Abstract
Background
Nausea and vomiting is a very prevalent condition during pregnancy. Combination of doxylamine and pyridoxine is placed as first-line pharmacological option for its treatment in most clinical guidelines. Among different release forms available, Cariban® is a fixed-dose combination of doxylamine/pyridoxine 10/10 mg, formulated as modified-release capsules.
Objectives
In the present study, we aimed to characterize the bioavailability performance of Cariban® in vitro and in vivo.
Methods
An in vitro dissolution test was performed to evaluate the release profile of Cariban®, together with immediate- and delayed-release formulations available on the market. A single-center, single-dose, open-label bioavailability study following Cariban® administration in 12 healthy adult female patients was carried out to explore the drug behavior in vivo (protocol NBR-002-13; EUDRA-CT 2013-005422-35). These data were additionally used to perform a computational pharmacokinetic simulation of the posology approved for this drug.
Results
Cariban® capsules demonstrate a prolonged-release performance, with an early, gradual, and progressive release of both actives until reaching a complete dissolution after 4–5 h in solution. The pharmacokinetic features of these capsules show that doxylamine and pyridoxine metabolites are early absorbed, being all detectable in plasma within 1 h following oral administration. Computational pharmacokinetic simulation predicts that different posology provides distinct profiles of metabolites in plasma, with 1–1–2 (morning–midafternoon–night) being the one that concentrates higher plasma levels but lower dose dumping for 24 h.
Conclusion
Cariban® behaves as a prolonged-release formulation, which correlates with rapid absorption and arising of the actives in the plasma, but also long-lasting and sustained bioavailability, especially when administered following the complete posology. These results would underlie its demonstrated efficacy to relieve nausea and vomiting of pregnancy (NVP) under clinical settings.
Similar content being viewed by others
Cariban® formulation provides early and progressive release and rapid absorption of doxylamine/pyridoxine. |
1–1–2 posology provides the most increased and sustained plasma concentration. |
1 Introduction
Nausea and vomiting of pregnancy (NVP) is a very common disorder reported in 50–90% of all pregnant women [1]. The usual onset for NVP is between 4 weeks and 9 weeks gestational age with maximal symptomatology at 12–15 weeks that usually subside by week 20. A small percentage of women suffer from NVP during the whole pregnancy [1]. Despite being classically termed as “morning sickness,” up to 80% of women suffering NVP experience symptoms throughout the day [2], with a peak of nausea and vomiting in the morning hours [2, 3].
The severity of NVP symptoms ranges from mild-to-severe nausea and vomiting to an extreme complication termed as hyperemesis gravidarum, which usually requires hospitalization [1]. Women with NVP are more likely to develop pregnancy complications, including pelvic girdle pain, proteinuria, high blood pressure, or preeclampsia [4, 5]. Despite a frequent normalization of the pathology, different studies have demonstrated that NVP has a noteworthy impact on the quality of life (QoL) of pregnant women, particularly in those experiencing more severe symptoms [6,7,8]. Effect on QoL includes negative impact on employment, household duties, parenting, partnership, social relationships, increased feelings of depression, and considering termination of pregnancy [9, 10]. Finally, NVP results in a significant impact on healthcare resources; overall economic burden was estimated at more than $1.7 billion annually in the USA, including direct (e.g., medical care, hospitalization) and indirect costs (e.g., work loss, caregiver time) [11, 12]. All these data suggest that NVP-associated morbidity, healthcare, and economic burden are significant; however, nowadays NVP is still a condition that tends to be overlooked and under-treated [8, 13].
According to symptoms, the early management of NVP pursues the following goals: reducing the incidence, severity, and impact of symptomatology; reducing progression to more severe NVP; correcting complications; and minimizing the effects of the treatment on the fetus [14]. Treatment with doxylamine and pyridoxine (vitamin B6) has been widely studied for the management of NVP [15]. Doxylamine exerts its effects by competitively antagonizing the binding of histamine at the H1-receptor, reducing the stimulation of the vomiting center [16]. Vitamin B6 is the collective term for a group of three related compounds, pyridoxine, pyridoxal, and pyridoxamine, and their phosphorylated derivatives, with pyridoxal 5′-phosphate being considered the most biological active metabolite [17]. The antiemetic effect of vitamin B6 has been documented since 1942, however, the underlying mechanisms have not been completely elucidated [18]. Several randomized placebo-controlled trials, as well as observational studies, have demonstrated the early and sustained efficacy of the fixed-dose combination of doxylamine/pyridoxine 10/10 mg in reducing NVP [15, 19, 20], as it is safe and well tolerated both by mothers and fetuses [15, 21, 22]. On that basis, combined treatment of doxylamine and pyridoxine is placed as a first-line pharmacologic option for the management of NVP clinical guidelines in the USA, Canada, the UK, or Spain, among others [23,24,25,26].
