher goal as unattainable. Medication adherence was monitored using the medication tracker eCAP™ (Information Mediary Corporation, Ottawa, ON, Canada), a medication container mounted with an electronic cap that records the time whenever the cap is closed. Each glaucoma eye drop bottle was stored in a separate eCAP™.
Participants learned about their arm allocation by phone the month following their enrolment in order to collect 1 month of baseline data on medication adherence using the medication trackers. At Months 3 and 6, the medication trackers were collected and medication adherence was assessed for Months 2–3 and Months 4–6, respectively. The patient timeline is described in detail in Fig. 1. Adherence reports were sent to the adherence-contingent rebates group in efforts to increase the salience of the payments and generate regret among those participants who were not sufficiently adherent. Similar adherence reports were sent to the control group, with the only difference being that there was no mention of financial incentive. We also sent standardized SMS reminders to all participants at the end of Week 1 and Months 1–5, in addition to making follow-up phone calls. Samples of the adherence reports and SMS reminders sent to the participants and phone call prompts are found in Fig. 2.
2.4 Outcomes
The primary outcome of this study was the mean change from baseline in the percentage of adherent days at the end of the intervention period (Month 6). As secondary outcomes, we measured the monthly proportion of participants meeting the adherence target set for the intervention group to earn rebates, which is ≥ 75% and ≥ 90% of adherent days, respectively. Although power was limited, we also measured changes from baseline in IOP, generic health status EQ-5D-5L [32] score, and Glaucoma Quality of Life-15 (GQL-15) [33] score. We generated the EQ-5D-5L score by mapping the EQ-5D-3L Singapore value set by Luo and Wang [34] using the transition probability matrix by van Hout et al. [35]. Other secondary outcomes were incentive amount earned at Month 6, which corresponds to the adherence level recorded by the primary outcome, and the mean change in the percentage of adherent days from Months 2–5. Explanatory outcomes included alternative measures of medication adherence even though these were not incentivized directly. These included the mean change from baseline in adherent days irrespective of time windows and mean change from baseline in dose adherence both according to and irrespective of the time windows. As control and moderating variables, we also administered the Brief Illness Perception Questionnaire (BIPQ) [36] and the specific subscale of the Beliefs about Medication Questionnaire (BMQ) [37] at baseline and calculated the corresponding scores.
2.5 Statistical Analysis
Mean change from baseline in monthly adherence, IOP, EQ-5D-5L score, and GQL-15 score were all linearly regressed on a binary variable indicating group membership (adherence-contingent rebates vs. control), their baseline level, average number of prescribed doses per day at baseline, change from baseline in the average number of daily doses prescribed, sociodemographic characteristics at baseline (age, language spoken, household monthly income), and IOP, BIPQ, and BMQ scores at baseline. The proportions of participants with a percentage of adherent days above 75% and 90% were regressed on the same variables using a logistic regression. Next, the models described earlier were extended by adding interaction terms between potential intervention moderators (presence of co-morbidities, glaucoma- and non-glaucoma-related medication regimen, potential incentive amount, BIPQ and BMQ variables, and patient sociodemographic characteristics) and the binary variable indicating study arms. Lastly, medication adherence levels at baseline were linearly regressed on potential factors of medication adherence (presence of co-morbidities, glaucoma- and non-glaucoma-related medication regimen, BIPQ and BMQ variables, and patient sociodemographic characteristics). As the perspective of the analysis was that of an intention to treat, all missing data were imputed by a Markov chain Monte Carlo method using all the variables included in the models.
3 Results
The 100 participants were recruited through doctor referral; 64.5% of eligible patients who were referred agreed to take part in the study. Figure 3 shows the CONSORT (Consolidated Standards of Reporting Trials) flow diagram that details the path to enrollment in the study and Fig. 4 provides more information on missing data patterns for the primary outcome. Overall, 92% of participants completed the study and the primary outcome was successfully recorded for 83% of the participants at Month 6.
