Abstract
Amantadine extended release (ER) [GOCOVRI®], an NMDA receptor antagonist, is an important treatment option in the management of levodopa-induced dyskinesia (LID) and OFF episodes in patients with Parkinson’s disease (PD). Taken at bedtime, amantadine ER provides low plasma levels overnight and high plasma concentrations during the waking day (i.e. delayed release). In randomized, double-blind, placebo-controlled phase III trials in adults with PD, once-daily amantadine ER was effective in improving LID, reducing OFF time and increasing ON time without troublesome dyskinesia, compared with placebo. Control of motor complications with amantadine ER was evident throughout the day, with fewer transitions between motor states. The clinical benefits of amantadine ER in reducing the duration and impact of dyskinesia and OFF episodes were durable with long-term treatment. Amantadine ER is generally well tolerated, including in the long term, with the majority of adverse events being mild or moderate in severity.
Plain Language Summary
Parkinson’s disease (PD), a common neurodegenerative disease, is associated with motor and non-motor symptoms and thus exerts a considerable burden on the health system. Levodopa is considered the gold standard treatment for PD; however, prolonged use is associated with reduced efficacy in the setting of more advanced disease and the emergence of motor complications of dyskinesia and OFF episodes. Management of these motor complications often involves the trade-off between treating one or the other. Amantadine extended release (ER) capsules (GOCOVRI®), administered once daily at bedtime, are the first to be approved in the USA to treat both dyskinesia and OFF episodes in PD. Evidence for the efficacy of amantadine ER has been demonstrated in two randomized, placebo-controlled phase III trials in PD patients with levodopa-induced dyskinesia (LID), where it reduced dyskinesia and OFF time and increased ON time without troublesome dyskinesia, relative to placebo. Benefits with amantadine ER were durable over long-term treatment. Amantadine ER is generally well tolerated in patients with LID, with no new safety concerns identified during longer-term use. Given its efficacy and tolerability profile, amantadine ER is an important treatment option for the management of LID and OFF episodes in PD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this Adis Drug Q&A can be found at https://doi.org/10.6084/m9.figshare.19546876. |
Delayed-release, extended-release (DR/ER) oral formulation administered once daily at bedtime |
Significantly improves dyskinesia and OFF time relative to placebo |
Significantly increases ON time without troublesome dyskinesia relative to placebo |
Durable clinical benefits with long-term treatment |
Generally well tolerated |
What is the rationale for using amantadine extended release (ER) [GOCOVRI®] in Parkinson’s disease (PD)?
The incidence of Parkinson’s disease (PD), the second most frequent neurodegenerative disease, is expected to continue to rise over the next decade, and with it the burden it places on the health system [1, 2]. Nigral dopaminergic degeneration leads to the cardinal motor symptoms of the disease (including bradykinesia, rigidity, resting tremor, and postural instability), while non-motor symptoms (e.g. depression or apathy) of the disease may reflect non-dopaminergic neurotransmitter derangements and can sometimes be treated by directly targeting the relevant neurotransmitter [1,2,3,4]. Some non-motor symptoms remain a treatment challenge as they are poorly understood [2].
Levodopa, the gold standard treatment option for PD, is initially highly effective in improving motor symptoms but prolonged use is associated with reduced efficacy in more advanced PD and the appearance of motor complications including periods of dyskinesia (mainly arising when symptoms are controlled, i.e. ON time) and OFF episodes [1, 2]. OFF episodes are periods within a day when the current levodopa dose wears off and parkinsonian signs and symptoms return and functional status declines; motor fluctuations occur between ON times (periods within a day when PD signs and symptoms are reduced and functional status is improved) and OFF episodes [3]. After 10 years of levodopa treatment, up to 90% of treated patients will have levodopa-induced dyskinesia (LID) and/or OFF episodes, both of which have an adverse effect on health-related quality of life, activities of daily living, care-partner burden, incidence of fall risk and healthcare costs [3, 5].
Peak-dose dyskinesia is the most common type of LID, and its management involves decreasing individual doses of levodopa; increasing the frequency of a lower dose of levodopa; adding a dopamine agonist while decreasing the dose of levodopa; switching to immediate-release (IR) formulations; and reducing the doses of or discontinuing dopamine-enhancing medications (e.g. monoamine oxidase B inhibitors) [6]. Conversely, the management of OFF episodes typically involves increasing the dosage or frequency of levodopa with or without the addition of adjunctive dopaminergic medication; however, such adjustments can cause an increase in the incidence of dyskinesia and thus presents a challenge when treating patients with both OFF episodes and dyskinesia [2]. The different approaches to treating dyskinesia and OFF episodes has meant that a trade-off between managing one or the other was necessary.
An extended-release (ER) capsule formulation of amantadine (GOCOVRI®), an NMDA receptor antagonist, was the first drug to be approved in the USA for the treatment of dyskinesia in patients with PD receiving levodopa-based therapy, with or without dopaminergic medications; the use of amantadine ER in this indication has been reviewed previously [7]. Amantadine ER has since been approved in the USA as adjunctive treatment to levodopa/carbidopa in patients with PD experiencing OFF episodes, and is the first agent to be approved for both dyskinesia and OFF episodes in patients with PD [8]. Table 1 provides a summary of the US prescribing information for the use of amantadine ER for the approved indications in patients with PD. Consult local prescribing information for further details. Discussion of the use of other oral amantadine formulations [including IR (SYMMETREL® [9]) and IR/ER (OSMOLEX® [10]) formulations] in their approved indications (i.e. the treatment of PD and the treatment of drug-induced extrapyramidal reactions) is beyond the scope of this article.
How should amantadine ER (GOCOVRI®) be used?