Cariban® is a fixed-dose combination of doxylamine succinate/pyridoxine hydrochloride 10/10 mg, formulated as modified-release hard capsules containing two types of pellets, one per active ingredient (Fig. 1) [27]. Cariban® was first introduced in Spain in 1967 [27], and approved in many other European countries such as Austria, Germany, Belgium, Ireland, and Italy, among others, under the names Nuperal®, Navalit®, or Cariban® from 2017 onward [28]. Therefore, it accounts for more than 50 years of clinical experience within healthcare professionals in Europe. Accounting for this vast experience, more than 21.5 million packs (containing 24 and 48 capsules) have been provided to pregnant women in all of Europe since 1983, i.e., > 500 million doses, with more than 430 million doses of Cariban® delivered only in Spain in this period [29].
Cariban® posology permits a gradual dose titration to assist the persistence of symptoms during the day. According to the summary of product characteristics (SmPC), adoption of maximal posology (four capsules, administered in a 1–1–2 morning–midafternoon–night pattern) would take place during the first 4 days of drug administration as follows: days 1 and 2, two capsules at bedtime (0–0–2); day 3, adding an additional capsule in the morning (1–0–2); day 4, adding an additional capsule in the midafternoon (1–1–2) [27]. Therefore, there is an adaptable and flexible dosage ranging from two to four capsules per day according to the timing, duration, severity, and frequency of the symptoms experienced by each pregnant woman.
Regarding pharmacological properties, modified-release formulations encompass different types of release performances, including delayed- and prolonged-release forms [30]. Delayed-release forms delivers a drug with a specific delay after its administration, but the subsequent release is similar to an immediate form [30]. Conversely, prolonged or sustained-release formulations are developed to release the drug gradually during a certain period of time [30]. However, an important challenge in the characterization of these formulations is the prediction of the in vivo behavior based on in vitro properties [31].
To date, in contrast with other types of doxylamine and pyridoxine formulations, the bioavailability of fixed-dose modified-release capsules is not deciphered and available in the literature. This includes the description of early drug performance, in other words, data on the release and absorption of actives. Description of drug dynamics following different posologies or drug titration is also lacking for any doxylamine/pyridoxine combination. In the present study, we fully characterized the in vitro dissolution profile of Cariban® modified-release capsules and assessed its pharmacokinetic profile in vivo.
2 Methods
2.1 In Vitro Dissolution Testing
The dissolution profiles were performed with samples of Benadon® 300 mg film-coated immediate-release tablets (pyridoxine hydrochloride) (Teofarma, Lot No. 025, exp. 10/2024), Dormidina® 25 mg film-coated immediate-release tablets (doxylamine succinate) (Esteve, Lot No. 212131, exp. 11/2024), Xonvea® 10 mg/10 mg delayed-release tablets (pyridoxine hydrochloride/doxylamine succinate) (Exeltis Healthcare, Lot 1575V-3, exp. 06/2025), and Cariban® 10 mg/10 mg modified-release hard-gelatin capsules (pyridoxine hydrochloride/doxylamine Succinate) (Effik, Navalit® Lot No. T24, exp. 03/2024), purchased in 2022 in pharmacies, kept in laboratory environmental conditions, and analyzed within their expiry dates. A single unit was defined according to the leaflet of each product, corresponding to a half-tablet for Benadon®, a tablet for Dormidina® and Xonvea®, and a capsule for Cariban®/Navalit®. The assay was performed with six units per product.
The dissolution media used in the assays included an acidic media (HCl pH 1.2) and a neutral media (phosphate buffer pH 7.0). The dissolution was performed for 3 h in acidic pH followed by 4 h at neutral pH to mimic pH and transit times as described elsewhere [32].
All tests were performed in an Agilent 708-DS apparatus, according to the following conditions:
Medium temperature | 37 ± 0.5 °C |
Volume | 900 ml |
Apparatus | Paddles (USP type II) |
Rotation speed | 100 rpm |
Sampling volume | 5 ml |
Samples from the dissolution media were automatically extracted hourly and the extracted volume was replaced each time with fresh media, keeping the total volume of the vessel constant. Doxylamine and pyridoxine content release was measured by Reverse Phase High-Performance Liquid Chromatography–Photodiode Array Detector (RP-HPLC/DAD).
The percentage of dissolved active substances was calculated from the concentration of the samples at each time point over the theoretical content of actives per formulation. Summary statistics are presented as mean and standard deviation (SD).
2.2 Pharmacokinetic Study
2.2.1 Study Design and Patients
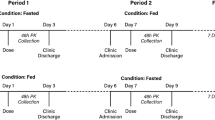
This was a single-center, randomized, single-dose, open-label, two-way crossover relative bioavailability study of Doxylamine/Pyridoxine 10 mg/10 mg (Cariban® hard capsules) following a 2 × 10 mg/10 mg dose in healthy adult non-pregnant females under fasting and fed conditions (Protocol NBR-002-13; EUDRA-CT 2013-005422-35). Treatment periods were separated by a washout period of at least 18 days. Two capsules of Cariban® were used as the recommended evening dose [27]. Data were blinded to the analytical facility. For the purpose of this manuscript, only data obtained under fasted conditions are presented, according to SmPC administration instructions [27].
