Abstract
Background
Heart failure with preserved ejection fraction (HFpEF) is common in elderly people and is increasing in prevalence. No specific treatment for this condition exists. Coenzyme Q10 (CoQ10) is an essential cofactor for energy production, with reduced levels being noted in HF. Previous studies have suggested a possible role for CoQ10 in the treatment of HF. This study examined the effect of CoQ10 supplementation on diastolic function in HFpEF patients.
Methods
We conducted a prospective, randomized, double-blind, placebo-controlled trial including patients aged > 55 years presenting with New York Heart Association class II–IV heart failure symptoms and left ventricular ejection fraction > 50%, with impaired diastolic function. Echocardiography and levels of serum N-terminal pro-B-type natriuretic peptide (NT-proBNP) were performed at baseline and following 4 months of CoQ10 or placebo supplementation.
Results
A total of 39 patients were enrolled—19 in the CoQ10 group and 20 in the placebo group. Baseline clinical characteristics were similar between groups, while compliance was high and also similar between the CoQ10 and placebo groups. There was no significant effect of treatment on indices of diastolic function (difference in the lateral E/e' ratio: −0.86 ± 6.57 in the CoQ10 group, +0.18 ± 3.76 in the placebo group; p = 0.561) or on serum NT-proBNP levels (− 72 pg/mL vs. − 42 pg/mL; p = 0.195).
Conclusions
In this pilot trial in elderly patients with HFpEF, treatment with CoQ10 did not significantly affect echocardiographic indices of diastolic function and serum NT-proBNP levels.
Trial Registration
This trial was registered in the US National Institutes of Health Clinical Trials Registry (ClinicalTrials.gov identifier: NCT02779634).
Similar content being viewed by others
Previous studies have suggested a potential role for coenzyme Q10 (CoQ10) therapy in the treatment of elderly patients with heart failure with preserved ejection fraction (HFpEF). |
Thirty-nine elderly patients with HFpEF were randomized to placebo or CoQ10. |
There were no differences in diastolic function or biomarkers. |
Our results do not support routine use of CoQ10 in HFpEF. |
1 Introduction
Heart failure (HF) is a leading cause of morbidity and mortality worldwide. The American Heart Association estimates 6.5 million subjects over the age of 20 years have HF in the US alone, and the prevalence is expected to increase by 46% to more than 8 million adults by 2030 [1]. Despite recent advancements in treatment, the prognosis of HF patients continues to be dire, with up to 50% of patients dying < 5 years from the time of diagnosis and an annual mortality rate of 30% in patients with advanced HF. The quality of life for surviving patients remains significantly impaired [2,3,4].
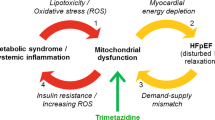
Heart failure is a complex clinical syndrome caused by various structural and functional impairments. Diastolic dysfunction associated with HF with preserved ejection fraction (HFpEF) is a leading cause of HF in the elderly population, and prevalence is increasing as the population ages and obesity, diabetes mellitus, and metabolic syndrome epidemics continue to spread. Approximately 40–70% of all HF patients have HFpEF [5, 6] and there are no specific proven treatments. Current therapies are aimed at relieving symptoms and treating comorbidities [7, 8]. One pathophysiologic mechanism that may significantly contribute to diastolic dysfunction in HFpEF is mitochondrial dysfunction leading to energy starvation of the cardiac myocyte [9, 10]. Coenzyme Q10 (CoQ10) is an essential cofactor for energy production, with reduced levels being noted in HF [11]. Low plasma concentrations of CoQ10 were shown to be an independent predictor of mortality in a relatively elderly cohort of patients with HF [12]. Previous studies have suggested a possible role for CoQ10 in the treatment of HF [13].
Q-SYMBIO, a prospective randomized controlled trial, evaluated CoQ10 supplementation to standard therapy in 420 HF patients with a mean left ventricular ejection fraction (LVEF) of 31%.
Overall, long-term treatment with CoQ10 was found to improve symptoms and reduce major adverse cardiovascular events (MACE). Furthermore, a positive trend with favorable treatment effects was found in elderly patients, LVEF >30%, New York Heart Association (NYHA) class III, and N-terminal pro-brain natriuretic peptide (NT-proBNP) levels >300 pg/mL. Given the relatively encouraging results of the Q-SYMBIO trial in the subset of elderly patients without significant left ventricular (LV) dysfunction, we designed this study to evaluate the effect of CoQ10 supplementation on diastolic function in patients with HFpEF.