Amantadine ER, available as an ER capsule for oral administration, should be swallowed whole once daily at bedtime at the recommended dosage (Table 1) [8]. Alternatively, the contents of the capsule may be sprinkled on a small amount of soft food (e.g. applesauce) and swallowed immediately without chewing; the drug/food mixture should not be stored for future use. Sudden discontinuation of amantadine ER should be avoided, and to avoid adverse reactions associated with a rapid dose reduction or withdrawal, the dosage of amantadine ER should be reduced by half for the final week of dosing (if possible) in patients who have been receiving the drug for longer than 4 weeks. Amantadine ER is not interchangeable with other amantadine IR or ER products [8].
Given the antiviral properties of amantadine ER and its potential interference with the efficacy of live attenuated influenza vaccines, the use of these vaccines is not recommended during treatment. Inactivated influenza vaccines may be used, as appropriate [8].
What are the pharmacological properties of amantadine ER (GOCOVRI®)?
The exact mechnism by which amantadine exerts its efficacy in the treatment of LID or OFF episodes in patients with PD is unknown [8]. Amantadine, a weak uncompetitive antagonist of the NMDA receptor, may exert its efficacy by reducing excessive glutamergic activity which contributes to dyskinesia or OFF episodes [13, 14]. Amantadine also has dopamine reuptake inhibiting properties [15]. In humans, amantadine is associated with dopaminergic-like adverse events (AEs) such as hallucinations and dizziness, indicating that it may directly and indirectly affect dopamine signalling [8]; these AEs may also be due to antiglutamatergic and/or anticholinergic activity [16, 17]. In animal studies, amantadine has not demonstrated direct anticholinergic activity; however, anticholinergic-like AEs have been observed with amantadine in humans [8]. The potential for central nervous system effects may be increased with concomitant alcohol consumption, and is thus not recommended [8].
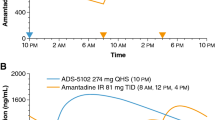
Amantadine ER (68.5 mg, 137 mg and 274 mg) demonstrated dose-porportional pharmacokinetics in healthy volunteers [8]. The ER formulation of amantadine has a markedly different pharmacokinetic profile to that of the IR formulation, with the latter showing a more rapid increase in the mean plasma amantadine concentration [18]. Following administration of amantadine ER at bedtime, the mean plasma concentration-time profile for amantadine shows a ≈3 h lag time for absorption (i.e. delayed release) followed by a slow rise in amantadine plasma concentrations (i.e. extended release); the maximum concentrations occur in the morning [18] and are sustained throughout the day [19], while lower concentrations occur in the evening [18]. The aforementioned distinctive pharmacokinetic profile of amantadine ER may contribute to its efficacy in reducing both dyskinesia and OFF time [19]. The pharmacokinetic properties of amantadine ER have been reviewed previously [7], and are summarized in Table 2.
What is the efficacy of amantadine ER (GOCOVRI®) in PD?
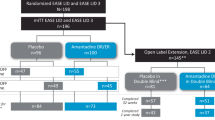
Amantadine ER 274 mg capsules administered once daily at bedtime are effective in the treatment of LID in PD and as adjunctive treatment to levodopa/carbidopa in patients with PD experiencing OFF episodes. The efficacy of amantadine ER was demonstrated in the randomized, double-blind, placebo-controlled phase III, 13-week EASE LID 3 [21] and 25-week EASE LID [22] trials, and further supported by efficacy data from the long-term, open-label 101-week EASE LID 2 safety trial which included patients who had completed the double-blind studies [23]. The amantadine dose of 274 mg was selected for the phase III trials, based on results of an earlier phase II/III dose-ranging EASED trial [24].
The EASE LID and EASE LID 3 studies enrolled patients who were aged 30–85 years (mean age 63.9–65.5 years), diagnosed with PD based on the UK Parkinson Disease Society Brain Bank Clinical Diagnostic Criteria (mean time since PD diagnosis 9.0–10.7 years), had a score of ≥2 (i.e. at least a mild functional impact of dyskinesia) on a scale 0–4 based on the Movement Disorder Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), part IV, item 4.2, at screening and baseline, and at least two 0.5 h periods of ON time with troublesome dyskinesia between 9 a.m. and 4 p.m. recorded in a 24-h PD patient diary on each of two consecutive days just before the first day of treatment (i.e. day 1) [21, 22].
All antiparkinsonian medications, including levodopa preparations (which were to be administered ≥3 time daily), were to remain unchanged for ≥30 days before screening and during participation in the trials [21, 22]. Among the key exclusion criteria in EASE LID and EASE LID 3 were amantadine use within 30 days before screening, or a documented intolerance or lack of dyskinesia response to amantadine, neurosurgical intervention related to PD, current treatment with apomorphine hydrochloride or dopamine receptor blocking agents, rimantadine or medications that prolong the QT interval and having a known risk of torsades de pointes, atypical parkinsonism, levodopa or dopamine agonist-induced psychosis, and cognitive (Mini-Mental State Examination score of <24 during screening) or abnormal kidney function [estimated glomerular filtration rate (eGFR) < 50 mL/min/1.73 m2][21, 22].
Eligible patients in EASE LID and EASE LID 3 were randomized to receive amantadine ER or placebo, once daily at bedtime [21, 22]. In the amantadine ER arm, patients received 137 mg/day for the first week of treatment, followed by 274 mg/day from the second week of treatment until the last week of treatment, when the dosage was reduced to 137 mg/day. The prespecified efficacy analysis population was the modified intent-to-treat population (mITT) [Table 3], which included patients who received treatment and provided ≥1 postbaseline assessment of the Unified Dyskinesia Rating Scale (UDysRS). In EASE LID and EASE LID 3, the primary efficacy endpoint was change in UDysRS total score from baseline to week 12; key secondary endpoints were assessed hierarchically (Table 3) [21, 22].