Missing data patterns for the primary outcome. Note: by the end of Month 6, 538 (89.7%) monthly observations of the primary outcome were collected. Among the participants, 8% had missing observations due to withdrawal and discontinuation from the study and 9% due to a faulty or lost eCAP™ (source: author calculations using the data collected from the study)
Table 1 displays the characteristics of the enrolled participants. The study participants were 64.7 years old on average. The most common participant was male, married, and with a primary education or less. Roughly half of the participants were Mandarin-speaking, while the other half were English-speaking. Of the participants, 43% lived in a household with monthly income less than 2000 Singapore dollars ($S). On average, the MMAS-8 score was 3.80, indicating low adherence, while participants were adherent on 76% of the days during the 1-month baseline assessment, likely due to the initial effect from monitoring adherence and joining the study. In addition, 31.8% of participants did not meet the goal of 75% adherent days and 61.5% did not achieve the goal of 90% of adherent days. At 18.3 mmHg, average IOP was close to the upper bound (20 mmHg) of the normal range for this risk factor and GLQ and EQ 5D-5L scores were 19.3 and 0.88, respectively. The average medication regimen consisted in taking 2.3 doses of eye drops per day. These include the following common medications for glaucoma: β-blockers, adrenergic agonists, parasympathetic agonists, carbonic anhydrase inhibitors, prostaglandin analogs, and hyperosmotic agents [38]. There was no significant difference in baseline characteristics between the two study groups.
Table 2 shows the average outcomes pre- and post-intervention along with the intervention effect at Month 6. It first should be noted that measurement of medication adherence dropped by 14.9 percentage points in the control group between baseline and Month 6. Month 6 medication adherence in the control group was 61.6% after the likely vanishing of the initial effect from joining the study and receiving medication trackers. As is discussed in Sect. 4 with regards to the limitations of the study, we think that this figure represents a reasonable assessment of medication adherence in the absence of rebates in the real world.
Our main finding is that medication adherence at Month 6 was 12.2 (p = 0.027) percentage points higher in the adherence-contingent rebates group than in the control group after adjusting for baseline differences. The proportion of participants with more than 75% and 90% of adherent days was higher in the intervention group by 8.2 (p = 0.372) and 12.5 (p = 0.169) percentage points, respectively, but the sample size was likely too low for these secondary outcomes to achieve statistical significance. Only non-clinically meaningful and statistically insignificant differences in IOP (0.687), GQL score (0.452), and EQ5D-5L (0.001) were observed at Month 6. Adherence-contingent rebates averaged $S8.07 ($US5.94 as of 2 November 2017) among all intervention group participants during Month 6 when the primary outcome was recorded.
The incremental effect of adherence-contingent rebates dropped to 5.7 percentage points (p = 0.26) when medication adherence was defined as the percentage of days where all doses were taken irrespective of time. This means that approximately half the intervention effect came from encouraging participants that were already taking all their daily medications to take them during the appropriate time windows, while the other half came from encouraging participants not to miss entire days. Though not statistically significant, the adherence-contingent rebates group also had greater levels of the total number of doses taken on time and total doses taken irrespective of time windows than the control group by 8.2 (p = 0.09) and 3.8 (p = 0.34) percentage points, respectively.
Figure 5 shows the monthly evolution of the unadjusted mean of adherence in both study groups (Fig. 5a) and of the incremental effect of adherence-contingent rebates (Fig. 5b). During Months 2 and 3, the incremental effect of adherence-contingent rebates amounted to only 0.61 (p = 0.84) and 3.34 (p = 0.47) percentage points. What mainly took place during these 2 months is the wearing off of the monitoring effect, with adherence rates decreasing from around 76% to around 65% in both groups. After the first incentive payment was made between Months 3 and 4, the intervention effect kicked in as shown by the marked increase at Month 4 (10.4 percentage points; p = 0.04). The intervention effect then slightly dropped during Month 5 and increased again during Month 6 following the text message promoting study compliance to all participants and reminding intervention group participants of the incentive scheme that was sent between Months 5 and 6.
As an additional analysis, we explored whether various factors moderated the effect of adherence-contingent rebates by including interaction terms between potential moderators and the dummy variable indicating the study group. Results from these estimations are shown in Table 3. While the statistical power was limited for such an analysis and the null hypothesis cannot be rejected, results nonetheless suggest that the intervention effect may increase with the number of glaucoma-related medication doses to be taken given the observed 3.8 percentage points increase in adherent days per dose (p = 0.26). Furthermore, our results suggest that each dollar of incentivized treatment cost may increase adherent days, given the observed 0.092 percentage points increase during the month (p = 0.29). Regarding potential distributional issues, our study did not reveal any clear association between intervention effect and household income.
Lastly, we explored determinants of medication adherence at baseline. The results are reported in Table 4. The only factor that we found to have a statistically significant effect is the number of medication doses to be taken daily by the participants. An increase by one daily dose decreased adherent days by 4.8 percentage points (p = 0.01).