2 Methods
The current study was an investigator-initiated, prospective, randomized, double-blind, placebo-controlled trial conducted at two tertiary care centers between June 2017 and December 2019. The study was approved by the Institutional Review Board and the regional Ethics Committee of each participating institution and conformed to the ethical guidelines of the Declaration of Helsinki. The trial was conducted according to Good Clinical Practice guidelines and all patients provided written informed consent. The trial was registered in the US National Institutes of Health Clinical Trials Registry (ClinicalTrials.gov identifier: NCT02779634).
Potential candidates were screened from the medical charts of general cardiology clinics, heart failure clinics, and emergency department visits cross-checked with medical records from the primary physician and echocardiography examinations.
Patients were aged > 55 years and presented with typical signs and symptoms of stage C heart failure (NYHA functional class II–IV) and a preserved ejection fraction on echocardiography (LVEF ≥ 50%) with evidence of diastolic dysfunction as defined by the Heart Failure and Echocardiography Associations of the European Society of Cardiology consensus statement [14]. This definition included lateral mitral valve annulus tissue Doppler e' <0.09 cm/s, average E:e' > 13, or average E:e' > 8 with additional markers of impaired diastolic function such as early/late (E/A) < 0.5, elevated deceleration time, left atrial volume index > 40 mL/m2, elevated LV mass index (>122 g/m2 ♀ or 149 g/m2 ♂), or pulmonary hypertension. All included patients were receiving stable medical therapy for at least 4 weeks before randomization.
Acute coronary syndrome or coronary revascularization within 60 days, clinically significant valvular disease, known infiltrative cardiomyopathy, hypertrophic cardiomyopathy, and chronic pericardial disease were causes for exclusion, as were treatment with chronic renal replacement therapy, CoQ10 use 2 months before randomization, and inability or refusal to provide informed consent.
Following informed consent, a physical examination was performed, a serum sample of NT-proBNP was drawn, and a full echocardiography examination was completed. Randomization was performed by a predefined computer-generated randomization code. Both the investigators and the subjects were blinded to the treatment allocation. Treatment in the CoQ10 arm consisted of 100 mg three times daily supplemented with the patient’s current medical therapy. The CoQ10 (stabilized Ubiquinol) was manufactured and supplied by Kaneka Corporation, Japan. Treatment in the placebo arm consisted of identical capsules. Compliance to therapy was evaluated for each participant by way of pill count at the middle and end of the 4-month trial. Repeat echocardiography examination and serum sampling for NT-proBNP measurements were repeated after 4 months of therapy.
The primary endpoint was defined as changes in diastolic tissue Doppler velocities, including early (e') diastolic mitral annular tissue velocities and the E:e' ratio, while the secondary endpoint was defined as a change in the serum NT-proBNP levels.
All subjects underwent standard two-dimensional and Doppler echocardiography with measurements of the interventricular septum, posterior wall, and LV end-systolic and end-diastolic diameters according to the recommendations of the European Association of Echocardiography/American Society of Echocardiography [15]. Measurements were performed for three consecutive cardiac cycles and then averaged. Subject height and weight at the time of examination were recorded and body surface area (BSA) was calculated. LV mass was calculated according to a necropsy-validated formula of LV mass (grams) = 0.8 × {1.04 × (septal thickness [cm] + LV internal diameter [cm] + posterior wall thickness [cm])3 – (LV internal diameter)3} + 0.6 g, and indexed to BSA.
Ejection fraction was calculated by averaging the measurements of end-diastolic and end-systolic LV volumes from the apical 4-chamber and 2-chamber views using Simpson's biplane method for three consecutive beats. In patients with atrial fibrillation, measurements were averaged for five consecutive beats.
Diastolic parameters were measured in a standard fashion from the apical 4-chamber view using pulsed-wave Doppler at the level of the mitral annulus and tissue Doppler imaging of the septal and lateral myocardial walls, and included early (E) and late (A) transmitral flow velocities, the ratio of early to late velocities (E/A), deceleration time of E velocity and isovolumic relaxation time. Early (e') and late (a') diastolic mitral annular tissue velocities at both the septum and lateral walls were obtained, and the E:e' ratio using the average of septal and lateral tissue velocities was calculated [16].
Serum samples were drawn for the measurement of NT-proBNP levels (Siemens, Erlangen Germany; limit of detection 2.4 pg/mL) on the day of randomization and following 4 months of treatment.