Amantadine ER significantly improved the UDysRS total score from baseline relative to placebo at week 12 (primary endpoint; Table 3) in EASE LID and EASE LID 3 [21, 22]; a significant improvement from baseline was also evident at week 24 in EASE LID (key secondary endpoint; Table 3) [22]. In the two trials, significant improvements in the UDysRS total scores with amantadine ER relative to placebo were consistent across several subgroups of patients based on age, baseline kidney function and dyskinesia severity, where the upper limit of the 95% confidence interval (CI) of the least-squares mean (LSM) for the treatment difference was <1 [21, 22]. The historical (duration and effect; patient-reported) and objective (impairment and disability; rater-assessed) UDysRS scores were significantly (p < 0.05) improved with amantadine ER versus placebo at week 12 in EASE LID and EASE LID 3 [21, 22], and at week 24 in EASE LID [22].
Treatment with amantadine ER significantly improved key secondary PD diary endpoints (ON time without troublesome dyskinesia and OFF time) relative to placebo [22]. Amantadine ER significantly prolonged ON time without troublesome dyskinesia and reduced OFF time compared with placebo at week 12 in EASE LID and EASE LID 3 (Table 3) [21, 22]; these improvements were maintained at week 24 in EASE LID (Table 3) [22].
For ON time with troublesome dyskinesia, the LSM reductions from baseline with amantadine ER and placebo were –3.6 h versus –2.5 h (p = 0.09) in EASE LID 3 [21]. Conversely, in EASE LID, the improvement in ON time with troublesome dyskinesia was significantly (p < 0.01) greater among amantadine ER versus placebo recipients at weeks 12 (LSM changes of –3.2 vs –1.6 h) and 24 (–3.3 vs –1.9 h) [22].
Amantadine ER significantly (p ≤ 0.001) improved motor complications, as assessed by the MDS-UPDRS part IV, compared with placebo at week 12 in EASE LID (LSM changes of –4.4 vs –2.5) [22] and EASE LID 3 (–4.3 vs –1.3) [21], and at week 24 in EASE LID (–4.2 vs –2.0) [22]. For assessments of non-motor and motor experiences of daily living and motor examination using the MDS-UPDRS parts I–III combined scores, amantadine ER was associated with significant (p < 0.05) improvements compared with placebo at week 12 in EASE LID 3 [21]. In contrast, there were no marked differences between amantadine ER and placebo in MDS-UPDRS parts I–III combined scores at weeks 12 and 24 in EASE LID [22].
Improvements in overall PD symptoms, including dyskinesia, as assessed by the Clinician’s Global Impression of Change (CGI-C) scale were seen in significantly (p < 0.001 for overall distribution) more amantadine ER than placebo recipients at week 12 in EASE LID (81% vs 36%) [22] and EASE LID 3 (68% vs 39%) [21]. At week 24 in EASE LID, 68% and 47% of amantadine ER and placebo recipients were considered to be improved from baseline, albeit no longer statistically significant [22].
Pooled analyses
Results of pooled analyses of EASE LID and EASE LID 3 (pooled mITT population = 196) were consistent with results of the individual studies, for the primary and key secondary endpoints; the improvements in the UDysRS total score from baseline with amantadine ER compared with placebo were significant at each time point from week 2 and sustained through to week 12 [25].
In a subgroup of patients with substantial (≥2.5 h) OFF time at baseline (n = 102), the LSM between-group difference was significant (p < 0.01) in favour of amantadine ER at week 12 and was –1.2 h for OFF time and +3.4 h for ON time without troublesome dyskinesia [26]. At week 12 in the ≥2.5 h subgroup, amantadine ER was associated with improvements in overall PD symptoms (as assessed by CGI-C; p < 0.0001), motor complications (MDS-UPDRS part IV) and motor experiences of daily living (MDS-UPDRS part II) relative to placebo; statistically significant differences favouring amantadine ER were evident at all double-blind visits in the ≥2.5 h subgroup. There was no significant overall change in MDS-UPDRS parts I or III scores in the ≥2.5 h subgroup [26].
The efficacy of amantadine ER was also demonstrated in patients meeting the 5-2-1 (≥5 levodopa doses, ≥2-h OFF and ≥1-h dyskinesia per day) criteria for advanced PD (n = 64; mITT), at week 12 [27]. The 5-2-1 criteria identify patients with PD who are uncontrolled on oral therapies and may therefore benefit from device-aided therapies. In the 5-2-1 cohort, amantadine ER significantly (p ≤ 0.002) improved the UDysRS scores (LSM between-group difference −9.57) and ON time without troublesome dyskinesia (LSM between-group difference +2.90 h) from baseline relative to placebo at week 12. At week 12, amantadine ER was also associated with significant (p < 0.05) improvements from baseline relative to placebo in OFF time, ON time with troublesome dyskinesia and MDS-UPDRS parts II and IV [27].
With respect to the effects of amantadine ER in PD patients beyond the primary and secondary outcomes in the phase III trials, amantadine ER offered the following benefits over placebo at week 12 in pooled analyses:
-
Item analysis of the UDysRS (n = 196) Amantadine ER significantly (p < 0.05) improved the patient-rated impact of dyskinesia from baseline compared with placebo for 6/10 activities of daily living (ADLs) [walking and balance, eating tasks, doing hobbies and other activities, public and social settings, exciting or emotional settings and speech] itemised in UDysRS part IB [28]. Treatment differences for all scale items numerically favoured amantadine over placebo. Significant (p < 0.05) improvements were also observed for the clinician-rated intensity of dyskinesia in all seven body regions itemised in UDysRS part III and clinician-rated disability during 3/4 ADLs (ambulation, dressing and drinking) tasks itemised in part IV. UDysRS parts IB, III and IV total scores were also significantly (p < 0.05) improved [28], as was dystonia on UDysRS part II [29].