4 Discussion
Our main finding is that, after 5 months, participants in the adherence-contingent rebates group had higher medication adherence than in the control group by 12.2 percentage points (p = 0.027) after controlling for baseline differences. While this is the first study applying monetary incentives to improve medication adherence in glaucoma, the behavioral change we found is larger than those reported in other disease areas. A prior study found that cash incentives improved adherent days to antihypertensive medications by 6.2 percentage points [39]. Various designs of lottery incentives have been shown to improve adherence to anticoagulation medications by 2.6 [40], 4.3 [41], and 7.4 [40] percentage points and to hypertension/hyperlipidemia medications by 2.6 [42] percentage points, but only the largest effect achieved statistical significance. One study found that deposit contracts did not change adherence to hypertension and hyperlipidemia medications [42]. While a head-to-head trial would be needed to test this hypothesis, theory and our results suggest that leveraging multiple insights from behavioral economics can improve outcomes compared to relying on a single incentive strategy. Moreover, leveraging loss aversion, regret, and salience via the novel adherence-contingent rebates we propose can be achieved at virtually no additional cost.
As in other studies that aimed at improving adherence to glaucoma medications [18, 19], the duration of our study was too short to observe improvements in health outcomes. It should also be noted that, as adherence rebates were awarded as a percentage of treatment costs, the average rebate amount is dependent on the cost of drug therapy of each participant, and might therefore not generalize well outside the study. Our intervention nonetheless has the potential to be cost effective in the long-term as, with an average incentive amount of $US5.94 per month per patient, the behavioral change achieved by adherence-contingent rebates came at a low cost. Furthermore, the supporting medication tracker used costs around $US60 per unit; a device that can operate for up to 3 years based on the manufacturer’s specifications. Labor costs were also kept at a minimum by automating adherence and incentive calculations. Taking a conservative estimate of $US250 per year including incentive payments, rebate would only need to improve average quality-adjusted life-year (QALY) weights by 0.005 to be cost effective using a willingness to pay of $US50,000 per QALY. By significantly promoting the adequate use of effective medications that slow or stop disease progression, adherence-contingent rebates have the potential to generate such improvement in QALY weights in the long-term. A longer study is needed to detect potential improvements in quality of life and clinical outcomes, and assess the long-term cost effectiveness of the adherence-contingent rebates. Showing health improvements is especially challenging in chronic diseases such as glaucoma, diabetes mellitus, and hypertension as these conditions are asymptomatic in their early stages and quality of life is initially not affected. For glaucoma, a relatively rapid progression such as a loss of 2 decibels in visual sensitivity per year would take about 15 years to evolve from full normal field to perimetric blindness [43].
It should be highlighted that our study focused on non-adherent patients. In the real world, the intervention effect would be diluted as those patients who are already adherent would receive rebates without behavioral change. Thus, a crucial factor to consider is the proportion of patients who are not adherent to their medications. With high rates of non-adherence reported globally and across countless disease areas, it is not expected that the effect of adherence-contingent rebates would be overly diluted, but dilution is still a consideration to bear in mind when setting adherence goals.
In the real world, new technology can be used to further reduce the cost of adherence monitoring via wireless pill boxes that can store multiple medications combined with a smartphone application or a cloud service [44,45,46]. Medication adherence for a wide range of chronic diseases such as diabetes and hypertension can be monitored using such technology [47,48,49], which can then serve as the vehicle for monetary incentives based on behavioral economics principles such as in our study [50]. Technological progress will also make it easier to identify the users and integrated information systems will allow automatic comparison of medication adherence with medication purchases to prevent cheating.
In an effort to make such a strategy more affordable to healthcare payers, instead of awarding new rebates to adherent patients as in this study, one alternative would be to make existing public financing contingent on medication adherence. In many health systems, medications are fully or partially reimbursed via public insurance schemes and/or public subsidies are used to decrease the price of medications at the point of consumption. The idea would be to leverage such public financing to improve medication adherence and, ultimately, health outcomes. This approach would have the advantage of providing a sustained incentive for medication adherence, which is especially important given the lack of evidence for habit formation following discontinuation of monetary incentives [51]. A further rationale for making existing public financing contingent on medication adherence is that many countries use cost-effectiveness thresholds to include healthcare in their insurance coverage and packages of subsidized care. By focusing their reimbursements and subsidies on those medications that are appropriately taken, these countries could obtain a more favorable cost-effectiveness ratio.
