Abstract
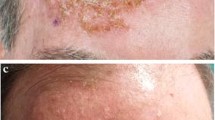
Ingenol mebutate gel (Picato®) is indicated for the topical treatment of actinic keratosis. Field-directed therapy with topical ingenol mebutate (150 µg/g gel applied once daily for 3 consecutive days for actinic keratoses on the face or scalp, and 500 µg/g gel applied once daily for 2 consecutive days for actinic keratoses on the trunk or extremities) resulted in significantly higher complete and partial clearance rates at day 57 than treatment with vehicle gel. Clearance was generally sustained during 1-year follow-up. Moreover, relative to cryosurgery alone, a 3-day course of ingenol mebutate 150 µg/g gel applied to the face and scalp 3 weeks after cryosurgery improved clearance of actinic keratosis at 11 weeks. Transient local skin responses are very common after initiation of ingenol mebutate gel.
Similar content being viewed by others
References
Rosen T, Lebowohl MG. Prevalence and awareness of actinic keratosis: barriers and opportunities. J Am Acad Dermatol. 2013;68(1 Suppl 1):S2–9.
Ceilley RI, Jorizzo JL. Current issues in the management of actinic keratosis. J Am Acad Dermatol. 2013;68(1 Suppl 1):S28–38.
Uhlenhake EE. Optimal treatment of actinic keratoses. Clin Interv Aging. 2013;8:29–35.
Del Rosso JQ. Current regimens and guideline implications for the treatment of actinic keratosis: proceedings of a clinical roundtable at the 2011 Winter Clinical Dermatology Conference. Cutis. 2011;88(Suppl 1):1–8.
Nashan D, Meiss F, Muller M. Therapeutic strategies for actinic keratoses: a systematic review. Eur J Dermatol. 2013;23(1):14–32.
Rigel DS, Stein Gold LS. The importance of early diagnosis and treatment of actinic keratosis. J Am Acad Dermatol. 2013;68(1 Suppl 1):S20–7.
Rosen RH, Gupta AK, Tyring SK. Dual mechanism of action of ingenol mebutate gel for topical treatment of actinic keratoses: rapid lesion necrosis followed by lesion-specific immune response. J Am Acad Dermatol. 2012;66(3):486–93.
Ramsay JR, Suhrbier A, Aylward JH, et al. The sap from Euphorbia peplus is effective against human nonmelanoma skin cancers. Br J Dermatol. 2011;164(3):633–6.
Rizk AM, Hammouda FM, El-Missiry MM, et al. Biologically active diterpene esters from Euphorbia peplus. Phytochem. 1985;24(7):1605–6.
Picato® (ingenol mebutate) 150 and 50 micrograms/gram gel: summary of product characteristics. London: European Medicines Agency; 2014.
Stahlhut M, Bertelsen M, Hoyer-Hansen M, et al. Ingenol mebutate: induced cell death patterns in normal and cancer epithelial cells. J Drugs Dermatol. 2012;11(10):1181–92.
Ogbourne S, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64(8):2833–9.
Challacombe JM, Suhrbier A, Parsons PG, et al. Neutrophils are a key component of the antitumor efficacy of topical chemotherapy with ingenol-3-angelate. J Immunol. 2006;177(11):8123–32.
Hampson P, Kavanagh D, Smith E, et al. The anti-tumor agent, ingenol-3-angelate (PEP005), promotes the recruitment of cytotoxic neutrophils by activation of vascular endothelial cells in a PKC-d dependent manner. Cancer Immunol Immunother. 2012;57(8):1241–51.
Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366(11):1010–9.
Berman B, Marmur E, Larsson T, et al. Three-day topical treatment with ingenol mebutate gel 0.015% for actinic keratoses on the face and scalp: analysis of data pooled from two trials [poster no. 5623]. In: 70th Annual Meeting of the American Academy of Dermatology; 16–20 Mar 2012; San Diego.
Anderson LL, Schmieder GJ, Xu Z, et al. Two-day topical treatment with ingenol mebutate gel 0.05% for actinic keratoses on the trunk and extremities: analysis of data pooled from two trials [poster no. 5640]. In: 70th Annual Meeting of the American Academy of Dermatology; 16–20 Mar 2012; San Diego.
Lebwohl M, Shumack S, Stein Gold L, et al. Long-term follow-up study of ingenol mebutate gel for the treatment of actinic keratosis. JAMA Dermatol. 2013;149(6):666–70.
Berman B, Goldenberg G, Hanke W, et al. Efficacy and safety of ingenol mebutate 0.015% gel 3 weeks after cryosurgery of actinic keratosis: 11-week results. J Drugs Dermatol. 2014;13(2):154–60.
Leo Pharma. FIELD study 1 now complete: evaluating one year treatment outcomes of actinic keratosis after a combined lesion- and field-directed treatment approach with cryosurgery and ingenol mebutate gel [media release]. 26 Sep 2013. http://www.leo-pharma.com/Home/LEO-Pharma/Media-Centre/News/News-2013/LEO-Pharma-completes-FIELD-Study-1.aspx.
Stockfleth E, et al. A consensus approach to improving patient adherence and persistence with topical treatment for actinic keratosis [poster no.IST13-1460]. In: 22nd Congress of the European Academy of Dermatology and Venereology; 2–6 Oct 2013; Istanbul.
Augustin M, et al. Effect of ingenol mebutate gel on treatment satisfaction and quality of life in actinic keratosis [poster no.IST13-1530]. In: 22nd Congress of the European Academy of Dermatology and Venereology; 2–6 Oct 2013; Istanbul.
Acknowledgments
The manuscript was reviewed by: S. Saluja, Consultant in Internal Medicine, Saran Ashram, Agra, India; W.P. Werschler, Department of Medicine/Dermatology, University of Washington School of Medicine, Seattle, Washington, USA.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from comments received were made by the authors on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lyseng-Williamson, K.A., Keating, G.M. Ingenol mebutate gel: a guide to its use in actinic keratosis in the EU. Drugs Ther Perspect 30, 155–160 (2014). https://doi.org/10.1007/s40267-014-0121-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-014-0121-9