-
The number and duration of daily episodes of OFF and troublesome dyskinesia, and the number of daily transitions between PD states (n = 162 with evaluable diary data) Amantadine ER offered a greater reduction from baseline than placebo in the mean number of episodes and mean duration of an individual episode of OFF (treatment difference vs placebo: –0.4 episodes and –0.3 h, respectively) and troublesome dyskinesia (–1.0 episodes and –0.6 h), assessed in 30-min intervals [30]. The mean duration of an episode of ON without troublesome dyskinesia was increased by 5.0 h from baseline (vs 2.0 h for placebo). Relative to baseline, amantadine recipients experienced 49% fewer transitions between states (vs 26% for placebo). A greater proportion of amantadine ER than placebo recipients reported no episodes of OFF time (27.3% vs 20.0%), no episodes of ON time with troublesome dyskinesia (57.1% vs 24.7%) and neither OFF time nor troublesome dyskinesia (19.5% vs 3.5%) [30].
-
ON time without any dyskinesia (n = 165) Treatment with amantadine ER increased ON time without (troublesome or non-troublesome) dyskinesia from baseline, with an LSM increase relative to placebo of +2.9 h [31]; a significantly (p = 0.003) higher proportion of amantadine ER recipients spent ≥50%, ≥75%, and 100% of their waking day without any dyskinesia relative to placebo (56% vs 23%, 22% vs 8%, and 4% vs 0%, respectively) [31, 32]. Moreover, amantadine ER reduced ON time with (troublesome + non-troublesome) dyskinesia from baseline, with a treatment difference relative to placebo of –1.9 [31]. Relative to placebo, significantly (p < 0.05) more patients in the amantadine group had ≥50% and ≥75% reduction of ON time with any dyskinesia (53% vs 26% and 27% vs 14%); notably, 13% and 7% of amantadine ER and placebo recipients had 100% reduction in ON time with dyskinesia (i.e. no dyskinesia) [31, 32]. The treatment effects with amantadine ER with respect to ON time with or without dyskinesia relative to placebo were significant (p ≤ 0.002) from week 2 and sustained through to week 12 [31].
-
Motor aspects of experiences of daily living (n = 196) Amantadine ER significantly improved MDS-UPDRS part II total score compared with placebo, with a placebo-adjusted treatment difference for the overall LSM change from baseline in MDS-UPDRS part II in favour of amantadine ER (–2.0; 95% CI –3.3 to –0.7; p = 0.004) [33]. The change in score from baseline for amantadine ER (–3.4) exceeded the threshold for a minimal clinically important difference for improvement of MDS-UPDRS part II of –3.05 (previously published [34]) [33]. Several motor activities (MDS-UPDRS part II item scores) were significantly (p < 0.05) improved with amantadine ER relative to placebo, including eating tasks, tremor, getting out of bed/car/chair and freezing; tremor and freezing of gait represent unmet needs in patients with PD [33].
-
Non-motor symptoms (n = 196) The LSM treatment difference significantly (p < 0.05) favoured amantadine ER over placebo for the daytime sleepiness and depressed mood items in MDS-UPDRS part I, but favoured (p < 0.05) placebo for hallucinations and psychosis and cognitive impairment [35]. For the MDS-UPDRS part I total score, the LSM treatment difference between amantadine ER and placebo was –0.8 (in favour of amantadine ER) [p = 0.2]. In the amantadine ER group, a correlation was observed between improvement in non-motor symptoms and improvement in dyskinesia (r +0.39; p < 0.001), whereas no correlation was evident in the placebo group (r +0.12; p = 0.29) [35].
Further to the aforementioned pooled analyses, amantadine ER also demonstrated additional clinical benefits in PD patients as a first/early add-on to levodopa in subgroups of patients who, at baseline, were receiving levodopa monotherapy (n = 44) and who were diagnosed with PD ≤5 years (n = 27) [36]. Results of an evaluation of the collective interference of dyskinesia and OFF time across daily activities and the ability of amantadine ER to reduce this interference indicate that multiple areas of function are impacted by dyskinesia and OFF time [37]. Reductions in dyskinesia and OFF time resulting from treatment with amantadine ER were accompanied by significant (p < 0.01) reductions from baseline in the mean number of UDysRS part IB and MDS-UPDRS part II items affected, at week 12 [37].
Long-term efficacy
Amantadine ER provided a sustained reduction in motor complications (dyskinesia and OFF time) through ≈2 years of treatment in patients continuing therapy after double-blind use in EASED, EASE LID and EASE LID 3 [23]. Sustained improvements in dyskinesia and OFF time were also observed for amantadine ER-naïve patients [23].
The open-label EASE LID 2 safety trial enrolled patients who had previously received amantadine ER (n = 60; continuing amantadine ER group) or placebo (n = 78; previous placebo) in EASE LID or EASE LID 3 and entered the current trial without interruption; patients who had completed treatment in EASED, EASE LID or EASE LID 3 but were enrolled following a time gap (n = 24; participation-gap group); and patients who were excluded from double-blind amantadine ER trials due to deep brain stimulation surgery (DBS) [n = 61; DBS group] [23]. Patients were treated with amantadine ER once daily at bedtime for up to 101 weeks, and received 137 mg/day for the first week treatment, 274 mg/day from the second week of treatment through week 100, then 137 mg/day for the last week of treatment (i.e. week 101). Changes to PD medications (including levodopa dosage) were permitted. The use of other amantadine formulations was not permitted [23].