Whether existing or new public financing is used to pay for adherence-contingent rebates, it is crucial to avoid unintended redistributional consequences. A potential equity concern is that low-income patients might be more affected by the out-of-pocket cost of medications and could be less adherent as a result. Other factors such as education might also play a role. While we did not find any clear association between socioeconomic status and intervention benefit, this is not guaranteed to be the case in other contexts, especially where individuals may be liquidity constrained and unable to pay in advance for medicines. If implemented, it would be important to monitor the benefit incidence of the program and consider corrective actions such as tying the rebate size and/or adherence goals to patient income if distributional concerns arose.
It is worth mentioning that incentives can be implemented in countless ways [51]. We chose a theory-based approach that we postulated to be both effective and cost effective in the context of chronic disease management in a rich country. Future research can explore other strategies in an effort to improve upon this design. Further research should also look into how to adapt our strategy to low-income countries where patients may have little disposable income to buy their medications. A potential idea to explore would be to include adherence-contingent incentives into existing conditional cash transfer schemes which have been widely used in low-income settings [52].
A limitation of the study is that measurement of adherence is confounded by the effect of the medication trackers that are required for the measurement. However, as medication trackers are used in both study groups, this Hawthorne effect [53] does not bias the measurement of the incremental effect of adherence-contingent rebates compared with the control group. Still, both groups likely increased adherence at the start of the study beyond normal levels both because of adherence monitoring and due to joining a study. Regarding measurement of adherence in the absence of rebates in the real world, we think that this is more reliably captured by the adherence levels recorded in the control group at the end of the study when the initial effect from being a study participant and being observed is likely to have vanished.
Furthermore, measurement of the primary outcome critically depended on the participants keeping their medications stored in their tracking devices. This study requirement was emphasized at recruitment and reiterated during follow-up calls. A potential downside of the follow-up calls, though, is that these reminders might have contributed to improving medication adherence in the control group. In addition, the adherence reports sent to the participants might have acted as non-monetary rewards, and might also have contributed to improving medication adherence in the control group. However, as both study groups received the same reminders and adherence reports, we were able to measure the incremental effect of adherence-contingent rebates compared with the control group without bias. Further, a recent literature review suggests that reward frequency has an effect, with more frequent incentive payments being more effective at generating behavioral changes [51]. Real-world implementation of our study intervention could, therefore, have additional effectiveness if the rebates were paid at each medication refill instead of waiting for 2–3 months. Furthermore, in efforts to counter and detect potential gaming, participants were asked to sign a participation oath [54] and their medication purchases were monitored by the study team. Lastly, with 100 participants, the study has limited power for secondary and explanatory analyses. As such, results that were not statistically significant have been interpreted with caution.
5 Conclusion
Adherence-contingent rebates extend the concepts of accountability, value-based insurance designs, and conditional cash transfers by creating a strategy where cost sharing depends not only on the effectiveness of the medications but also on how effectively these medications are used. With the explosion of healthcare costs that are increasingly driven by modifiable risk factors, including poor medication adherence, it is time to extend accountable care all the way to the patient level.
References
De Geest S, Sabaté E. Adherence to long-term therapies: evidence for action [letter]. Eur J Cardiovasc Nurs. 2003;2(4):323. https://doi.org/10.1016/s1474-5151(03)00091-4.
Viswanathan M, Golin CE, Jones CD, Ashok M, Blalock SJ, Wines RCM, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157(11):785–95. https://doi.org/10.7326/0003-4819-157-11-201212040-00538.
Naderi SH, Bestwick JP, Wald DS. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients. Am J Med. 2012;125(9):8827.e1. https://doi.org/10.1016/j.amjmed.2011.12.013.
Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28(4):437–43. https://doi.org/10.1592/phco.28.4.437.
Kini V, Ho PM. Interventions to improve medication adherence: a review. JAMA. 2018;320(23):2461–73. https://doi.org/10.1001/jama.2018.19271.
Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014. https://doi.org/10.1002/14651858.CD000011.pub4.
Loewenstein G, Asch DA, Volpp KG. Behavioral economics holds potential to deliver better results for patients, insurers, and employers. Health Aff (Millwood). 2013;32(7):1244–50. https://doi.org/10.1377/hlthaff.2012.1163.