The study protocol was approved by the Independent Ethics Committee on Clinical Research of the Hospital Universitario La Paz in January 2014. The study was conducted and data were processed in accordance with procedures of UAM Clinical Trials Unit, Spanish legislation, the International Council on Harmonization (ICH) Guidelines on Good Clinical Practice E6 (R2) [33], and the Revised Declaration of Helsinki [34].
All volunteers signed an informed consent prior to initiation of study procedures. Healthy non-pregnant women of childbearing potential aged 18–35 years old were included. Main exclusion criteria were body mass indices < 18.5 kg/m2 or > 30 kg/m2, suffering from physical or psychic pathologies or any analytical abnormalities, pregnancy, or breastfeeding status, prior systemic pharmacological treatment (within 30 days) or potential interfering over-the-counter medication, smoking, daily alcohol consumption, positive alcohol, or drug testing, and having donated blood in the previous 3 months.
No concomitant pharmacological treatments were allowed, except those without any known interactions with doxylamine and pyridoxine, and oral contraceptives. Subject screening procedures included informed consent, inclusion/exclusion criteria, medical history, demographic data, physical examination and vital signs, 12-lead ECG, clinical laboratory tests such as hematology, biochemistry, urinalysis, and serology, alcohol and drug testing, and urine pregnancy test.
Volunteers received two capsules of Cariban® for a total dose of doxylamine succinate/pyridoxine hydrochloride 20 mg/20 mg with 170 mL of water. Fluid intake was not allowed 1 h before and 1 h after drug administration. No food intake was allowed for at least 4 h post-dose. All blood samples were collected at the following timepoints: within 30 min, 15 min, and 0 min predose (baseline) and 0.25 h, 0.50 h, 0.75 h, 1.00 h, 1.50 h, 2.00 h, 2.33 h, 2.66 h, 3.00 h, 3.33 h, 3.66 h, 4.00 h, 4.50 h, 5.00 h, 5.50 h, 6.00 h, 8.00 h, 12.0 h, 24.0 h, 48.0 h, 72.0 h, 144.0 h, 240.0 h, 360.0 h and 432.0 h post-dose.
Venous blood samples (6 mL) were taken from a peripheral vein of the forearm using an indwelling catheter. Blood samples were collected in EDTA-containing tubes, centrifuged at 3000 rpm for 10 min at 4 °C to obtain plasma, and samples were stored frozen at −20 °C (doxylamine metabolites) or −80 °C (pyridoxine metabolites), until shipment to the analytical facility (Anapharm Europe, SLU) for determination. Quantification limits of the analytical method are as follows: doxylamine enantiomers, 0.25 ng/mL; pyridoxine, 0.2 ng/mL; pyridoxal, 1 ng/ml; pyridoxal 5′-phosphate, 0.5 ng/mL. Concentrations below these limits were considered as zero for analysis.
Safety analyses included assessment of all adverse events, which were individually examined to evaluate their onset, duration, severity, intensity, causality, and outcome. All subjects performed a follow-up visit in which they were subjected to a safety analysis.
2.2.2 Pharmacokinetic Analysis
The following pharmacokinetic values were calculated by standard non-compartmental methods for doxylamine and pyridoxine metabolites: Cmax, Tmax, Tfirst, Tlast, AUC0–t, AUC0–∞, and t1/2el, using a model-independent approach with the software WinNonlin Pro 2.0 (Pharsight Corporation, Cary, USA). For pyridoxal 5′-phosphate, basal concentrations were subtracted.
Summary statistics are presented as median (min–max range) or mean (SD), according to normal distribution.
2.2.3 Pharmacokinetic Simulation
A simulation of a 24-h PK profile of Cariban® 10/10 mg was performed for each analyte of interest on the basis of nonparametric superposition methodology, using Phoenix® WinNonlin® 8.2 (Certara USA Inc, Princeton, NJ) by BlueClinical Ltd (Porto, Portugal) for 104 h.
Nonparametric superposition is used to predict drug concentrations after multiple dosing at steady state, based on non-compartmental results describing single-dose data and not assuming any PK model [35]. For this simulation, the data from the single-dose pharmacokinetic study described above was used.
The simulation was carried out with the gradual dose titration defined in Cariban® posology [27]. Time 0 h represents the first two capsules taken at day 1 (bedtime). Simulation was performed until reaching the maximum recommended daily dose of four capsules distributed according to 8-h dosing interval: one capsule in the morning, one capsule at midafternoon, and two capsules at bedtime.
3 Results
3.1 In Vitro Dissolution Testing
Doxylamine and pyridoxine are both reported as being absorbed along the gastrointestinal tract, mainly in the jejunum [36]. To reproduce the potential gastrointestinal transit experience of Cariban® in vitro, a 7-h dissolution profile at different pH conditions was performed. The profile was designed to mimic the transit time reported in women [32] and therefore be close to the real-life patient receiving this drug. The profile consists of 3-h dissolution in acidic media, mimicking stomach conditions, followed by 4 h in neutral media, to simulate the small intestine environment. The dissolution profile of Cariban® was evaluated alongside with immediate- and delayed-release formulations (Fig. 2).
