Abstract
Purpose
Angiogenesis plays a key role in glioblastoma, but most anti-angiogenic therapy trials have failed to change the poor outcome of this disease. Despite this, and because bevacizumab is known to alleviate symptoms, it is used in daily practice. We aimed to assess the real-life benefit in terms of overall survival, time to treatment failure, objective response, and clinical benefit in patients with recurrent glioblastoma treated with bevacizumab.
Methods
This was a monocentric, retrospective study including patients treated between 2006 and 2016 in our institution.
Results
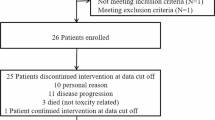
202 patients were included. The median duration of bevacizumab treatment was 6 months. Median time to treatment failure was 6.8 months (95%CI 5.3–8.2) and median overall survival was 23.7 months (95%CI 20.6–26.8). Fifty percent of patients had a radiological response at first MRI evaluation, and 56% experienced symptom amelioration. Grade 1/2 hypertension (n = 34, 17%) and grade one proteinuria (n = 20, 10%) were the most common side effects.
Conclusions
This study reports a clinical benefit and an acceptable toxicity profile in patients with recurrent glioblastoma treated with bevacizumab. As the panel of therapies is still very limited for these tumors, this work supports the use of bevacizumab as a therapeutic option.



Similar content being viewed by others
Data availability
Data available on request. The data presented in this study are available on request from the corresponding author.
References
Stupp R, Masson WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Koshy M, Villano JL, Dolecek TA et al (2012) Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J Neurooncol 107:207–212
Di Carlo DT, Cagnazzo F, Benedetto N, Morganti R, Perrini P (2017) Multiple high-grade gliomas: epidemiology, management, and outcome. A systematic review and meta-analysis. Neurosurg Rev. https://doi.org/10.1007/s10143-017-0928-7
Bahadur S, Sahu AK, Baghel P et al (2019) Current promising treatment strategy for glioblastoma multiform: a review. Oncol Rev 13(2):417
Chen S, Le T, Harley BAC, Imoukhuede PI (2018) Characterizing glioblastoma heterogeneity via single-cell receptor quantification. Front Bioeng Biotechnol 6:92
Hanif F, Muzzafar K, Perveen K, Malhi SM, Simjee ShU (2017) Glioblastoma multiforme: a review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev 18(1):3–9
Shergalis A, Bankhead A 3rd, Luesakul U, Muangsin N, Neamati N (2018) Current challenges and opportunities in treating glioblastoma. Pharmacol Rev 70(3):412–445
Perrin SL, Samuel SM, Koszyca B et al (2019) Glioblastoma heterogeneity and the tumour microenvironment: implications for preclinical research and development of new treatments. Biochem Soc Trans 47(2):625–638
Han S, Liu Y, Cai SJ et al (2020) IDH mutation in glioma: molecular mechanisms and potential therapeutic targets. Br J Cancer 122(11):1580–1589
Saadeh FS, Mahfouz R, Assi HI (2018) EGFR as a clinical marker in glioblastomas and other gliomas. Int J Biol Markers 33(1):22–32
Lu VM, O’Connor KP, Shah AH et al (2020) The prognostic significance of CDKN2A homozygous deletion in IDH-mutant lower-grade glioma and glioblastoma: a systematic review of the contemporary literature. J Neurooncol 148(2):221–229
Brito C, Azevedo A, Esteves S et al (2019) Clinical insights gained by refining the 2016 WHO classification of diffuse gliomas with: EGFR amplification, TERT mutations, PTEN deletion and MGMT methylation. BMC Cancer 19(1):968
Cakar B, Muslu U, Bozkurt E et al (2018) Angiogenesis inhibition on glioblastoma multiforme cell lines (U-87 MG and T98G) by AT-101. J Oncol Sci 4(2):65–69
Das S, Marsden PA (2013) Angiogenesis in glioblastoma. N Engl J Med 369(16):1561–1563
Folberg R, Maniotis AJ (2004) Vasculogenic mimicry. APMIS 112(7–8):508–525
Kreisl TN, Kim L, Moore K et al (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27(5):740–745
Field KM, Simes J, Nowak AK et al (2015) Randomized phase 2 study of carboplatin and bevacizumab in recurrent glioblastoma. Neuro Oncol 17(11):1504–1513