A total of 60 subjects were planned. This sample size was calculated to provide 90% power to detect a 20% change in indices of diastolic function, assuming a dropout rate of up to 10%. In practice, due to low recruitment, 39 patients consented and were subsequently included. This actual sample size provided 80% power to detect a 20% change in indices of diastolic function, with an in-practice dropout rate of 18% encountered. Analysis of all data was performed according to the intention-to-treat principle, and all patients were included as randomized. Baseline clinical characteristics were described by means of percentages or ratios for categorical data, mean and standard deviation for continuous normally distributed data, and median and interquartile range for non-normally distributed data. Groups were compared using t-tests for normally distributed variables (with Levene's test for equality of variances), and Mann–Whitney U tests (in case of non-normal distribution) for continuous variables. Pearson's Chi-square test or Fisher’s exact test were used for categorical variables. For intragroup comparison, before and after treatment, paired t-tests and Wilcoxon signed ranks test were used. A p-value ≤ 0.05 was considered significant. Due to the small sample sizes, multivariate regression models were not performed.
3 Results
A total of 100 patients were identified and 39 patients consented to participate in the study, of whom 19 patients were randomly assigned to CoQ10 supplementation and 20 patients to placebo (see Fig. 1).
3.1 CoQ10 Group
From the 19 patients randomized, 15 patients received the designated intervention and 4 patients discontinued CoQ10 supplementation; of these 4 patients, 2 agreed to complete control echocardiography and serum sampling for NT-proBNP measurement, 1 patient withdrew consent and was lost to follow-up, and another patient died of sepsis 1 month following discontinuation of CoQ10 supplementation. During the trial, 1 patient was hospitalized due to non-ST-elevation acute coronary syndrome and another patient was hospitalized due to HF exacerbation.
3.2 Placebo Group
Of the 20 patients randomized, 17 patients received the designated intervention and 3 discontinued supplementation; of these 3 patients, 2 withdrew consent and 1 patient had non-ST-elevation acute coronary syndrome, leading to the withdrawal of consent. Control echocardiography and blood sampling for NT-proBNP measurement were only performed in one of the patients whose consent was withdrawn.
3.3 Baseline Clinical Characteristics
Baseline clinical characteristics were similar (see Table 1). Significant differences between groups were observed for rates of β-blocker use, which was higher in the CoQ10 group (84.2% vs. 50%; p = 0.023), and rates of thyroid supplement use, which was lower in the CoQ10 group (15.8% vs. 45%; p = 0.048. Compliance was high and similar between groups (mean 208 ± 108 mg/day in the CoQ10 group vs. 212 ± 95 mg/day in the placebo group; p = 0.915.
3.4 Echocardiographic Measurements
No significant effect within either group was observed on indices of diastolic function following 4 months of CoQ10 use, and no significant difference between intragroup changes was observed (see Table 2). The mean lateral E:e' ratio decreased slightly from 12.57 ± 5.08 to 11.72 ± 5.7 in the treatment group (95% confidence interval [CI] −2.64 to + 4.36; p = 0.6) and increased slightly from 8.10 ± 3.11 to 8.28 ± 4.5 in the placebo group (95% CI −1.99 to +1.62; p = 0.8). A significant intergroup difference was only observed for the lateral E/e' ratio at baseline (p = 0.004) and at 4 months (p = 0.0125), which was higher in the CoQ10 group.
3.5 NT-proBNP Measurements
No intergroup significant effect was observed on serum NT-proBNP values following 4 months of CoQ10 use, although substantial heterogeneity existed, as depicted by the wide interquartile range. Mean NT-proBNP levels decreased from 767 pg/mL (246–1714) to 695 pg/mL (306–1903) in the treatment group (95% CI − 1339 to +599; p = 0.72) and from 511 pg/mL (125–1318) to 469 pg/mL (119–1588) in the placebo group (95% CI − 497 to +903; p = 0.16).
4 Discussion
In this pilot, randomized, double-blind, placebo-controlled trial in patients older than 55 years of age with HFpEF, treatment with CoQ10 did not significantly affect diastolic function as assessed by echocardiography or serum NT-proBNP.
HFpEF is a complex and heterogeneous clinical syndrome involving cardiac and skeletal muscle abnormalities, leading to reduced peak oxygen consumption and exercise intolerance, the main driver of morbidity and reduced quality of life [9]. Increased LV end-diastolic pressure due to decreased ventricular compliance, in the absence of LV systolic dysfunction, is the hallmark of diastolic dysfunction in HFpEF. Current evidence suggests that HFpEF and diastolic dysfunction represent, in part, a disorder of abnormal energetics, resulting, among others, from mitochondrial dysfunction, and the known role of CoQ10 in mitochondrial bioenergetics provided the pathophysiologic rationale for examining CoQ10 as a possible treatment for HFpEF [9, 10, 17].