In vitro dissolution profile of different type of release forms. Seven-h in vitro dissolution profile of doxylamine (A) and pyridoxine (B) active substances from immediate- (pink line), delayed- (black line), and prolonged-release (Cariban®, blue line) formulations. Below the graphs is a picture of the gastrointestinal tract parallelly disposed to the pH conditions used in the test to illustrate the mimicked anatomical region: acidic pH for the stomach and neutral pH for small intestine. The mean value ± SD of the percentage of actives dissolved over time is shown
As expected, the immediate-release formulations of doxylamine and pyridoxine liberate most of their content during the first hours in acidic pH. Conversely in the delayed-release formulation, both actives remain undissolved in acidic pH, being fully released only in neutral conditions, during the first hour following the pH change. Instead, Cariban® capsules show a prolonged/ or extended-release profile: the formulation starts to liberate both actives since the very beginning of the dissolution test, being gradually released until they are completely dissolved at 4–5 h (Fig. 2).
3.1.1 Pharmacokinetic Study
Dynamics of drug absorption in vivo are hardly predictable for modified-release formulations. To understand how the extended release observed in vitro was translated into in vivo performance, the pharmacokinetics of doxylamine-, pyridoxine-, and pyridoxine-related metabolites were evaluated following oral administration of Cariban® capsules under fasting conditions.
Out of 45 healthy women assessed for eligibility, 12 were enrolled, received the study medication, and completed the study. Demographics and baseline characteristics are presented in Table 1.
Pharmacokinetics parameters following the administration of Cariban® are presented in Table 2 and shown in Fig. 3.
Plasma concentration–time curves for doxylamine and pyridoxine metabolites following Cariban® capsules administration under fasted conditions in healthy volunteers. A, B Full profile of plasmatic levels of doxylamine (A) and pyridoxine (B) for 432 h post-administration. C, D Data extracted from A and B showing the plasmatic level of doxylamine (C) and pyridoxine (D) zoomed on the first 10 h post-administration. Data are shown as mean concentration (ng/ml)/time (h)
AUC0–∞ of doxylamine R and S enantiomers was 936.32 ± 261.93 ng·h/ml (mean ± SD) and 936.45 ± 199.50 ng·h/ml, respectively, which would result in an overall extent of exposure over 1.800 ng·h/ml for doxylamine. Mean peak values (Cmax) of 47.30 ± 6.25 ng/ml (R form) and 43.78 ± 5.64 ng/ml (S form) were achieved at a median of 6.00–7.00 h, being 90.88 ± 11.54 ng/ml for the whole doxylamine metabolite (including both R- and S forms).
Interestingly, doxylamine was first detected (Tfirst) within 1 h following administration (median 45 min). This is in line with the profile observed in vitro, where about 30% of doxylamine was already dissolved in the first hour in acidic pH, which imitates the gastric compartment. Finally, the elimination half-life of doxylamine ranged between 10.84 h and 12.33 h, being undetectable after 72 h (Tlast).
Pyridoxine is a prodrug rapidly metabolized into different metabolites, pyridoxal and pyridoxal 5′-phosphate (PLP), with PLP being the main reported active metabolite [17]. Following oral administration, pyridoxine extent of exposure (AUC0–∞) was 59.14 ± 11.56 ng·h/ml. The mean peak value (Cmax) of 15.80 ± 2.96 ng/ml was achieved at a median of 4.00 h. Mean elimination half-life of pyridoxine was 1.90 ± 1.38 h, and was detectable up to a median of 8.00 h (Tlast). Pyridoxal extent of exposure (AUC0–∞) was 1003.75 ± 389.55 ng·h/ml, with a Cmax of 35.85 ± 9.51 ng/ml achieved at a median of 5.00 h.
In parallel, the extent of exposure (AUC0–∞) of the main metabolite PLP was 15,912.24 ± 19,983.31 ng·h/ml. The maximum concentration (Cmax) was 64.99 ± 45.17 ng/ml. Mean plasma concentration at 6 h post-dose was 37.34 ± 12.03 ng/ml.
Similarly to doxylamine, pyridoxine and its metabolites were already detectable (Tfirst) within the first 30 min following Cariban® administration. Also in this case, this result correlates with the in vitro profile, where about 17% of pyridoxine was found dissolved already within the first hour in acidic pH.
In terms of safety, no treatment-emergent adverse events were reported.
3.1.2 Pharmacokinetic Simulation of Drug Posology
On the basis of Cariban® SmPC, daily dosage ranges from two to four capsules, depending on symptom persistence, with adoption of maximal posology (1–1–2 capsules at morning–midafternoon–night) by day 4 [27]. To better understand the pharmacokinetics during this gradual scale up, we performed a pharmacokinetic simulation based on the above-mentioned in vivo pharmacokinetic data (Table 2 and Fig. 3).