Carra E, Barbieri F, Marubbi D et al (2013) Sorafenib selectively depletes human glioblastoma tumor-initiating cells from primary cultures. Cell Cycle 12(3):491–500
Batchelor TT, Mulholland P, Neyns B et al (2013) Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol 31(26):3212–3218
Lombardi G, Zustovich F, Farina P et al (2013) Hypertension as a biomarker in patients with recurrent glioblastoma treated with antiangiogenic drugs: a single-center experience and a critical review of the literature. Anticancer Drugs 24(1):90–97
Wick W, Platten M, Wick A et al (2015) Current status and future directions of anti-angiogenic therapy for gliomas. Neuro Oncol 18(3):315–328
Cohen MH, Shen YL, Keegan P et al (2009) FDA drug approval summary: bevacizumab (Avastin®) as treatment of recurrent glioblastoma multiforme. Oncologist 14(11):1131–1138
Balañá C, Etxaniz O, Bugés C et al (2011) Approval denied by the European Medicines Agency (EMA) for bevacizumab in the treatment of high-grade glioma recurrence: a good idea or a grave error? Clin Transl Oncol 13(3):209–210
Dirven L, van den Bent MJ, Bottomley A et al (2015) The impact of bevacizumab on health-related quality of life in patients treated for recurrent glioblastoma: results of the randomised controlled phase 2 BELOB trial. Eur J Cancer 51(10):1321–1330
Gramatzki D, Roth P, Rushing EJ et al (2018) Bevacizumab may improve quality of life, but not overall survival in glioblastoma: an epidemiological study. Ann Oncol 29(6):1431–1436
Wick W, Gorlia T, Bendszus M et al (2017) Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med 377(20):1954–1963
Friedman HS, Prados MD, Wen PY et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27(28):4733–4740
Desjardins A, Reardon DA, Coan A et al (2012) Bevacizumab and daily temozolomide for recurrent glioblastoma. Cancer 118(5):1302–1312
Desjardins A, Herndon JE 2nd, McSherry F et al (2019) Single-institution retrospective review of patients with recurrent glioblastoma treated with bevacizumab in clinical practice. Health Sci Rep 2(4):e114
Li Y, Ali S, Clarke J et al (2017) Bevacizumab in recurrent glioma: patterns of treatment failure and implications. Brain Tumor Res Treat 5(1):1–9
Ranjan S, Skorupan N, Ye X et al (2020) Patterns of bevacizumab use in patients with glioblastoma: an online survey among experts in neuro-oncology. Neurooncol Pract 7(1):52–58
Nagpal S, Harsh G, Recht L (2011) Bevacizumab improves quality of life in patients with recurrent glioblastoma. Chemother Res Practice 2011:1–6
Torres IJ, Mundt AJ, Sweeney PJ et al (2003) A longitudinal neuropsychological study of partial brain radiation in adults with brain tumors. Neurology 60(7):1113–1118
Brown PD, Jensen AW, Felten SJ et al (2006) Detrimental effects of tumor progression on cognitive function of patients with high-grade glioma. J Clin Oncol 24(34):5427–5433
Chinot OL, Wick W, Mason W et al (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370(8):709–722
Gilbert MR, Dignam JJ, Armstrong TS et al (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370(8):699–708
Sharma A, Low J, Mrugala MM (2019) Neuro-oncologists have spoken—the role of bevacizumab in the inpatient setting. A clinical and economic conundrum. Neurooncol Pract 6(1):30–36
Allegra CJ, Yothers G, O’Connell MJ et al (2013) Bevacizumab in stage II-III colon cancer: 5-year update of the National Surgical Adjuvant Breast and Bowel Project C-08 trial. J Clin Oncol 31(3):359
Herrlinger U, Schäfer N, Steinbach JP et al (2016) Bevacizumab plus irinotecan versus temozolomide in newly diagnosed O6-methylguanine–DNA methyltransferase nonmethylated glioblastoma: the randomized GLARIUS trial. J Clin Oncol 34(14):1611–1619
Boxerman JL, Zhang Z, Safriel Y et al (2013) Early post-bevacizumab progression on contrast-enhanced MRI as a prognostic marker for overall survival in recurrent glioblastoma: results from the ACRIN 6677/RTOG 0625 Central Reader Study. Neuro Oncol 15(7):945–954