The median duration of exposure to amantadine ER in EASE LID 2 was 1.9 years; of enrolled patients, 76% completed ≥52 weeks of treatment and 58% completed the trial [23].
In the continuing amantadine ER group, improvements in the MDS-UPDRS part IV achieved during the double-blind trials were maintained for up to 100 weeks in EASE LID 2 [23]. At 52 and 100 weeks, the mean MDS-UPDRS part IV change from double-blind baseline was –4.7 and –4.0, respectively; similar changes were evident at other visits. In the groups with no previous exposure to amantadine ER, marked improvements in the MDS-UPDRS part IV scores were evident and resulted in total scores comparable to those in patients continuing amantadine ER by week 8, and maintained at other visits throughout the study. A sustained improvement from baseline in the MDS-UPDRS part IV scores was also evident in a subgroup of patients who were receiving amantadine IR at the time of enrollment (n = 32; from the participation-gap and DBS groups). The majority (71–87%) of patients in the four groups had ≥ 1-point improvement in MDS-UPDRS score from pre-amantadine ER baseline, at week 100, and 50–76% of patients had ≥ 2-point improvement from baseline [23].
When evaluating the individual MDS-UPDRS part IV subscores relating to dyskinesia and OFF (4.1 time spent with dyskinesia, 4.2 functional impact of dyskinesia, 4.3 time spent with OFF and 4.4 functional impact of OFF), results indicate that improvements in MDS-UPDRS part IV derived from improvements in both dyskinesia and OFF items [23]. The overall change for all subscores remained below pre-amantadine ER baseline, at week 100; functional impact of dyskinesia demonstrated the largest reductions [23].
Amantadine ER improved mean MDS-UPDRS part IV scores at weeks 52 and 100 from baseline, regardless of levodopa dose adjustments (i.e. unchanged, increased or decreased dose) [23].
For all four groups, fluctuations in the MDS-UPDRS parts I–III scores were seen across study visits and mean scores had increased by the end of the trial [23].
Consistent efficacy of amantadine ER in terms of MDS-UPDRS part IV scores improvements from baseline was also evident throughout open-label treatment to week 100 in the ≥2.5h subgroup [26], as well as patients meeting the 5-2-1 criteria [27].
What is the tolerability profile of amantadine ER (GOCOVRI®)?
Amantadine ER 274 mg administered once daily is generally well tolerated in patients with PD and LID, based on data from the 13-week EASE LID 3 trial [21], 25-week EASE LID trial [22], their pooled analysis [8, 25] and the 101-week, open-label EASE LID 2 safety trial [23].
The safety population in the pooled analysis of data from the EASE LID and EASE LID 3 trials included 100 amantadine ER and 98 placebo recipients [8, 25]. Adverse events (AEs) occurred in 87.0% of amantadine ER recipients and 56.1% of placebo recipients [21, 22], and led to treatment discontinuation in 20% [most commonly (incidence >3%) due to hallucinations (8%), dry mouth, peripheral oedema and blurred vision (3% each)] and 8% of patients in these respective groups [8]. The majority of AEs were mild or moderate in severity [21, 22].
The most common AEs in the pooled analysis (occurring in >10% of patients in either treatment group) were hallucination (visual and auditory; 21% in the amantadine ER group vs 3% in the placebo group), dizziness (16% vs 1%), dry mouth (16% vs 1%), peripheral oedema (16% vs 1%), falls (13% vs 7%), constipation (13% vs 3%) and orthostatic hypotension (including orthostatic hypotension, postural dizziness, syncope, presyncope and hypotension; 13% vs 1%) [8].
A number of adverse reactions were reported more frequently in patients aged ≥65 years (n = 52) compared with those aged <65 years (n = 48) in the pooled analysis, including hallucinations (31% vs 10%), falls (17% vs 8%) and orthostatic hypotension (8% vs 2%) [8]. Similarly, there was a difference in the frequency of adverse reactions by gender; dry mouth (22% in women vs 11% in men), nausea (13% vs 4%), livedo reticularis (13% vs 0%), abnormal dreams (9% vs 0%) and cataracts (7% vs 0%) occurred more frequently in women (n = 46) than men (n = 54), while dizziness (20% in men vs 11% in women), peripheral oedema (19% vs 11%), anxiety (11% vs 2%), orthostatic hypotension (7% vs 2%) and gait disturbance (6% vs 0%) were reported more frequently in men than women [8]. Visual hallucinations were reported more frequently in patients with an eGFR of 50–89 mL/min/1.73 m2 than in in those with an eGFR of ≥90 mL/min/1.73 m2 (26% vs 11%) [25].
Serious AEs (SAEs) occurred in 11% of amantadine ER recipients; constipation and urinary retention that occurred in one patient were the only SAEs considered to be related to treatment [25]. One death occurred in the amantadine ER group, which was due to advanced PD during hospice care (i.e. not treatment-related) [25].
A number of warnings and precautions pertain to the use of amantadine ER (Table 4) [8]. In addition to hallucinations, dizziness and orthostatic hypotension, other clinically relevant adverse drug reactions included depression and depressed mood (6% of amantadine ER recipients vs 1% of placebo recipients), somnolence and fatigue (4% vs 1%), confusional state (3% vs 2%) and apathy (2% vs 0%). Where specified, monitoring is recommended with subsequent dosage modifications as required (Table 4) [8].
Long-term safety
The long-term tolerability and safety profile of amantadine ER in patients with PD in EASE LID 2 was consistent with that identified in double-blind phase III trials and their pooled analysis, with no new safety signals identified during the open-label safety trial [23].