According to the computational simulation, the continued treatment and the gradual increase of the dose produce an accumulation of doxylamine, pyridoxal, and PLP (Fig. 4). In fact, the concentration of doxylamine in plasma virtually achieves a steady state following a second complete administration of the 1–1–2 posology (Fig. 4A). Instead, pyridoxine plasma level tends to decay to baseline every 24 h (Fig. 4B), correlating with its fast elimination half-life. The pyridoxine metabolites, pyridoxal and PLP, accumulate overtime, as expected according to the longer half-life observed in vivo (Fig. 4C, D).
Predicted plasma concentration–time curves for doxylamine (A) and pyridoxine (B–D) metabolites following Cariban® administration posology. Simulation of the plasma concentrations of doxylamine enantiomers (A), pyridoxine (B), and the related metabolites pyridoxal (C) and pyridoxal 5′-phosphate (D), during the first 4 days following the posology instructions of the SmPC. The numbers in the graphs refer to the number of capsules taken at a given timepoint according to the progressive posology indicated in the SmPC. Shadowed boxes make up a whole-day (24 h) morning-to-night posology (i.e., capsules taken in the morning–afternoon–night). Data are shown as mean concentration (ng/ml)/time (h)
Overall, the simulation shows that for a 24-h window (morning–to–night), time reduction in the dosing interval to 8 h, which means following the 1–1–2 posology, leads to less-pronounced plasma level fluctuations or declines (Fig. 4). This posology (total daily dose 40/40 mg doxylamine/pyridoxine) also favored a higher daily increase of pyridoxal and PLP levels compared with reduced use of capsules (Fig. 4C, D).
4 Discussion
Cariban® is a fixed-dose combination of doxylamine/pyridoxine 10/10 mg, formulated as modified-release hard capsules, that has been available for the symptomatic treatment of NVP for more than 50 years. Nevertheless, dynamics of drug gastrointestinal dissolution and absorption had never been studied and presented in detail.
In the present study, we demonstrated that Cariban® presents a prolonged-release performance, with an early, gradual, and progressive liberation of both actives for 5 h until reaching complete dissolution. The release of the actives starts in acidic pH, suggesting their availability already at stomach level. There is barely probability of degradation in the acid environment of the stomach, as the stability of doxylamine and pyridoxine in acidic pH has been well established and reported [38,39,40,41]; furthermore, no significant degradation was observed in the Cariban® dissolution profile since the actives are fully recovered at the end of the assay. Both doxylamine and pyridoxine are absorbed along the gastrointestinal tract, mainly in the jejunum [16, 36]; therefore, early dissolution of doxylamine and pyridoxine opens the possibility to some absorption of the actives at the stomach and duodenal level [42], as described for similar prolonged-release formulations [43], as well as to speed up the absorption in the jejunum, as the actives reach the site already dissolved. The concept of a fast absorption derived from a fast release of the formulation is proven by the correlation between the in vitro dissolution and in vivo data that show appearance of the actives in plasma at early timepoints. Early onset of dissolution and absorption could be the result of the multiparticle formulation of Cariban®, and is in line with the European Medicines Agency (EMA), who concluded that modified-release forms that do not disintegrate in the stomach would result in a prolonged and highly erratic gastric emptying [30].
The extended dissolution profile of Cariban® lasts for at least 5 h, as designed in accordance with gastrointestinal transit time described in the literature [32, 43]. This means that doxylamine and pyridoxine are continuously released and available to be absorbed for a period of time after administration.
In contrast to immediate- and delayed-release forms, the extended dissolution profile of Cariban® produces a gradual and sustained release over time, thus avoiding dose dumping or high local concentration of actives. This advantage of extended-release formulations is recognized by the European Medicines Agency, since they offer several advantages over instant-release formats, including reduced plasma fluctuations, more continuous effects, and/or reduced side effects [30].
In fact, pellet-containing formulations such as Cariban® have been shown to provide many of these advantages in comparison with single-units: controlled-release technology, free dispersion in the GI tract and reduced gastric transit time, lowered dose dumping and peak plasma fluctuations, and decreased intra- and inter-variability, thus maximizing drug absorption as well as minimizing potential side effects [43, 44]. This is of special interest at nighttime, when extended gastric residence time of single units has been reported [43].
Cariban® pharmacokinetic features under fasting conditions show that both doxylamine and pyridoxine metabolites are early absorbed and detected in plasma. Pyridoxal and PLP are also quickly detected, suggesting a rapid metabolization of pyridoxine, with long-lasting presence in plasma. Thus, all actives and related metabolites are detectable within the first hour following administration, indicating that early dissolution tightly correlates with fast absorption. This fact would further reinforce the hypothesis of absorption of the actives at the stomach level.