Arevalo OD, Soto C, Rabiei P et al (2019) Assessment of glioblastoma response in the era of bevacizumab: longstanding and emergent challenges in the imaging evaluation of pseudoresponse. Front Neurol 10:460
Kim WY, Lee HY (2009) Brain angiogenesis in developmental and pathological processes: mechanism and therapeutic intervention in brain tumors. FEBS J 276(17):4653–4664
Gatto L, Franceschi E, Tosoni A et al (2021) Distinct MRI pattern of “pseudoresponse” in recurrent glioblastoma multiforme treated with regorafenib: Case report and literature review. Clin Case Reports 9(8):e04604
Österlund P, Soveri L, Isoniemi H et al (2011) Hypertension and overall survival in metastatic colorectal cancer patients treated with bevacizumab-containing chemotherapy. Br J Cancer 104(4):599–604
Lombardi G, De Salvo GL, Brandes AA et al (2019) Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol 20(1):110–119
Zhong J, Ali AN, Voloschin AD et al (2015) Bevacizumab-induced hypertension is a predictive marker for improved outcomes in patients with recurrent glioblastoma treated with bevacizumab. Cancer 121(9):1456–1462
Carvalho B, Lopes RG, Linhares P et al (2020) Hypertension and proteinuria as clinical biomarkers of response to bevacizumab in glioblastoma patients. J Neurooncol 147(1):109–116
Rahbar A, Cederarv M, Wolmer-Solberg N et al (2016) Enhanced neutrophil activity is associated with shorter time to tumor progression in glioblastoma patients. Oncoimmunology 5(2):e1075693
Bambury R, Teo M, Power D et al (2013) The association of pre-treatment neutrophil to lymphocyte ratio with overall survival in patients with glioblastoma multiforme. J Neurooncol 114(1):149–154
Massara M, Persico P, Bonavita O et al (2017) Neutrophils in gliomas. Front Immunol 8:1349
Tecchio C, Cassatella MA (2014) Neutrophil-derived cytokines involved in physiological and pathological angiogenesis. Angiogenesis, Lymphangiogenesis Clin Implic 99:123–137
Bertaut A, Truntzer C, Madkouri R et al (2016) Blood baseline neutrophil count predicts bevacizumab efficacy in glioblastoma. Oncotarget 7(43):70948
Trifiletti DM, Alonso C, Grover S et al (2017) Prognostic implications of extent of resection in glioblastoma: analysis from a large database. World Neurosurg 103:330–340
Gittleman H, Lim D, Kattan MW et al (2017) An independently validated nomogram for individualized estimation of survival among patients with newly diagnosed glioblastoma: NRG Oncology RTOG 0525 and 0825. Neuro Oncol 19(5):669–677
Chaichana KL, Jusue-Torres I, Lemos AM et al (2014) The butterfly effect on glioblastoma: is volumetric extent of resection more effective than biopsy for these tumors? J Neurooncol 120(3):625–634
Puhalla S, Elmquist W, Freyer D et al (2015) Unsanctifying the sanctuary: challenges and opportunities with brain metastases. Neuro Oncol 17(5):639–651
Louveau A, Smirnov I, Keyes TJ et al (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523(7560):337–341
Weenink B, French PJ, Sillevis Smitt PA et al (2020) Immunotherapy in glioblastoma: current shortcomings and future perspectives. Cancers 12(3):751
Yu MW, Quail DF (2021) Immunotherapy for glioblastoma: current progress and challenges. Front Immunol 12:676301
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
CS wrote the article under the supervision of SD. ER carried out the statistical analysis. FD reviewed the article. All the other authors had an equal contribution. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Ethical approval
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the Gustave Roussy Cancer Center.
Informed consent
Patient consent was waived due to the death of the patients. We sent a letter to the families of the patients to inform them of this study.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Smolenschi, C., Rassy, E., Pallud, J. et al. Bevacizumab in real-life patients with recurrent glioblastoma: benefit or futility?. J Neurol 270, 2702–2714 (2023). https://doi.org/10.1007/s00415-023-11600-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11600-w