In EASE LID 2, although the majority (92%) of patients reported at least one AE, most of these AEs were mild or moderate in severity [23]. The most commonly (≥10% incidence) reported AEs were falls (33%), hallucinations (24%; mostly visual), peripheral oedema (16%), constipation (14%) and urinary tract infection (10%); these AEs were reported more frequently in patients aged ≥65 years compared with patients <65 years. Other AEs of specific interest to PD patients included orthostatic hypotension and/or hypotension (3%), impulse control disorder (2%; most of whom were concomitantly using dopamine agonists) and suicidality (1%; attempted suicide and suicide ideation with intent to self-harm). Drug-related AEs were reported in 56% of patients, with the most common (≥5% incidence) being visual hallucinations (18%), peripheral oedema (11%), livedo reticularis (8%), dry mouth (7%) and falls (6%) [23].
In the safety population, AEs that led to study-drug discontinuation or death occurred in 22% [most commonly (≥2% incidence) due to visual hallucinations (3%) and falls (2%)] of patients, and were considered to be related to study drug in 14% of patients [23]. AEs that led to treatment discontinuation occurred more frequently amongst patients who initiated amantadine ER treatment in EASE LID 2 compared with those who continued amantadine ER treatment from the phase III trials [23].
SAEs occurred in 27% of the safety population, with the most common (≥2% incidence) being fractures (6%; typically due to falls) and worsening PD symptoms (2%); SAEs considered to be related to treatment occurred in five patients (suicidal ideation, postoperative confusion, worsening PD symptoms, urinary tract infection and confusional state) [23]. There were nine deaths during EASE LID 2, none of which were considered to be treatment-related [23].
One-year post-launch pharmacovigilance data were generally consistent with those reported in phase III clinical trials; an age-event relationship was evident for the majority of AEs [38]. Seizures were identified as an adverse reaction during post-approval use of amantadine ER and, consistent with other preparations of amantadine [2], have subsequently been included in the prescribing information [8].
What is the current clinical position of amantadine ER (GOCOVRI®) in PD?
Amantadine ER is an important treatment option in the management of motor complications and fluctuations in patients with PD, offering the advantage of being clinically effective in reducing both dyskinesia as well as OFF episodes. Taken at bedtime, this delayed-release ER formulation of amantadine provides low plasma levels overnight so as to minimise negative impact on sleep, with higher plasma concentrations (and therefore, efficacy) occurring during the waking day [2].
Dyskinesia and/OFF episodes are commonly reported by persons with PD and adversely impact their everyday life. In a Parkinson and Movement Disorder Alliance survey designed to evaluate the impact of motor complications for persons with PD or their care partners, dyskinesia, OFF episodes or both (at least occasionally) were reported by 51%, 76% and 48% of evaluable respondents (n = 775), respectively [39]. In those acknowledging dyskinesia or OFF episodes, 86% and 90% reported motor complications were present daily, and 61% and 60% reported having to change plans and activities to accommodate these complications. Motor complications can also make interactions difficult, be isolating and lead to feelings of loneliness [39].
Overcoming the usual challenge of treating dyskinesia and OFF episodes has traditionally required a trade-off due to opposing treatment approaches [40], until the approval of amantadine ER. Amantadine ER reduces both dyskinesia and OFF episodes and increases ON time without troublesome dyskinesia, without a requirement to adjust existing levodopa or other dopaminergic therapies [21, 22]. In addition, amantadine ER provides control of motor complications throughout the day, and is associated with fewer and shorter episodes of troublesome dyskinesia and OFF with a resultant increase in the duration of ON time without troublesome dyskinesia and fewer transitions between motor states [30]. The efficacy of amantadine ER in reducing the duration and functional impact of dyskinesia and OFF episodes is evident with up to ≈2 years of treatment in the open-label safety trial, and regardless of previous therapy [23].
The efficacy of amantadine ER in patients with PD and motor complication was further evaluated using indirect treatment comparisons [41, 42]. Amongst amantadine formulations (including IR administered 2–3 times/day and IR/ER administered in the morning), only the delayed-release formulation of amantadine ER administered at bedtime was associated with significant (p ≤ 0.0006) improvements in both dyskinesia and OFF time [41]. Similarly, amantadine ER improved time with dyskinesia by 30% and OFF time by 36% from baseline, whereas oral dopaminergic treatments and levodopa/carbidopa intestinal gel (CLIG) improved OFF by 15–29% and worsened (increased by 12–31% with oral treatments) or did not impact (CLIG) dyskinesia [42]. Improvements in dyskinesia and OFF time were also seen with DBS [42].
Amantadine ER is generally well tolerated [8], including during long-term treatment [23], with the majority of AEs being mild or moderate in severity. The EASE LID [22] and EASE LID 3 [21] trials excluded patients with cognitive impairment who are at a higher risk of mental status changes and agitational states [43]; therefore, the safety of amantadine ER should be carefully considered in such real-life settings.
When using a cost-utility model with a 10-year base-case patient time horizon from a US payer perspective to assess the potential cost-effectiveness of amantadine ER for the treatment of dyskinesia, the incremental cost-utility ratio was $US98,905/quality-adjusted life year in the base case, and varied by time horizon ($US166,201 at 1 year and $US123,261 at 5 years) [44]. This appears to be acceptable by commonly-cited thresholds of societal willingness to pay. Results were sensitive to PD patient utilities (OFF time, ON time and ON time with dyskinesia), rate of disease progression, adjustment for placebo effects and impact on supportive case costs [44]. Further cost-effectiveness analyses would be of interest.