To date, only a single study examined the effect of CoQ10 use on diastolic dysfunction in a population of patients with hypertrophic cardiomyopathy, demonstrating a positive effect. In this study, the diastolic function was evaluated by out-of-date non-quantitative criteria, limiting the applicability of the results [18].
Q-SYMBIO was the first prospective, randomized, double-blind trial utilizing CoQ10 to treat HF, powered to address MACE [13]. Most of these patients had HFrEF (LVEF 31 ± 10%), while only 7% of patients (n = 29) had LVEF > 45%. In a follow-up period of 2 years, there was a significant relative risk reduction of 43% in MACE (p = 0.003) and a 42% relative risk reduction in all-cause mortality (p = 0.036) with CoQ10 supplementation versus placebo. Additionally, the treatment group showed a significant improvement in NYHA class and a reduction in NT-proBNP serum levels following 2 years of treatment. These benefits were confirmed in a recent analysis of the European subgroup (n = 231) of the Q-SYMBIO trial, and a mild improvement of 6% in LVEF was also noted [19]. In Q-SYMBIO, positive, non-significant trends were observed in males, the elderly, NYHA functional class III, LVEF ≥ 30% (p = 0.065), and NT-proBNP ≥ 300 pg/mL. Overall, CoQ treatment in patients with chronic HF was found to be well tolerated, with a reduction in symptoms and major adverse cardiac events. These results supported a possible clinical benefit for elderly HFpEF patients. However, our study was specifically designed to investigate the HFpEF population and failed to demonstrate any clinical advantage of treatment with CoQ10.
There are several possible explanations for our findings. One possibility is the relatively short treatment duration. In a recent review of mitochondrial dysfunction and strategies for modulation of mitochondrial function, it was inferred that the use of mitochondrial-targeted therapeutic agents, including CoQ10, are required to be used for a minimum period of at least 4–6 weeks in HFpEF patients to induce a clinical effect [9]. In the current study, 4 months of CoQ10 supplementation should be adequate to induce a clinical effect if one exists. The CoQ10 dose chosen (100 mg three times daily) was similar to that used in the Q-SYMBIO trial, although in the latter, a different formulation was used (Ubiquinone [Pharma Nord ApS] was used in the Q-SYMBIO trial, versus stabilized ubiquinol [Kaneka Corporation, Japan] in the current trial). In one study, ubiquinol had superior bioavailability when compared with ubiquinone and was therefore used in our study [20]. However, other studies demonstrated large differences in the bioavailability of different CoQ10 formulations, which were related to the matrix utilized and the proportion of preservatives [21]. The Q-SYMBIO dosing rationale was based on prior clinical trials and aimed for a therapeutic serum concentration of at least 2 μg/mL, as positive clinical effects are visible with CoQ10 serum concentrations > 2.5 μg/mL [12, 22, 23]. The dosage of CoQ10 200–300 mg daily was also confirmed in the clinical trial by Belardinelli et al. [24]. Therefore, a dose of 300 mg/day should be adequate to achieve a positive clinical effect. Compliance with CoQ10 supplementation was high throughout the present trial, with an average dosage of over 200 mg/day in the CoQ10 group.
Another explanation for the lack of benefit may be the heterogeneous nature of HFpEF, which may have different clinical phenotypes responding to different therapeutic interventions [25]. It is possible that selective assessment of myocardial energetics in the general population of HFpEF patients may identify a subgroup of responders to CoQ10 therapy.
4.1 Limitations
A relatively small sample size and high dropout rate are the main limitations of this study. Nonetheless, the sample size retained sufficient power (80%) to detect a clinically relevant (20%) change in diastolic indices. We did not measure blood levels of CoQ10, however the dose used was similar to that used in previous trials of CoQ10 in a heart failure population. The well-defined patient population, clear endpoints, and the double-blind nature comprise the strengths of this study.
5 Conclusion
In this pilot trial, treatment with CoQ10 did not significantly affect diastolic function as determined by echocardiography or NT-proBNP levels in elderly patients with HFpEF. Further study is necessary to determine whether modulation of myocardial energetics may be beneficial in the HFpEF population.
References
Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–603.
Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–50.
Levy D, Kenchaiah S, Larson MG, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–402.
