Abstract
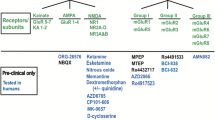
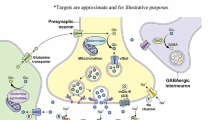
Treatment-resistant depression (TRD) affects many people with major depressive disorder or bipolar disorder. Glutamate is the key excitatory neurotransmitter in the CNS, and the confirmed rapid-acting antidepressant (RAAD) efficacy of the glutamatergic agent ketamine has fuelled extensive research into other glutamatergic agents. While mixed results have been reported in clinical trials, several glutamate receptor antagonists, including glycine site modulators, show promise as RAADs for severe TRD. The place of glutamatergic agents in the hierarchy of treatment for TRD is yet to be determined as more robust long-term efficacy and safety data are required. Nevertheless, ketamine and esketamine appear to be useful options for the rapid treatment of TRD.
Similar content being viewed by others
References
Henter ID, Park LT, Zarate CA. Novel glutamatergic modulators for the treatment of mood disorders: current status. CNS Drugs. 2021;35(5):527–43.
Delfino RS, Surjan J, Bandeira ID, et al. NMDA antagonists and their role in the management of bipolar disorder: a review. Curr Behav Neurosci Rep. 2020;7(2):76–85.
Kasper S, Cubala WJ, Fagiolini A, et al. Practical recommendations for the management of treatment-resistant depression with esketamine nasal spray therapy: basic science, evidence-based knowledge and expert guidance. World J Biol Psychiatry. 2021;22(6):468–82.
López-Díaz Á, Murillo-Izquierdo M, Moreno-Mellado E. Off-label use of ketamine for treatment-resistant depression in clinical practice: European perspective. Br J Psychiatry. 2019;215(2):447–8.
Karabatsiakis A, Schönfeldt-Lecuona C. Depression, mitochondrial bioenergetics, and electroconvulsive therapy: a new approach towards personalized medicine in psychiatric treatment—a short review and current perspective. Transl Psychiatry. 2020;10(1):226.
Sanacora G, Frye MA, McDonald W, et al. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiat. 2017;74(4):399–405.
Correia-Melo FS, Leal GC, Vieira F, et al. Efficacy and safety of adjunctive therapy using esketamine or racemic ketamine for adult treatment-resistant depression: a randomized, double-blind, non-inferiority study. J Affect Disord. 2020;264:527–34.
Kishimoto T, Chawla JM, Hagi K, et al. Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med. 2016;46(7):1459–72.
Janssen Pharmaceutical Companies. Spravato (esketamine) US Prescribing Information. 2020. https://www.accessdata.fda.gov/. Accessed 9 Nov 2021.
Leal GC, Bandeira I, Correia-Melo FS, et al. Intravenous arketamine for treatment resistant depression: open-label pilot study. Eur Arch Psychiatry Clin Neurosci. 2021;271(3):577–82.
Axsome Therapeutics. Axsome Therapeutics announces AXS-05 achieves primary endpoint in phase 2 trial in major depressive disorder [media release]. 7 Jan 2019. https://www.globenewswire.com/.
The Pharmaletter. Axsome sees mixed top-line ph III results with AXS-05 in TR depression [media release]. 31 Mar 2020. https://www.thepharmaletter.com/.
Relmada Therapeutics. Relmada Therapeutics announces top-line results from REL-1017 phase 2 study in individual with treatment resistant depression [media release]. 15 Oct 2019. https://www.prnewswire.com/.
Nagele P, Duma A, Kopec M, et al. Nitrous oxide for treatment-resistant major depression: a proof-of-concept trial. Biol Psychiatry. 2015;78(1):10–8.
Heresco-Levy U, Gelfin G, Bloch B, et al. A randomized add-on trial of high-dose d-cycloserine for treatment-resistant depression. Int J Neuropsychopharmacol. 2013;16(3):501–6.
Kantrowitz JT, Halberstam B, Gangwisch J. Single-dose ketamine followed by daily d-cycloserine in treatment-resistant bipolar depression. J Clin Psychiatry. 2015;76(6):737–8.
NeuroRx. NeuroRx: phase 3 drug for suicidal bipolar depression to present at BIO CEO conference [media release]. 10 Feb 2020. https://www.prnewswire.com/.
Huang CC, Hua Wei I, Huang CL, et al. Inhibition of glycine transporter-I as a novel mechanism for the treatment of depression. Biol Psychiatry. 2013;74(10):734–41.
US National Library of Medicine. ClinicalTrials.gov. 2021. https://clinicaltrials.gov/. Accessed 9 Nov 2021.
GlaxoSmithKline. WELLBUTRIN SR (bupropion) US Prescribing Information. 2020. https://www.accessdata.fda.gov/. Accessed 9 Nov 2021.
The National Institute for Health and Care Excellence. Esketamine for treatment-resistant depression (ID1414). 2020. https://www.nice.org.uk/. Accessed 9 Nov 2021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
C. Fenton, a contracted employee of Adis International Ltd/Springer Nature, and A. Lee, a salaried employee of Adis International Ltd/Springer Nature, declare no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent for publication, Availability of data and material, Code availability
Not applicable.
Rights and permissions
About this article
Cite this article
Fenton, C., Lee, A. Glutamatergic modulators are poorly understood, but promising therapies in depressive disorders. Drugs Ther Perspect 38, 7–12 (2022). https://doi.org/10.1007/s40267-021-00882-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-021-00882-7