Laibson D. Golden eggs and hyperbolic discounting. Q J Econ. 1997;112(2):443–78. https://doi.org/10.1162/003355397555253.
Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47(2):263–91. https://doi.org/10.2307/1914185.
Loomes G, Sugden R. Regret theory: an alternative theory of rational choice under uncertainty. Econ J. 1982;92(368):805–24. https://doi.org/10.2307/2232669.
Pizzi LT, Tran J, Shafa A, Waisbourd M, Hark L, Murchison AP, et al. Effectiveness and cost of a personalized reminder intervention to improve adherence to glaucoma care. Appl Health Econ Health Policy. 2016;14(2):229–40. https://doi.org/10.1007/s40258-016-0231-8.
Pizzi LT, Zangalli CS, Murchison AP, Hale N, Hark L, Dai Y, et al. Prospective randomized controlled trial comparing the outcomes and costs of two eyecare adherence interventions in diabetes patients. Appl Health Econ Health Policy. 2015;13(2):253–63. https://doi.org/10.1007/s40258-015-0159-4.
Basch CE, Walker EA, Howard CJ, Shamoon H, Zybert P. The effect of health education on the rate of ophthalmic examinations among African Americans with diabetes mellitus. Am J Public Health. 1999;89(12):1878–82.
Newman-Casey PA, Dayno M, Robin AL. Systematic review of educational interventions to improve glaucoma medication adherence: an update in 2015. Expert Rev Ophthalmol. 2016;11(1):5–20. https://doi.org/10.1586/17469899.2016.1134318.
Newman-Casey PA, Weizer JS, Heisler M, Lee PP, Stein JD. Systematic review of educational interventions to improve glaucoma medication adherence. Semin Ophthalmol. 2013;28(3):191–201. https://doi.org/10.3109/08820538.2013.771198.
Glanz K, Beck AD, Bundy L, Primo S, Lynn MJ, Cleveland J, et al. Impact of a health communication intervention to improve glaucoma treatment adherence. Results of the interactive study to increase glaucoma adherence to treatment trial. Arch Ophthalmol. 2012;130(10):1252–8. https://doi.org/10.1001/archophthalmol.2012.1607.
Lim MC, Watnik MR, Imson KR, Porter SM, Granier AM. Adherence to glaucoma medication: the effect of interventions and association with personality type. J Glaucoma. 2013;22(6):439–46. https://doi.org/10.1097/IJG.0b013e31824cd0ae.
Okeke CO, Quigley HA, Jampel HD, Ying GS, Plyler RJ, Jiang Y, et al. Interventions improve poor adherence with once daily glaucoma medications in electronically monitored patients. Ophthalmology. 2009;116(12):2286–93. https://doi.org/10.1016/j.ophtha.2009.05.026.
Gray TA, Fenerty C, Harper R, Spencer AF, Campbell M, Henson DB, et al. Individualised patient care as an adjunct to standard care for promoting adherence to ocular hypotensive therapy: an exploratory randomised controlled trial. Eye (Lond). 2012;26(3):407–17. https://doi.org/10.1038/eye.2011.269.
Nordstrom BL, Friedman DS, Mozaffari E, Quigley HA, Walker AM. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140(4):598–606. https://doi.org/10.1016/j.ajo.2005.04.051.
Sleath B, Robin AL, Covert D, Byrd JE, Tudor G, Svarstad B. Patient-reported behavior and problems in using glaucoma medications. Ophthalmology. 2006;113(3):431–6. https://doi.org/10.1016/j.ophtha.2005.10.034.
Lai JSM, Tham CCY. Medication adherence in glaucoma patients. Asia Pac J Ophthalmol (Phila). 2013;2(6):354–5. https://doi.org/10.1097/apo.0000000000000025.
Bilger M, Wong TT, Howard KL, Lee JY, Toh AN, John G, et al. Study on Incentives for Glaucoma Medication Adherence (SIGMA): study protocol for a randomized controlled trial to increase glaucoma medication adherence using value pricing. Trials. 2016;17(1):316. https://doi.org/10.1186/s13063-016-1459-1.
Krousel-Wood M, Islam T, Webber LS, Re RN, Morisky DE, Muntner P. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. Am J Manag Care. 2009;15(1):59–66.
Morisky DE, DiMatteo MR. Improving the measurement of self-reported medication nonadherence: response to authors. J Clin Epidemiol. 2011;64(3):255–63. https://doi.org/10.1016/j.jclinepi.2010.09.002.
Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich). 2008;10(5):348–54.
Mills RP, Budenz DL, Lee PP, Noecker RJ, Walt JG, Siegartel LR, et al. Categorizing the stage of glaucoma from pre-diagnosis to end-stage disease. Am J Ophthalmol. 2006;141(1):24–30. https://doi.org/10.1016/j.ajo.2005.07.044.
Coleman AL, Miglior S. Risk factors for glaucoma onset and progression. Surv Ophthalmol. 2008;53(6):S3–10. https://doi.org/10.1016/j.survophthal.2008.08.006.
Nouri-Mahdavi K, Hoffman D, Coleman AL, Liu G, Li G, Gaasterland D, et al. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology. 2004;111(9):1627–35. https://doi.org/10.1016/j.ophtha.2004.02.017.
De Moraes CGV, Juthani VJ, Liebmann JM, Teng CC, Tello C, Susanna R Jr, et al. Risk factors for visual field progression in treated glaucoma. Arch Ophthalmol. 2011;129(5):562–8. https://doi.org/10.1001/archophthalmol.2011.72.
Le A, Mukesh BN, McCarty CA, Taylor HR. Risk factors associated with the incidence of open-angle glaucoma: the visual impairment project. Invest Ophthalmol Vis Sci. 2003;44(9):3783–9. https://doi.org/10.1167/iovs.03-0077.
The EuroQol Group. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. https://doi.org/10.1016/0168-8510(90)90421-9.
Nelson P, Aspinall P, Papasouliotis O, Worton B, O’Brien C. Quality of life in glaucoma and its relationship with visual function. J Glaucoma. 2003;12(2):139–50.
Luo N, Wang P. Estimating an EQ-5D-3L value set in Singapore. Value Health. 2013;16(3):A34. https://doi.org/10.1016/j.jval.2013.03.195.
van Hout B, Janssen MF, Feng Y-S, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708–15. https://doi.org/10.1016/j.jval.2012.02.008.
Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006;60(6):631–7. https://doi.org/10.1016/j.jpsychores.2005.10.020.
Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47(6):555–67. https://doi.org/10.1016/s0022-3999(99)00057-4.
Ministry of Health Singapore. Clinical practice guidelines: glaucoma. Singapore: Ministry of Health Singapore; 2005.
Petry NM, Alessi SM, Byrne S, White WB. Reinforcing adherence to antihypertensive medications. J Clin Hypertens (Greenwich). 2015;17(1):33–8. https://doi.org/10.1111/jch.12441.
Kimmel SE, Troxel AB, French B, Loewenstein G, Doshi JA, Hecht the, et al. A randomized trial of lottery-based incentives and reminders to improve warfarin adherence: the Warfarin Incentives (WIN2) trial. Pharmacoepidemiol Drug Saf. 2016;25(11):1219–27. https://doi.org/10.1002/pds.4094.
Kimmel SE, Troxel AB, Loewenstein G, Brensinger CM, Jaskowiak J, Doshi JA, et al. Randomized trial of lottery-based incentives to improve warfarin adherence. Am Heart J. 2012;164(2):268–74. https://doi.org/10.1016/j.ahj.2012.05.005.
Garza KB, Owensby JK, Braxton Lloyd K, Wood EA, Hansen RA. Pilot study to test the effectiveness of different financial incentives to improve medication adherence. Ann Pharmacother. 2016;50(1):32–8. https://doi.org/10.1177/1060028015609354.
Heijl A. Concept and importance of visual field measurements to detect glaucoma progression. Glaucoma Now. 2010;2:2–4.
WHO. Digital health for the end TB strategy: an agenda for action. Technical documents. Geneva: World Health Organization; 2015.
Kvedar J, Coye MJ, Everett W. Connected health: a review of technologies and strategies to improve patient care with telemedicine and telehealth. Health Aff (Millwood). 2014;33(2):194–9. https://doi.org/10.1377/hlthaff.2013.0992.
Paterson M, Kinnear M, Bond C, McKinstry B. A systematic review of electronic multi-compartment medication devices with reminder systems for improving adherence to self-administered medications. Int J Pharm Pract. 2017;25(3):185–94. https://doi.org/10.1111/ijpp.12242.
Conway CM, Kelechi TJ. Digital health for medication adherence in adult diabetes or hypertension: an integrative review. JMIR Diabetes. 2017;2(2):e20. https://doi.org/10.2196/diabetes.8030.
