Abstract
Background and Objective
Glioblastoma is a cranial malignant tumor with a high recurrence rate after surgery and a poor response to chemoradiotherapy. Bevacizumab has demonstrated efficacy in the treatment of glioblastoma by inhibiting vascular endothelial growth factor, but the efficacy of vascular endothelial growth factor receptor tyrosine kinase inhibitors varies in treating glioblastoma. This single-arm prospective study aimed to explore the efficacy and safety of the vascular endothelial growth factor receptor tyrosine kinase inhibitor apatinib in treating recurrent glioblastoma after chemoradiotherapy.
Methods
A total of 15 patients with recurrent glioblastoma (2016 World Health Organization grade IV) after chemoradiotherapy were enrolled in this study from September 2017 to September 2019 and treated with apatinib 500 mg once daily. Responses were evaluated according to the Response Assessment in Neuro-Oncology criteria, and adverse events were recorded according to the National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.0.
Results
The overall response rate was 33.3%, and the disease control rate was 66.6%. The median progression-free survival was 2 months, and the median overall survival was 6.5 months. The apatinib dose was adjusted in seven patients because of adverse events (46.6%). The most common adverse events were thrombocytopenia (53.3%), asthenia (40%), and hand-foot syndrome (33.3%).
Conclusions
Apatinib might be effective in treating recurrent glioblastoma after chemoradiotherapy in terms of the overall response rate, but the efficacy is not durable and the clinical benefit is limited. The adverse effects of apatinib were acceptable.
Clinical Trial Registration
ChiCTR-ONC-17013098, date of registration: 24 October, 2017.
Similar content being viewed by others
Apatinib had limited efficacy in the treatment of recurrent glioblastoma, and the adverse effects should also be paid attention to. | |
Moreover, significant individual variations were observed in its response. |
1 Introduction
Glioblastoma (GBM, World Health Organization grade IV) is the most common intracranial malignancy, accounting for half of all primary brain tumors. Glioblastoma is also the most malignant type of glioma and is aggressive and easy to recurrent. Glioblastoma shows a poor response to various treatments, including surgery, radiotherapy, and chemotherapy. The median survival of patients with GBM is 14–16 months, and is only 3–9 months after recurrence [1]. The best supportive care merely achieves a median survival time of 3.1 months. Therefore, effective treatments for recurrent GBM are still lacking [2].
Glioblastoma is one of the most richly vascularized tumors in the central nervous system, and hence can express a variety of specific tumor angiogenesis regulators, including hypoxia-inducible factor-1 subunit alpha, angiopoietin 1/2, transforming growth factor β1, platelet-derived growth factor, and fibroblast growth factor [3]. Most of these regulators exert an angiogenic effect through the vascular endothelial growth factor (VEGF) pathway. The expression of VEGF in the tumor tissues is closely related to the grade and prognosis of glioma [4].
Bevacizumab binds to VEGF and inhibits the activation of the angiogenesis pathway. It was approved for treating GBM by the US Food and Drug Administration in 2009. The addition of bevacizumab to the standard regimen of temozolomide and radiotherapy improved the progression-free survival (PFS) of treatment-naïve GBM by 3.4–4.4 months [5]. For recurrent GBM, additional use of bevacizumab improved the PFS by 2.7 months compared with lomustine alone [6]. The overall safety of adding bevacizumab to the treatment of GBM is acceptable. A meta-analysis of 480 patients with GBM showed the most frequent adverse events (AEs) associated with bevacizumab were asthenia, headache, diarrhea, and hypertension [7]. However, treatment interruption because of AEs was 20% in patients treated with bevacizumab plus chemotherapy, which is significantly higher than the 5.5% in patients treated with bevacizumab alone [7].