Current treatment guidelines precede the approval of amantadine ER for the treatment of dyskinesia and OFF episodes [45]. The guidelines indicate that although many treatment options are available for motor fluctuations, there are only a few clinically available pharmacological options that specifically target dyskinesia; there are no recommendations for the use of a single agent to treat both dyskinesia and motor fluctuations [45]. Head-to-head trials comparing extended- and immediate- formulations of amantadine would be of interest, as would trials exploring the use of amantadine ER in early PD.
Change history
29 November 2022
A Correction to this paper has been published: https://doi.org/10.1007/s40267-022-00973-z
References
Müller T, Möhr JD. Recent clinical advances in pharmacotherapy for levodopa-induced dyskinesia. Drugs. 2019;79(13):1367–74.
Isaacson SH, Lyons KE, Amjad F, et al. Development, efficacy and safety of once-daily, bedtime, extended-release amantadine (Gocovri®) to treat dyskinesia and OFF time in Parkinson’s disease. touchREVIEWS Neurol. 2021;17(1):36–47.
Aradi SD, Hauser RA. Medical management and prevention of motor complications in Parkinson’s disease. Neurotherapeutics. 2020;17(4):1339–65.
Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8(5):464–74.
Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16(3):448–58.
Vijayakumar D, Jankovic J. Drug-Induced dyskinesia, part 1: treatment of levodopa-induced dyskinesia. Drugs. 2016;76(7):759–77.
Paik J, Keam SJ. Amantadine extended-release (GOCOVRI™): a review in levodopa-induced dyskinesia in Parkinson’s disease. CNS Drugs. 2018;32(8):797–806.
Adamas Pharma LLC. GOCOVRI® (amantadine) extended-release capsules, for oral use: US prescribing information. 2021. https://www.gocovrihcp.com/pdf/Gocovri_Prescribing_Information.pdf. Accessed 31 Mar 2022.
Endo Pharmaceuticals Inc. SYMMETREL® (amantadine hydrochloride) tablets and syrup: Us prescribing information 2009. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/016023s041,018101s016lbl.pdf. Accessed 31 Mar 2022.
Adamas Pharma LLC. OSMOLEX® ER (amantadine) extended-release tablets, for oral use: US prescribing information 2021. https://www.osmolexhcp.com/wp-content/uploads/2021/01/Prescribing_Information.pdf. Accessed 31 Mar 2022.
Postma JU, Van Tilburg W. Visual hallucinations and delirium during treatment with amantadine (Symmetrel). J Am Geriatr Soc. 1975;23(5):212–5.
Schwab RS, England AC Jr, Poskanzer DC, et al. Amantadine in the treatment of Parkinson’s disease. JAMA. 1969;208(7):1168–70.
Duty S. Targeting glutamate receptors to tackle the pathogenesis, clinical symptoms and levodopa-induced dyskinesia associated with Parkinson’s disease. CNS Drugs. 2012;26(12):1017–32.
Parsons CG, Quack G, Bresink I, et al. Comparison of the potency, kinetics and voltage-dependency of a series of uncompetitive NMDA receptor antagonists in vitro with anticonvulsive and motor impairment activity in vivo. Neuropharmacology. 1995;34(10):1239–58.
Muller T. Experimental dopamine reuptake inhibitors in Parkinson’s disease: a review of the evidence. J Exp Pharmacol. 2021;13:397–408.
Mintzer J, Burns A. Anticholinergic side-effects of drugs in elderly people. J R Soc Med. 2000;93(9):457–62.
Muir KW, Lees KR. Clinical experience with excitatory amino acid antagonist drugs. Stroke. 1995;26(3):503–13.
Hauser RA, Pahwa R, Wargin WA, et al. Pharmacokinetics of ADS-5102 (amantadine) extended release capsules administered once daily at bedtime for the treatment of dyskinesia. Clin Pharmacokinet. 2019;58(1):77–88.
Hauser R, Isaacson S, Mittur A, et al. Analysis of the shape of the Gocovri steady-state PK profile: implications for an extended release product [abstract no. 107]. Mov Disord. 2019;34(Suppl 2):S47.
Guay DR. Amantadine and rimantadine prophylaxis of influenza A in nursing homes. A tolerability perspective. Drugs Aging. 1994;5(1):8–19.
Oertel W, Eggert K, Pahwa R, et al. Randomized, placebo-controlled trial of ADS-5102 (amantadine) extended-release capsules for levodopa-induced dyskinesia in Parkinson’s disease (EASE LID 3). Mov Disord. 2017;32(12):1701–9.
Pahwa R, Tanner CM, Hauser RA, et al. ADS-5102 (amantadine) extended-release capsules for levodopa-induced dyskinesia in Parkinson disease (EASE LID study): a randomized clinical trial. JAMA Neurol. 2017;74(8):941–9.
Tanner CM, Pahwa R, Hauser RA, et al. EASE LID 2: a 2-year open-label trial of Gocovri (amantadine) extended release for dyskinesia in Parkinson’s disease. J Parkinsons Dis. 2020;10(2):543–58.
Pahwa R, Tanner CM, Hauser RA, et al. Amantadine extended release for levodopa-induced dyskinesia in Parkinson’s disease (EASED study). Mov Disord. 2015;30(6):788–95.
Elmer LW, Juncos JL, Singer C, et al. Pooled analyses of phase III studies of ADS-5102 (amantadine) extended-release capsules for dyskinesia in Parkinson’s disease. CNS Drugs. 2018;32(4):387–98.
Hauser RA, Lytle J, Formella AE, et al. Amantadine delayed release/extended release capsules significantly reduce OFF time in Parkinson’s disease. NPJ Parkinsons Dis. 2022;8(1):29.