Pharmacokinetic analysis also demonstrates a high and sustained bioavailability of doxylamine and pyridoxine metabolites following Cariban® administration. This prolonged bioavailability further corroborates the stability of doxylamine and pyridoxine to exert sustained antiemetic effects over time. In this sense, PLP seems to be produced in different waves over time, and PK data could hint a further upstream trend after 400 h of drug administration. Compared with single-unit formulations, a longer residence time and spreading of pellets along the small intestine may also contribute to the high drug bioavailability observed [43]. Future comparative pharmacokinetic studies among different types of release formulations and galenic forms would be of outstanding interest.
The pharmacokinetic simulation exercise predicted profiles for doxylamine and pyridoxine metabolites following the SmPC dose titration. First, the different posology alternatives (0–0–2 to 1–1–2) provide distinct profiles in plasma. This suggests that posology might be flexibly adapted to concentrate plasma peaks on the basis of the need of each patient and presence of symptoms at different times of the day. In other words, the flexibility offered by Cariban® (10/10 mg) posology adapts to different patient needs according to their manifestations of nausea and vomiting.
Tmax values for doxylamine and pyridoxine and simulated administration of capsules indicate maximal presence in plasma by 4–6 h. This in turn would suggest that maximal antiemetic effect will occur 6–8 h after administration. Thus, the dosing schedule of this drug is important to ensure onset of action occurs when NVP symptoms are at their peak. A bedtime dose would be effective in the early morning, a morning dose would be effective around afternoon, and a mid-afternoon dose would be effective in the evening. Taking the medication before sleep (when symptoms are typically minimal or absent) is important to obtain relief in the morning when symptoms are maximal [3]. These data would preclude the use of this antiemetic formulation with the only purpose of immediate alleviation of symptoms, but would reinforce the need of a continued treatment to maximize efficacy. In addition, administration of two capsules led to the highest accumulation of metabolites; therefore administration of two capsules at bedtime is desirable for patients experiencing increased presence of symptoms in the morning [3].
Cariban® administered at a 1–1–2 posology provides the most sustained plasma concentration of any of the metabolites and reduced 24-h fluctuations, which may be desirable to control the symptoms in a patient experiencing nausea and vomiting throughout the whole day. Conversely, other posology alternatives lead to a drop of doxylamine and pyridoxine levels during the day. As far as we know, this pharmacokinetic profile (getting 24-h maintained plasma concentration curves) has not been shown for other formulations or strengths. Altogether, the Cariban® posology 1–1–2 (10–10–20 mg; three daily administrations) seems to better adapt to pathophysiology of NVP, which occurs throughout the day in up to 80% of cases, with increased intensity in the morning hours [2, 3].
Dose titration up to four capsules in a 1–1–2 posology also allows for the increased accumulation of plasma metabolites and was needed to reach the steady state following several administrations. In terms of plasma accumulation, similar results have been provided by other doxylamine/pyridoxine-containing formulations [18, 45]. This fact supports the need for continued treatment, rather than on-demand intake of this drug, to maximize efficacy. In this regard, women with NVP that scaled up their treatment with doxylamine/pyridoxine to an average of four daily units (40/40 mg) significantly reduced their symptomatology compared with their status with lower doses [46]. Maximizing efficacy is very important for the management of NVP, as symptom reduction has been shown to be a determinant for adherence to doxylamine/pyridoxine treatment [47]. Altogether, a complete posology (1–1–2) [27] might better ensure the 24-hour control of NVP symptoms, thus maximizing efficacy and adherence to the treatment.
Of note, no treatment-adverse events were reported in this clinical study. This further reinforces the excellent safety profile documented for this fixed-dose combination of doxylamine/pyridoxine (10/10 mg) in numerous clinical studies [15, 21, 22] and extensive clinical experience gained over decades. The overall safety of Cariban® is confirmed by its global use for decades. In the period 2013–2022, with over 7.7 million packs provided to women with NVP, a 0.003% rate of adverse reactions per Cariban® pack has been found (data on file, individual case safety reports, Corporate Pharmacovigilance Department, Italfarmaco Research Group). Altogether, this vast safety information should also serve as support for healthcare professionals and patients who are still reluctant to prescribe or receive this pharmacological treatment [8, 13].
5 Conclusions
The present study describes for the first time the drug bioavailability of Cariban® modified-release capsules, a fixed-dose combination of doxylamine/pyridoxine indicated for the management of NVP, with particular focus on early stages of drug release and absorption. Our results show that Cariban® behaves as a prolonged-release formulation, with an early, gradual, and progressive release of both drugs starting from the very beginning following oral administration until reaching complete dissolution. This sustained release tightly correlates with a rapid absorption of actives and prompt presence of metabolites in the plasma of women following oral administration, with overall high drug bioavailability. The administration of four Cariban® 10/10 mg hard capsules in a 1–1–2 posology is expected to provide the most increased and sustained plasma concentration, with reduced fluctuations of the actives. This unique early, constant, and long-lasting kinetics further supports this modified-release, hard-capsule, pellet-based Cariban® formulation, successfully used for more than 50 years to relieve nausea and vomiting of pregnant women in the clinical practice. Uncovering plasma profiles of different administration schedules may help physicians to better understand the dynamics of this drug and to improve prescriptions in real-life clinical settings, according to patients’ symptoms.