Apatinib, also known as rivoceranib, is a novel small-molecule drug approved in China for the treatment of advanced or metastatic gastric cancer. It blocks signal transduction by binding to VEGF through the intracellular ATP-binding site of the tyrosine receptor, thereby inhibiting tumor angiogenesis. Compared with VEGF antibody drugs, apatinib has a stronger inhibitory effect on the VEGF pathway in vitro [8]. Several case reports first demonstrated the efficacy of apatinib in treating recurrent GBM [9, 10]. The following small-scale studies reported further promising results. A clinical study reported a PFS of 8.3 months in nine patients with recurrent high-grade glioma who were treated with apatinib (500 mg once daily [qd]) concurrently with irinotecan (340 mg/m2 or 125 mg/m2 every 21 days) [11]. Another clinical study reported an objective response rate (ORR) of 45% and a PFS of 6 months in 20 patients with recurrent GBM who were treated with apatinib (500 mg qd) and temozolomide (100 mg/m2, 7 days on with 7 days off) [12]. Similar results of overall survival (OS) of 9.1 months and a disease control rate of 82.3% were achieved in 18 patients with recurrent high-grade glioma who were treated with apatinib (500 mg qd) and temozolomide (50 mg/m2 qd) [13].
However, combined therapy will inevitably increase the adverse reactions of patients with recurrent GBM with a poor performance status. It is not clear whether apatinib alone is equally treatment effective and has lower adverse reactions. Currently, only a few case reports and small sample size retrospective studies indicated that apatinib might be effective in the treatment of recurrent glioma [14]. Our prospective study aimed to further evaluate the efficacy and safety of apatinib monotherapy in treating recurrent GBM after chemoradiotherapy.
2 Methods
2.1 Patients
The inclusion criteria were as follows: aged 18–75 years; GBM (2016 World Health Organization grade IV) confirmed by surgical pathology; at least one intracranial target lesion defined according to the Response Assessment in Neuro-Oncology (RANO); failed previous intracranial lesion radiotherapy and temozolomide-based chemotherapy regimens (progressive disease confirmed with clear imaging evidence during treatment or within 6 months after treatment); no indications for reoperation; and Eastern Cooperative Oncology Group performance status scores of 0–2.
The exclusion criteria were as follows: resistant hypertension; severe cardiovascular diseases; abnormal blood coagulation or current gastrointestinal bleeding; major surgery in the previous 3 months; participation in other clinical trials in the previous 3 months; and renal insufficiency or liver dysfunction.
Written informed consent was obtained from all participants before enrollment. The study protocol was approved by the Ethics Committee of Fudan University Huashan Hospital. The study was conducted in compliance with Good Clinical Practice guidelines and the Declaration of Helsinki (Clinical Trial Registration No. ChiCTR-ONC-17013098).
2.2 Study Design
This single-center, prospective, single-arm clinical trial was performed to preliminarily investigate the efficacy and safety of apatinib mesylate in patients with recurrent GBM after surgery, radiotherapy, and temozolomide chemotherapy.
2.3 Procedure
Patients were treated with apatinib at an initial dose of 500 mg/day until progression, death, or serious AEs. In the case of an adverse reaction ≥ grade 3, the drug was discontinued until the adverse reaction returned to grade 1 and then was resumed from 250 mg qd orally. If an adverse reaction of grade 3 or above occurred again, the drug was withdrawn. Molecular pathological examinations were performed at the Huashan Hospitals’ pathology departments following primary surgery. Methyl-guanine methyl transferase promoter methylation status was evaluated by polymerase chain reaction and verified by quantitative pyrosequencing. Mutations in the IDH1 gene were investigated by Sanger sequencing. Ki67 was evaluated by immunohistochemistry. A blood test was completed at the College of American Pathologists-certified Central Laboratory of Huashan Hospital.
2.4 Endpoints
The primary endpoint was ORR. The secondary endpoints were PFS, OS, and AEs. Brain cranial magnetic resonance imaging was performed after apatinib treatment and every 1 month or when there were significant signs of progression. Magnetic resonance imaging was used for examining the lesions, and complete remission (CR), partial response (PR), stable disease (SD), and progressive disease (PD) evaluations were performed according to the RANO criteria. The ORR was calculated as (CR + PR)/total number of patients × 100%. The disease control rate was calculated as (CR + PR + SD)/total number of patients × 100%. Progression-free survival was the time from the start of treatment to PD or death. Overall survival was defined as the time from the start of treatment to death. Adverse events were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.0, and monitored by consultation.