Miller L, Schüz B, Walters J, Walters EH. Mobile technology interventions for asthma self-management: systematic review and meta-analysis. JMIR mHealth uHealth. 2017;5(5):e57. https://doi.org/10.2196/mhealth.7168.
Welch G, Balder A, Zagarins S. Telehealth program for type 2 diabetes: usability, satisfaction, and clinical usefulness in an urban community health center. Telemed J E Health. 2015;21(5):395–403. https://doi.org/10.1089/tmj.2014.0069.
Putt ME, Reese PP, Volpp KG, Russell LB, Loewenstein G, Yan J, et al. The Habit Formation trial of behavioral economic interventions to improve statin use and reduce the risk of cardiovascular disease: rationale, design and methodologies. Clinic Trials. 2019:1740774519846852. https://doi.org/10.1177/1740774519846852.
Finkelstein EA, Bilger M, Baid D. Effectiveness and cost-effectiveness of incentives as a tool for prevention of non-communicable diseases: a systematic review. Soc Sci Med. 2019;232:340–50. https://doi.org/10.1016/j.socscimed.2019.05.018.
Lagarde M, Haines A, Palmer N. Conditional cash transfers for improving uptake of health interventions in low- and middle-income countries: a systematic review. JAMA. 2007;298(16):1900–10. https://doi.org/10.1001/jama.298.16.1900.
McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67(3):267–77.
Mazar N, Amir O, Ariely D. The dishonesty of honest people: a theory of self-concept maintenance. J Mark Res. 2008;45(6):633–44. https://doi.org/10.1509/jmkr.45.6.633.
Acknowledgements
Open access funding provided by Vienna University of Economics and Business (WU). The authors would like to thank Andy Ang, Marlina Tay, Fong Yee Wei, Foo Mei Ling Valerie, and Toh Ai Nee for supporting the study on site. We would also like to thank Janessa Tan, Joanna Cheong, Angela Peh, Geraldine John, and Sylvia Kong for their assistance with the study at Duke-NUS. The use of the MMAS is protected by US copyright laws; permission for use is required. A license agreement is available from Donald E. Morisky, ScD, ScM, MSPH, Professor, Department of Community Health Sciences, UCLA Fielding School of Public Health, 650 Charles E. Young Drive South, Los Angeles, CA 90095–1772, USA, dmorisky@ucla.edu. The authors have obtained written permission from copyright owners for any excerpts from copyrighted works that are included and have credited the sources in the article or the Supplemental Materials. The credit footnote is located in the copyright agreement.
Author information
Authors and Affiliations
Contributions
MB and EF conceived and designed the study, conducted the analysis and interpretation of the data, and led the manuscript preparation. TTW contributed to the conception and design of the study and helped oversee data acquisition and implementation on site. JYL helped design the study protocol and was responsible for the acquisition of the data and daily implementation. KLH helped design the study protocol and coordinated the study and data collection. FGB contributed to the analysis and interpretation of data and helped in drafting and finalizing the manuscript. ELL contributed to the conception of the study. All authors reviewed and approved the final manuscript. MB will act as the overall guarantor.
Corresponding author
Ethics declarations
Funding
The study was supported by grant NMRC/HSRNIG/0006/2014 (principal investigator: Marcel Bilger) from the Ministry of Health of Singapore. The funding agency played no role in the study conception, design, writing of the manuscript, or decision to submit for publication.
Conflict of interest
Marcel Bilger, Tina T. Wong, Jia Yi Lee, Kaye L. Howard, Filipinas G. Bundoc, Ecosse L. Lamoureux, and Eric A. Finkelstein declare that they have no conflicts of interest.
Ethical approval
This research has received ethics approval from the SingHealth Centralised Institutional Review Board (ref: 2013/852/A).
Informed consent
Written informed consent were obtained from each participant prior to inclusion in the study.
Research reporting
The trial registration number is ClinicalTrials.gov identifier NCT02271269. This clinical trial was registered on 19 October 2014.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bilger, M., Wong, T.T., Lee, J.Y. et al. Using Adherence-Contingent Rebates on Chronic Disease Treatment Costs to Promote Medication Adherence: Results from a Randomized Controlled Trial. Appl Health Econ Health Policy 17, 841–855 (2019). https://doi.org/10.1007/s40258-019-00497-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-019-00497-0