References
Clark SM, Costantine MM, Hankins GD. Review of NVP and HG and early pharmacotherapeutic intervention. Obstet Gynecol Int. 2012;2012: 252676.
Liu C, et al. Emerging progress in nausea and vomiting of pregnancy and hyperemesis gravidarum: challenges and opportunities. Front Med (Lausanne). 2021;8: 809270.
Gadsby R, et al. Nausea and vomiting in pregnancy is not just ‘morning sickness’: data from a prospective cohort study in the UK. Br J Gen Pract. 2020;70(697):e534–9.
Chortatos A, et al. Pregnancy complications and birth outcomes among women experiencing nausea only or nausea and vomiting during pregnancy in the Norwegian Mother and Child Cohort Study. BMC Pregnancy Childbirth. 2015;15:138.
Temming L, et al. Adverse pregnancy outcomes in women with nausea and vomiting of pregnancy. J Matern Fetal Neonatal Med. 2014;27(1):84–8.
Lacasse A, Berard A. Validation of the nausea and vomiting of pregnancy specific health related quality of life questionnaire. Health Qual Life Outcomes. 2008;6:32.
Balikova MBR. Quality of women’s life with nausea and vomiting during pregnancy. Ošetř Porod Asist. 2014;5(1):29–35.
Tan A, Lowe S, Henry A. Nausea and vomiting of pregnancy: effects on quality of life and day-to-day function. Aust N Z J Obstet Gynaecol. 2018;58(3):278–90.
Mazzotta P, et al. Attitudes, management and consequences of nausea and vomiting of pregnancy in the United States and Canada. Int J Gynaecol Obstet. 2000;70(3):359–65.
Heitmann K, et al. The burden of nausea and vomiting during pregnancy: severe impacts on quality of life, daily life functioning and willingness to become pregnant again - results from a cross-sectional study. BMC Pregnancy Childbirth. 2017;17(1):75.
Piwko C, et al. Economic burden of nausea and vomiting of pregnancy in the USA. J Popul Ther Clin Pharmacol. 2013;20(2):e149–60.
Gadsby R, et al. Nausea and vomiting of pregnancy and resource implications: the NVP Impact Study. Br J Gen Pract. 2019;69(680):e217–23.
Heitmann K, et al. Nausea in pregnancy: attitudes among pregnant women and general practitioners on treatment and pregnancy care. Scand J Prim Health Care. 2016;34(1):13–20.
Niebyl JR, Briggs GG. The pharmacologic management of nausea and vomiting of pregnancy. J Fam Pract. 2014;63(2 Suppl):S31–7.
Madjunkova S, Maltepe C, Koren G. The delayed-release combination of doxylamine and pyridoxine (Diclegis(R)/Diclectin (R)) for the treatment of nausea and vomiting of pregnancy. Paediatr Drugs. 2014;16(3):199–211.
Brott NRRA. Doxylamine, ed. StatPearls. 2022, FL: Treasure Island.
Merrill AH Jr, Henderson JM. Vitamin B6 metabolism by human liver. Ann N Y Acad Sci. 1990;585:110–7.
Matok I, et al. Studying the antiemetic effect of vitamin B6 for morning sickness: pyridoxine and pyridoxal are prodrugs. J Clin Pharmacol. 2014;54(12):1429–33.
Koren G, et al. Effectiveness of delayed-release doxylamine and pyridoxine for nausea and vomiting of pregnancy: a randomized placebo controlled trial. Am J Obstet Gynecol. 2010;203(6):571 e1-571 e7.
Zhang R, Persaud N. 8-Way randomized controlled trial of doxylamine, pyridoxine and dicyclomine for nausea and vomiting during pregnancy: restoration of unpublished information. PLoS ONE. 2017;12(1): e0167609.
Boskovic R, et al. Is lack of morning sickness teratogenic? A prospective controlled study. Birth Defects Res A Clin Mol Teratol. 2004;70(8):528–30.
Koren G, et al. Maternal safety of the delayed-release doxylamine and pyridoxine combination for nausea and vomiting of pregnancy; a randomized placebo controlled trial. BMC Pregnancy Childbirth. 2015;15:59.
RCOG. The management of nausea and vomiting of pregnancy and hyperemesis gravidarum (Green-top Guideline No. 69). 2016: London.
SEGO. Documentos de consenso SEGO. Hiperémesis gravídica. 2008.
Campbell K, et al. The management of nausea and vomiting of pregnancy. J Obstet Gynaecol Can. 2016;38(12):1127–37.
ACOG. ACOG Practice Bulletin No. 189: Nausea And Vomiting Of Pregnancy. Obstet Gynecol 2018;131(1): e15–e30.
