Abstract
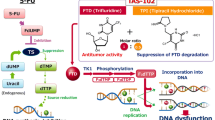
Trifluridine/tipiracil (Lonsurf®; also known as TAS-102), an oral antimetabolite agent, is a welcome addition to the options available to treat metastatic colorectal cancer in patients who are refractory to, or are not considered candidates for, currently available therapies. This novel fixed-dose formulation combines trifluridine, which inhibits cell proliferation after being incorporated into DNA via phosphorylation, with tipiracil, which increases systemic exposure to trifluridine by potently inhibiting thymidine phosphorylase. In clinical trials, the addition of trifluridine/tipiracil (35 mg/m2 twice daily on days 1–5 and 8–12 of each 28-day cycle) to best supportive care significantly improved overall survival, progression-free survival and disease control relative to placebo. Adverse events associated with trifluridine/tipiracil can generally be managed with dose modification and/or supportive measures.


Similar content being viewed by others
References
Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–422.
National Comprehensive Cancer Network®. Clinical Practice Guidelines in Oncology (NCCN Guidelines®): colon cancer (version 1.2017). Fort Washington: National Comprehensive Cancer Network®, Inc.; 2016.
Lonsurf (trifluridine/tipiracil) film-coated tablets: summary of product characteristics. London: European Medicines Agency; 2016.
Lonsurf (trifluridine and tipiracil) tablets for oral use: US prescribing information. Princeton (NJ): Taiho Oncology Inc.; 2015.
Lonsurf combination tablet. Japanese prescribing information. Tokyo: Taiho Pharmaceutical Co., Ltd.; 2015.
Emura T, Suzuki N, Fujioka A, et al. Potentiation of the antitumor activity of α, α, α-trifluorothymidine by the co-administration of an inhibitor of thymidine phosphorylase at a suitable molar ratio in vivo. Int J Oncol. 2005;27(2):449–55.
Lenz H-J, Stintzing S, Loupakis F. TAS-102, a novel antitumor agent: a review of the mechanism of action. Cancer Treat Rev. 2015;41(9):777–83.
Suzuki N, Emura T, Fukushima M. Mode of action of trifluorothymidine (TFT) against DNA replication and repair enzymes. Int J Oncol. 2011;39(1):263–70.
Matsuoka K, Iimori M, Niimi S, et al. Trifluridine induces p53-dependent sustained G2 phase arrest with its massive misincorporation into DNA and few DNA strand breaks. Mol Cancer Ther. 2015;14(4):1004–13.
Tanaka N, Sakamoto K, Okabe H, et al. Repeated oral dosing of TAS-102 confers high trifluridine incorporation into DNA and sustained antitumor activity in mouse models. Oncol Rep. 2014;32(6):2319–26.
Yamashita F, Komoto I, Oka H, et al. Exposure-dependent incorporation of trifluridine into DNA of tumors and white blood cells in tumor-bearing mouse. Cancer Chemother Pharmacol. 2015;76(2):325–33.
Yoshino T, Mizunuma N, Yamazaki K, et al. TAS-102 monotherapy for pretreated metastatic colorectal cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2012;13(10):993–1001.
Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909–19.
Suzuki S, Iwaizumi M, Hamaya Y, et al. Trifluridine to enhance cytotoxicity by DNA mismatch repair deficiency and subsequent MBD4 frameshift mutation in colorectal cancer [abstract no. 608]. J Clin Oncol. 2016;34(Suppl 4).
Emura T, Murakami Y, Nakagawa F, et al. A novel antimetabolite, TAS-102 retains its effect on FU-related resistant cancer cells. Int J Mol Med. 2004;13(4):545–9.
Emura T, Nakagawa F, Fujioka A, et al. An optimal dosing schedule for a novel combination antimetabolite, TAS-102, based on its intracellular metabolism and its incorporation into DNA. Int J Mol Med. 2004;13(2):249–55.
Sun W, Rosen LS, Rasco DW, et al. An open-label, randomized, parallel-group study of the pharmacokinetics of trifluridine as a component of TAS-102 versus FTD alone [abstract no. 751]. In: Gastrointestinal Cancers Symposium. 2016.
Yoshino T, Shinozaki E, Yamazaki K, et al. Final survival results and onset of neutropenia as an indicator of therapeutic effect in phase 2 of TAS-102 vs placebo with metastatic colorectal cancer (J003-10040030) [abstract no. PD-014]. Ann Oncol. 2016;27(Suppl 2):ii107.
Mayer RJ, Ohtsu A, Yoshino T, et al. TAS-102 versus placebo plus best supportive care in patients with metastatic colorectal cancer refractory to standard therapies: final survival results of the phase III RECOURSE trial [abstract no. 634]. J Clin Oncol. 2016;34(Suppl 4).
Committee for Medicinal Products for Human Use. Lonsurf: European public assessment report (EMA/CHMP/287846/2016). London: European Medicines Agency; 2016.
Van Cutsem E, Benedetti FM, Mizuguchi H, et al. TAS-102 vs placebo (PBO) in patients (pts) >65 years (y) with metastatic colorectal cancer (mCRC): an age-based analysis of the RECOURSE trial [abstract no. 638]. J Clin Oncol. 2016;34(Suppl 4).