Townsend N, Nichols M, Scarborough P, Rayner M. Cardiovascular disease in Europe–epidemiological update 2015. Eur Heart J. 2015;36:2696–705.
Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18–28.
Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14:591–602.
Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239.
Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200.
Kumar AA, Kelly DP, Chirinos JA. Mitochondrial dysfunction in heart failure with preserved ejection fraction. Circulation. 2019;139:1435–50.
Sharma A, Fonarow GC, Butler J, Ezekowitz JA, Felker GM. Coenzyme Q10 and heart failure: a state-of-the-art review. Circ Heart Fail. 2016;9:1–8.
Folkers K, Vadhanavikit S, Mortensen SA. Biochemical rationale and myocardial tissue data on the effective therapy of cardiomyopathy with coenzyme Q10. Proc Natl Acad Sci USA. 1985;82(3):901–4.
Molyneux SL, Florkowski CM, George PM, et al. Coenzyme Q10: An independent predictor of mortality in chronic heart failure. J Am Coll Cardiol. 2008;52:1435–41.
Mortensen SA, Rosenfeldt F, Kumar A, et al. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC Heart Fail. 2014;2:641–9.
Paulus WJ, Tschope C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–50.
Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–70.
Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–93.
Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: part II: causal mechanisms and treatment. Circulation. 2002;105:1503–8.
Adarsh K, Kaur H, Mohan V. Coenzyme Q10 (CoQ10) in isolated diastolic heart failure in hypertrophic cardiomyopathy (HCM). BioFactors. 2008;32:145–9.
Mortensen A, Rosenfeldt FL, Filipiak KJ. Effect of coenzyme Q10 in Europeans with chronic heart failure: a sub-group analysis of the QSYMBIO randomized double-blind trial. Cardiol J. 2019;26:147–56.
Langsjoen PH, Langsjoen AM. Comparison study of plasma coenzyme Q10 levels in healthy subjects supplemented with ubiquinol versus ubiquinone. Clin Pharmacol Drug Dev. 2014;3(1):13–7.
Lopez-lluch G, del Pozo-Cruz J, Sanchez-Cuesta A, Cortes-Rodriguez AB, Navas P. Bioavailability of coenzyme Q10 supplements depends on carrier lipids and solubilization. Nutrition. 2019;57:133–40.
Mortensen S. Overview on coenzyme Q10 as adjunctive therapy in chronic heart failure: rationale, design and end-points of “Q-symbio”—a multinational trial. BioFactors. 2003;18:79–89.
Langsjoen P. Lack of effect of coenzyme Q on left ventricular function in patients with congestive heart failure. J Am Coll Cardiol. 2000;35:816–7.
Belardinelli R, Muçaj A, Lacalaprice F, et al. Coenzyme Q10 and exercise training in chronic heart failure. Eur Heart J. 2006;27:2675–81.
Cohen JB, Schrauben SJ, Zhao L, et al. Clinical phenogroups in heart failure with preserved ejection fraction. JACC Heart Fail. 2020;8:172–84.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Partial funding for this study was provided by Kaneka Co., Japan. The funding source had no role in the collection of data or preparation of the manuscript.
Conflicts of interest
Tal Y. Samuel, Tal Hasin, Israel Gotsman, Tanya Weitzman, Fanny Ben Ivgi, Ziv Dadon, Elad Asher, Offer Amir, Michael Glikson, Ronny Alcalai, and David Leibowitz have no conflicts of interest to report.
Ethics approval
This study was approved by the Institutional Review Boards of the two participating centers (reference number 129-16).
Consent to participate and publish
All participants provided written informed consent to participate in this study and for the manuscript to be published.
Availability of data
All data generated in this study are included in the article. Further enquiries should be directed to the corresponding author.
Code availability
Not applicable.
Author contributions
DL, TH, IG, and TYS were involved in the design of the study, interpretation of the data, and drafting of the manuscript. TYS, TW, and ZD were responsible for the enrollment of patients. FBI performed the echocardiographic examinations. EA and RA were involved in interpretation of the data and manuscript revisions. MG and OA critically reviewed the manuscript prior to submission.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Samuel, T.Y., Hasin, T., Gotsman, I. et al. Coenzyme Q10 in the Treatment of Heart Failure with Preserved Ejection Fraction: A Prospective, Randomized, Double-Blind, Placebo-Controlled Trial. Drugs R D 22, 25–33 (2022). https://doi.org/10.1007/s40268-021-00372-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-021-00372-1