Inibsa Ginecología, S.A., Summary of Product Characteristics. Cariban 10 mg/10 mg modified-release hard capsules. 2020: Spain.
DataBase, E.U.H.A.o.M. Navalem 10 mg/10 mg modified release capsules, hard (ES/H/0343/001). 2022; https://www.drugfuture.com/hma/drugview.aspx. Accessed 13 Feb 2023.
IQVIA, Market data audit sell-in retail 1983-2022. 2022.
Committee for Medicinal Products for Human Use, E.M.A.E., Guideline on the pharmacokinetic and clinical evaluation of modified release dosage forms. 2014.
Heigoldt U, et al. Predicting in vivo absorption behavior of oral modified release dosage forms containing pH-dependent poorly soluble drugs using a novel pH-adjusted biphasic in vitro dissolution test. Eur J Pharm Biopharm. 2010;76(1):105–11.
Wang YT, et al. Regional gastrointestinal transit and pH studied in 215 healthy volunteers using the wireless motility capsule: influence of age, gender, study country and testing protocol. Aliment Pharmacol Ther. 2015;42(6):761–72.
Committee for Medicinal Products for Human Use, E.M.A.E., Guideline for good clinical practice E6(R2). 2017.
W.M.A., WMA Declaration of Helsinki – Ethical principles for medical research involving human subjects. Updated 2018 July.
Library, P.A. NonParametric Superposition. 2022. https://onlinehelp.certara.com/phoenix/8.2/topics/nonparasuper.htm.
Cada DJ, et al. Doxylamine succinate/pyridoxine hydrochloride. Hosp Pharm. 2013;48(9):762–6.
National Center for Biotechnology Information. PubChem Compound Summary for CID 1054, Pyridoxine. 2022. https://pubchem.ncbi.nlm.nih.gov/compound/Pyridoxine.
Harde MT, Lakade SH. A stability-indicating HPLC method for estimation of doxylamine succinate in tablets and characterization of its major alkaline stress degradation product. Fut J Pharm Sci. 2021;7:137.
And C. Stability of three forms of vitamin B6 to laboratory light conditions. J Assoc Off Anal Chem. 1979;62:1170–3.
Yessaad M, Bernard L, Bourdeaux D, Chennell P, Sautou V. Development of a stability indicating method for simultaneous analysis of five water-soluble vitamins by liquid chromatography. Pharm Technol Hosp Pharm. 2018;3:207–18.
Hochberg M, Oser B. On the stability of pyridoxine. JBC. 1944;155:129–36.
Yartsev A. Gastric drug absorption. In: Deranged Physiology. 2017.
Varum FJ, Merchant HA, Basit AW. Oral modified-release formulations in motion: the relationship between gastrointestinal transit and drug absorption. Int J Pharm. 2010;395(1–2):26–36.
Prabhakaran L, P.M., Sriganesan P, Pharmaceutical micropellets: an overview. Pharm Rev. 2009;7(4).
Koren G, V.M. Treating symptoms of morning sickness: the first dual release combination of doxylamine-pyridoxine. Int J Pharm. 2018;8(3):52–58.
Boskovic R, et al. Diclectin therapy for nausea and vomiting of pregnancy: effects of optimal dosing. J Obstet Gynaecol Can. 2003;25(10):830–3.
Costantine MM, et al. Determinants of adherence to delayed-release doxylamine and pyridoxine in patients with nausea and vomiting of pregnancy. Ther Drug Monit. 2012;34(5):569–73.
Acknowledgements
We thank Blue Clinical Ltd, for the pharmacokinetic simulation carried out in the present work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contributions
KU and LZ-D designed and performed the in vitro dissolution testing and analyzed data. JF, PG, MS-V, RR-J, and JN-T were involved in the design and performance of the pharmacokinetic study; PG analyzed the data and wrote the clinical study report. KU, LZ-D, TV, PS-L, and EG-A contributed to data interpretation and to the writing of the manuscript. All authors contributed to the interpretation of study data and critically reviewed and approved the final version of the manuscript.
Conflict of Interests
PS-L, LZ-D, EG-A, and TV are employees of ITF Research Pharma S.L.U. MS-V, RR-J and JN-T are employees of Inibsa Ginecologia S.A.
Funding
This study was funded by ITF Research Pharma S.L.U. and Inibsa Ginecologia S.A.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Ethical approval
The study protocol was approved by the Independent Ethics Committee on Clinical Research of the Hospital Universitario La Paz in January 2014.
Consent to participate
All volunteers signed an informed consent prior to initiation of study procedures.
Consent for publication
All authors gave consent for publication.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Saz-Leal, P., Zamorano-Domínguez, L., Frías, J. et al. Bioavailability of Cariban® Capsules: A Modified-Release Fixed-Dose Combination of Doxylamine and Pyridoxine to Relieve Nausea and Vomiting During Pregnancy. Drugs R D 23, 185–195 (2023). https://doi.org/10.1007/s40268-023-00425-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-023-00425-7