2.5 Statistical Analysis
The PFS and OS were estimated by the Kaplan–Meier method. The corresponding two-sided 95% confidence intervals were calculated via the Brookmeyer–Crowley method. All analyses were performed with SPSS version 25.0 (IBM Corporation, Armonk, NY, USA).
3 Results
3.1 Clinical Characteristics of the Patients
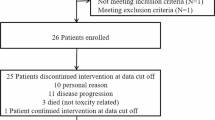
A total of 15 patients with local recurrent GBM were enrolled into the study from September 2017 to September 2019. The clinical characteristics of these patients are shown in Table 1. Female patients accounted for 26.6%. The median age was 52 years. Patients with an Eastern Cooperative Oncology Group performance status score of 2 accounted for 60%. The average duration of treatment was 3.4 months. The median time between the first surgery and recurrence was 7 months (range 2–27 months). All patients had completed the Stupp protocol. Dexamethasone was used at enrollment and during the treatment of apatinib in five patients with a dose of 5–7.5 mg/day.
3.2 Molecular Features of the Tumors
The isocitrate dehydrogenase 1 mutation was found in only one patient. Promoter methylation of the methyl-guanine methyl transferase gene was found in seven patients. The median of the Ki-67 labeling index was 30% (range 10–60%).
3.3 Efficacy
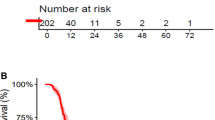
As of 21 July, 2020, data of 15 patients were available for analysis. Partial response and stable disease were achieved in 5 of the 15 patients, respectively. The treatment achieved an ORR of 33.3%, a disease control rate of 66.6%, a median PFS of 2 months (95% confidence interval 1.0–2.9), and a median OS of 6.5 months (95% confidence interval 3.6–9.3). Figure 1 shows the Kaplan–Meier OS and PFS curve. The pre- and post-treatment cranial magnetic resonance imaging scans of two patients with good response to apatinib are shown in Fig. 2.
Brain magnetic resonance imaging scans of two recurrent patients. A–D Patient #8; E–H patient #12; brain magnetic resonance imaging scan followed up before and 1 month after apatinib treatment. Patients #8 and #12 achieved partial remission after apatinib treatment. flair fluid-attenuated inversion recovery
3.4 AEs
The AEs included thrombocytopenia in eight patients, asthenia in six, hand-foot syndrome in five, elevated liver enzymes in five, hypertension in four, leukopenia in four, oral ulcers in two, and proteinuria in one. Dose adjustment was required for a total of seven patients because of thrombocytopenia ≥ grade 3 in two patients, hand-foot syndrome ≥ grade 3 in three, and leukopenia ≥ grade 3 in two.
4 Discussion
Anti-tumor angiogenesis therapy plays an important role in tumor treatment. Bevacizumab binds to VEGF-A and inhibits its binding to VEGFR-2, thereby inhibiting angiogenesis and tumor growth. Additional chemotherapy can further improve the anti-tumor effect of bevacizumab. This is also true in treating recurrent GBM. Bevacizumab plus irinotecan has better efficacy in treating recurrent GBM, but with more AEs, including gastrointestinal reactions and bone marrow suppression [7].
Vascular endothelial growth factor receptor tyrosine kinase inhibitors (TKIs) are novel anti-angiogenic drugs generally used in treating advanced tumors. They are multi-target TKIs that potently inhibit tumor angiogenesis pathways compared with anti-angiogenic antibody drugs. Vascular endothelial growth factor receptor TKIs have demonstrated significant inhibitory effects on a variety of solid tumors, including lung cancer, gastric cancer, bowel cancer, and liver cancer. Multiple VEGFR-TKIs have been used to treat patients with recurrent GBM.
A phase III clinical study showed a response rate of 56% for cediranib in treating recurrent GBM, with a 6-month PFS rate of 26% [15]. Sunitinib and other VEGFR-TKIs were not quite effective in treating recurrent GBM, with a 6-month PFS rate of only 10.4%. Sorafenib had a 7.9-month PFS and a 17.8-month OS in treating recurrent GBM in phase I clinical trials, but serious toxic effects were observed [16, 17]. Why the efficacy of sunitinib, cediranib, and sorafenib varies in patients with recurrent GBM is still unclear.