Hauser RA, Goud S, Formella AE. Potential utility of amantadine DR/ER in persons with Parkinson’s disease meeting 5-2-1 criteria for device aided therapy. Clin Park Relat Disord. 2022;6:100123.
Pahwa R, Isaacson S, Jimenez-Shaheed J, et al. Impact of dyskinesia on activities of daily living in Parkinson’s disease: results from pooled phase 3 ADS-5102 clinical trials. Parkinsonism Relat Disord. 2019;60:118–25.
Tanner C, Ostrem J, Lytle J, et al. Amantadine DR/ER improved OFF-state dystonia motor complications in PD [abstract no. P11.004 ]. In: 74th Annual Meeting of the American Academy of Neurology. 2022.
Hauser RA, Kremens DE, Elmer LW, et al. Prevalence of dyskinesia and OFF by 30-minute intervals through the day and assessment of daily episodes of dyskinesia and OFF: novel analyses of diary data from Gocovri pivotal trials. J Parkinsons Dis. 2019;9(3):591–600.
Hauser RA, Walsh RR, Pahwa R, et al. Amantadine ER (Gocovri®) significantly increases ON time without any dyskinesia: pooled analyses from pivotal trials in Parkinson’s disease. Front Neurol. 2021;12:645706.
Hauser R, Pawha R, Walsh R, et al. ADS-5102 significantly increases ON time without dyskinesia: pooled patient diary analyses from Parkinson’s disease pivotal trials [abstract no. 4718]. Neurology. 2021;96(15 Suppl):4718.
Hauser RA, Mehta SH, Kremens D, et al. Effects of Gocovri (amantadine) extended-release capsules on motor aspects of experiences of daily living in people with Parkinson’s disease and dyskinesia. Neurol Ther. 2021;10:739–51.
Horvath K, Aschermann Z, Kovacs M, et al. Minimal clinically important differences for the experiences of daily living parts of movement disorder society-sponsored unified Parkinson’s disease rating scale. Mov Disord. 2017;32(5):789–93.
Mehta SH, Pahwa R, Tanner CM, et al. Effects of Gocovri (amantadine) extended release capsules on non-motor symptoms in patients with Parkinson’s disease and dyskinesia. Neurol Ther. 2021;10:307–20.
Tanner CM, Lytle JM, Formella AE. Amantadine DR/ER efficacy as early add-on for motor complications in Parkinson’s disease [abstract no. 446]. Mov Disord. 2021;36(Suppl 1):S196.
Lyons KE, Pahwa R, Crouse N, et al. Amantadine DR/ER-related reduction in OFF and dyskinesia improved patient-rated interference with activities and social interactions [abstract no. 414]. Mov Disord. 2021;36(Suppl 1):S184–5.
Tanner C, Pahwa R, Vandevoorde V, et al. Safety of Gocovri in clinical practice: one-year post-launch pharmacovigilance data [abstract no. 213]. Mov Disord. 2019;34(Suppl 2):S89.
Farmer J, Bixby M, Brillman S, et al. Evaluating the impact of levodopa-induced dyskinesia and OFF time in persons affected by Parkinson’s disease: results of a PMD Alliance survey [abstract no. 4624 plus presentation]. Neurology. 2021;96(15 Suppl):4624.
Pajo AT, Espiritu AI, Jamora RDG. Efficacy and safety of extended-release amantadine in levodopa-induced dyskinesias: a meta-analysis. Neurodegener Dis Manag. 2019;9(4):205–15.
Oertel W, Pahwa R, Hauser RA, et al. Analysis of amantadine formulations for OFF and dyskinesia in Parkinson disease [abstract no. 520]. Movement Disorder. 2021;36(Suppl 1):S225.
Kremens DE, Oertel W, Pahwa R, et al. Indirect treatment comparison of adjunctive treatments for patients with Parkinson’s disease experiencing motor complications [abstract no. 500]. Mov Disord. 2021;36(Suppl 1):S216.
Rascol O, Fabbri M, Poewe W. Amantadine in the treatment of Parkinson’s disease and other movement disorders. Lancet Neurol. 2021;20(12):1048–56.
Pahwa R, Garrison L, Zimmerman M, et al. Assessing the potential cost-effectiveness of ADS-5102 (amantadine HCl) for the treatment of dyskinesia in Parkinson’s disease patients [abstract no. 877]. Mov Disord. 2018;33(Suppl 2):S398.
Fox SH, Katzenschlager R, Lim SY, et al. International Parkinson and Movement Disorder Society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 2018;33(8):1248–66.
Acknowledgements
The manuscript was reviewed by: J. Margolesky, Department of Neurology, University of Miami Miller School of Medicine, Miami, FL, USA; T. Müller, Department of Neurology, St. Joseph Hospital Berlin-Weissensee, Berlin, Germany; O. Rascol, Departments of Clinical Pharmacology and Neurosciences, University of Toulouse III, Toulouse, France. During the peer review process, Supernus Pharmaceuticals Inc., the marketing authorization holder of amantadine ER (GOCOVRI®), was also offered an opportunity to provide a scientific accuracy review of their data. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
Z. T. Al-Salama is a salaried employee of Adis International Ltd/Springer Nature and an editor of Drugs & Therapy Perspectives. She was not involved in any publishing decisions for the manuscript and declares no declare no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content
Ethics approval, Consent to participate, Consent for publication, Availability of data and material, Code availability
Not applicable.
Additional information
The original article has been revised due to retrospective open choice order.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Al-Salama, Z.T. Amantadine extended release capsules (GOCOVRI®) in Parkinson’s disease: a profile of its use in the USA. Drugs Ther Perspect 38, 203–214 (2022). https://doi.org/10.1007/s40267-022-00912-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-022-00912-y