Ohtsu A, Yoshino T, Wahba MM, et al. Phase III RECOURSE trial of TAS-102 versus placebo with best supportive care in patients with metastatic colorectal cancer: geographic subgroups [abstract no. 646]. J Clin Oncol. 2016;34(Suppl 4).
Longo-Muñoz F, Argiles G, Tabernero J, et al. Efficacy of trifluridine and tipiracil (TAS-102) versus placebo, with supportive care, in a randomized, controlled trial of patients with metastatic colorectal cancer from Spain: results of a subgroup analysis of the phase 3 RECOURSE trial. Clin Transl Oncol. 2017;19(2):227–35.
VanCutsem E, GarciaCarbonero R, Pastorino A, et al. RECOURSE trial: performance status at discontinuation in patients receiving trifluridine/tipiracil (TAS-102) [abstract no. 515P]. Ann Oncol. 2016;27(Suppl 6):vii74.
Falcone A, Ohtsu A, Van Cutsem E, et al. Integrated safety summary (ISS) for trifluridine/tipiracil (TAS-102) [abstract]. Ann Oncol. 2016;27(Suppl 6):555P.
Falcone A, Garcia-Carbonero R, Tabernero J, et al. Low rates of hospitalizations with TAS-102 in the European (EU) subregion of the Phase 3 RECOURSE trial in patients (pts) with metastatic colorectal cancer (mCRC) [abstract no. 2150]. Eur J Cancer. 2015;51(Suppl 3):S383.
Shinozaki E, Laurent S, Gravalos C, et al. Timing of adverse events (AEs) in the phase 3 RECOURSE trial of TAS-102 versus placebo in patients (pts) with metastatic colorectal cancer (mCRC) [abstract no. 2151]. Eur J Cancer. 2015;51(Suppl 3):S383–4.
Tabernero J, Mayer R, Ohtsu A, et al. RECOURSE trial: impact of adverse events on quality of life and duration of TAS-102 (trifluridine and tipiracil) treatment [abstract no. PD-025]. Ann Oncol. 2016;27(Suppl 2):ii117.
Mayer R, Van Cutsem E, Yoshino T, et al. Supportive treatment for hematologic toxicities in the phase 3 RECOURSE trial of TAS-102 vs placebo with best supportive care in patients with metastatic colorectal cancer [abstract no. e15021]. J Clin Oncol. 2016;34(Suppl 4).
Yoshino T, Uetake H, Furuta T, et al. TAS-102 safety in metastatic colorectal cancer: results from the first post-marketing surveillance study. Clin Colorectal Cancer. 2016;15(4):e205–11.
Bendell JC, Patel MR, Yoshida K, et al. Phase 1 study of cardiac safety of TAS-102 in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2016;77(6):1275–83.
Sakamoto K, Yokogawa T, Ueno H, et al. Crucial roles of thymidine kinase 1 and deoxyUTPase in incorporating the antineoplastic nucleosides trifluridine and 2′-deoxy-5-fluorouridine into DNA. Int J Oncol. 2015;46(6):2327–34.
Stivarga® (regorafenib) tablets: US prescribing information. Whippany (NJ): Bayer Healthcare Pharmaceuticals Inc.; 2015.
Masuishi T, Taniguchi H, Hamauchi S, et al. Regorafenib versus trifluridine/tipiracil for refractory metastatic colorectal cancer: a retrospective comparison. Clin Colorectal Cancer. 2016. doi:10.1016/j.clcc.2016.07.019.
Kimura M, Usami E, Iwai M, et al. Comparison of cost-effectiveness of regorafenib and trifluridine/tipiracil combination tablet for treating advanced and recurrent colorectal cancer. Mol Clin Oncol. 2016;5(5):635–40.
Kotani D, Shitara K, Kawazoe A, et al. Safety and efficacy of trifluridine/tipiracil monotherapy in clinical practice for patients with metastatic colorectal cancer: experience at a single institution. Clin Colorectal Cancer. 2016;15(3):e109–15.
Burness CB, Duggan ST. Trifluridine/tipiracil: a review in metastatic colorectal cancer. Drugs. 2016;76(14):1392–402.
Acknowledgements
This manuscript is updated from Drugs 2016;76(14):1392–1402 [37] and was reviewed by: B. Royer, Department of Pharmacology and Toxicology, Centre Hospitalier Régional Universitaire de Besançon, Besançon, France; S.V. Sakpal, Department of Surgery, Medical College of Wisconsin, Milwaukee, WI, USA; R.G. Watson, Alexion Pharmaceuticals, Inc., Lexington, MA, USA; K.-H. Yeh, Graduate Institute of Oncology, National Taiwan University College of Medicine, Taipei, Taiwan; A. Zanboni, Medical Oncology Department, Fondazione Poliambulanza, Brescia, Italy. During the peer review process, the manufacturer of trifluridine/tipiracil was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
KA. Lyseng-Williamson, C.B. Burness and S.T. Duggan are/were salaried employees of Adis/Springer, are responsible for the article content and declare no relevant conflicts of interest.
Rights and permissions
About this article
Cite this article
Lyseng-Williamson, K.A., Burness, C.B. & Duggan, S.T. Trifluridine/tipiracil in metastatic colorectal cancer: a guide to its use. Drugs Ther Perspect 33, 110–118 (2017). https://doi.org/10.1007/s40267-017-0385-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-017-0385-y