Both sunitinib and cediranib are multi-target receptor TKIs with inhibitory effects on VEGFR 1/2/3 and platelet-derived growth factor receptor pathways. Sorafenib is also a multi-target receptor TKI with inhibitory effects on VEGFR-2, c-Kit, fibroblast growth factor receptors, and the FLT-3 pathways. Recent studies have shown that VEGFR-1 is mainly responsible for the positive regulation of monocyte and macrophage migration, VEGFR-3 is mostly related to the formation of lymphatic vessels, while VEGFR-2 plays a primary role in tumor angiogenesis and therefore is the main target in treating tumor angiogenesis.
Sunitinib, cediranib, and sorafenib are not the same in terms of inhibitory activity against VEGFR-2 tyrosine kinase, with a half-maximal inhibitory concentration of 9 nM, 90 nM, and 5 nM, respectively, clearly indicating that cediranib has the strongest inhibitory effect on VEGFR-2 kinase. This partially explains why cediranib is the most effective agent in treating recurrent GBM [18, 19].
Apatinib is a compound derived from the small-molecule VEGFR-TKI PTK787 (vatalanib). It is chemically known as methane sulfonate N-[4-(cyanocyclopentyl) phenyl] [2-[(4-pyridinylmethyl)amino] (3-pyridine)]formamide, with a molecular formula of C25H27N5O3S and a molecular weight of 493.58 (methane sulfonate). Pharmacodynamic studies have shown that apatinib can inhibit the activity of VEGFR-2 tyrosine kinase to block the signal transduction after binding to VEGF through the intracellular ATP-binding site of the protein tyrosine receptor, thereby inhibiting tumor angiogenesis. Additionally, apatinib can effectively inhibit VEGFR-2 at a very low concentration, with a capacity of binding to VEGFR-2 that is more than ten times that of PTK787 as shown in the activity assay. With regard to the inhibitory activity against VEGFR-2 tyrosine kinase, apatinib has a half-maximal inhibitory concentration of 2 nM and is stronger than cediranib. The drug was approved by the China Food and Drug Administration in October 2014 for the third-line or later-line treatment of advanced metastatic gastric cancer/adenocarcinoma of the esophagogastric junction. Apatinib treatment significantly prolonged median PFS and OS compared with placebo (PFS 2.6 months vs 1.8 months; OS 6.5 months vs 4.7 months; ORR 2.8% vs 0%) [20].
As a novel agent, apatinib was previously applied to treat patients with recurrent GBM in China. Individual case reports indicated that apatinib monotherapy was effective in treating recurrent GBM [9]. In this prospective study, a remission rate of 33.3% and a disease control rate of 66.6%, along with a PFS of 2 months and an OS of 6.5 months, were established for apatinib monotherapy in treating advanced GBM after chemoradiotherapy. The PFS and OS data of this study were inferior to previous retrospective studies [14]. The incidence of AEs was slightly higher [14]. We believe the efficacy of apatinib in the treatment of recurrent GBM is worse than that of bevacizumab and temozolomide when comparing with historical data [21].
5 Conclusions
In this prospective research, we showed that apatinib might be effective in treating recurrent GBM after chemoradiotherapy in terms of the ORR, but the responses were not durable and the clinical benefit is limited. The adverse reactions, especially thrombocytopenia and hand-foot syndrome, need attention and prevention.
Some limitations could not be ignored in this research, especially the small sample size, single-central design, lack of a control group, and some potential biases. Further randomized controlled clinical studies are necessary to verify this finding.
References
Gil-Gil MJ, Mesia C, Rey M, et al. Bevacizumab for the treatment of glioblastoma. Clin Med Insights Oncol. 2013;7:123–35.
Van Meir EG, Hadjipanayis CG, Norden AD, et al. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60(3):166–93.
Schulte JD, Aghi MK, Taylor JW. Anti-angiogenic therapies in the management of glioblastoma. Chin Clin Oncol. 2020;10(4):37.
Chen W, He D, Li Z, et al. Overexpression of vascular endothelial growth factor indicates poor outcomes of glioma: a systematic review and meta-analysis. Int J Clin Exp Med. 2015;8(6):8709–19.
Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708.
Wick W, Gorlia T, Bendszus M, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–63.
Zhang G, Huang S, Wang Z. A meta-analysis of bevacizumab alone and in combination with irinotecan in the treatment of patients with recurrent glioblastoma multiforme. J Clin Neurosci. 2012;19(12):1636–40.
Peng S, Zhang Y, Peng H, et al. Intracellular autocrine VEGF signaling promotes EBDC cell proliferation, which can be inhibited by apatinib. Cancer Lett. 2016;373(2):193–202.
Zhang H, Chen F, Wang Z, et al. Successful treatment with apatinib for refractory recurrent malignant gliomas: a case series. Onco Targets Ther. 2017;10:837–45.
Yu D, Han G, Liu H, et al. Treatment of adult brainstem glioma with combined antiangiogenic therapy: a case report and literature review. Onco Targets Ther. 2019;12:1333–9.
Wang L, Liang L, Yang T, et al. A pilot clinical study of apatinib plus irinotecan in patients with recurrent high-grade glioma: clinical trial/experimental study. Medicine (Baltimore). 2017;96(49): e9053.
Wang Y, Meng X, Zhou S, et al. Apatinib plus temozolomide for recurrent glioblastoma: an uncontrolled, open-label study. Onco Targets Ther. 2019;12:10579–85.
Yao H, Liu J, Zhang C, et al. Clinical study of apatinib plus temozolomide for the treatment of recurrent high-grade gliomas. J Clin Neurosci. 2021;90:82–8.
Zhang H-H, Du X-J, Deng M-L, et al. Apatinib for recurrent/progressive glioblastoma multiforme: a salvage option. Front Pharmacol. 2022;13: 969565.
Batchelor TT, Mulholland P, Neyns B, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–8.
Kreisl TN, Smith P, Sul J, et al. Continuous daily sunitinib for recurrent glioblastoma. J Neurooncol. 2013;111(1):41–8.
Galanis E, Anderson SK, Lafky JM, et al. Phase II study of bevacizumab in combination with sorafenib in recurrent glioblastoma (N0776): a north central cancer treatment group trial. Clin Cancer Res. 2013;19(17):4816–23.
Li J, Zhao X, Chen L, et al. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer. 2010;10:529.
Cowey CL, Sonpavde G, Hutson TE. New advancements and developments in treatment of renal cell carcinoma: focus on pazopanib. Onco Targets Ther. 2010;3:47–55.
Li J, Qin S, Xu J, et al. Randomized, double-blind, placebocontrolled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34(13):1448–54.
Balañá C, Etxaniz O, Bugés C, et al. Approval denied by the European Medicines Agency (EMA) for bevacizumab in the treatment of high-grade glioma recurrence: a good idea or a grave error? Clin Transl Oncol. 2011;13(3):209–10.
Acknowledgments
The authors thank the Neurosurgery Department of Huashan Hospital for providing the patient data.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
The Baoshan District Science and Technology Innovation Fund supported project 18-E-23.
Conflict of interest
Hao Lin, Xinli Zhou, Xiaofang Sheng, and Xiaohua Liang have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
The study protocol was approved by the Ethics Committee of Fudan University Huashan Hospital. The study was conducted in compliance with the Good Clinical Practice guidelines and the Declaration of Helsinki (Clinical Trial Registration No. ChiCTR-ONC-17013098).
Consent to participate
Written informed consent was obtained from all participants before starting the study.
Consent for publication
Not applicable.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Code availability
Not applicable.
Author contributions
Conceptualization: XL, XS, HL; methodology: HL; formal analysis and investigation: HL; writing, original draft preparation: HL; writing, review and editing: XZ; supervision: XZ.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lin, H., Zhou, X., Sheng, X. et al. Efficacy and Safety of Apatinib in Patients with Recurrent Glioblastoma. Drugs R D 23, 239–244 (2023). https://doi.org/10.1007/s40268-023-00429-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-023-00429-3