Abstract
Objective
Temporomandibular disorders (TMDs) encompass several conditions that cause pain and impair function of the masticatory muscles (M-TMDs) and temporomandibular joints. There is a large interest among clinicians and researchers in the use of botulinum toxin-A (BoNT-A) as a treatment for M-TMD. However, due to the lack of consistent evidence regarding the efficacy as well as adverse events of BoNT-A, clinical decision making is challenging. Therefore, this umbrella review aimed to systematically assess systematic reviews (SRs) evaluating BoNT-A treatment effects on pain intensity, mandibular movements, and adverse events in patients with M-TMDs.
Method
An electronic search was undertaken in the databases MEDLINE, EMBASE, CINAHL, Cochrane Central Registry of Controlled Trials (CENTRAL), Web of Science, Epistemonikos, ClinicalTrials.gov, and ICTRP to identify SRs investigating BoNT-A effects on M-TMDs, published from the inception of each database until 6 December 2023. The quality of evidence was rated according to the critical appraisal checklist developed by the umbrella review methodology working group. Only high-quality SRs were included.
Results
In total, 18 SRs were included. BoNT-A was shown to be more effective than placebo to reduce pain intensity, but not compared to standard treatments. Additionally, BoNT-A was not superior to placebo or standard treatments regarding improvement of mandibular movements. BoNT-A was considered to have a higher risk for adverse events on muscle and bony tissue compared with other treatments.
Conclusion
The synthesis in this umbrella review provides the highest level of evidence present. Taken together, there are indications of effectiveness of BoNT-A for treatment of M-TMDs, supported by moderate evidence. However, considering the risk of causing serious adverse events, treatment with BoNT-A is recommended to be the last treatment alternative.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Evidence clearly indicates that BoNT-A has positive effects in the treatment of M-TMD but it is not superior to standard treatments. |
BoNT-A treatment for M-TMDs could cause adverse effects on muscle and bony tissue of major importance that must be considered. |
This umbrella review presents the highest level of evidence regarding BoNT-A treatment for M-TMDs, since it included only SRs with low risk of bias/high quality. |
1 Introduction
Botulinum toxin (BoNT) is a very potent biological toxin produced by several species of the Clostridia bacteria family, such as Clostridium botulinum [1, 2]. Intake of BoNT type A (BoNT-A) and other BoNT serotypes causes botulism, a condition that leads to flaccid paralysis of skeletal muscles and dysautonomia in humans. This effect is caused by interference with neurotransmitter release (acetylcholine) at presynaptic terminals [3, 4]. Because of the muscle paralysis, BoNT-A has become a common medical treatment used for autonomic disorders, spasticity, and hyperkinetic movement disorders, as well as in aesthetics for cosmetic purposes [5]. Recently, there has been a growing interest in its pain-reducing properties. Initially, the analgesic effect in neuromuscular disorders and musculoskeletal pain was attributed to the muscle relaxant effect, until the anti-hyperalgesic effect in non-muscular pain models was unequivocally demonstrated in human and animal models [6, 7].
In this regards experimental studies have proposed and demonstrated several antinociceptive mechanisms through local injections of BoNT-A that may reduce peripheral and central sensitization [8]. Some of the proposed analgesic effects of BoNT-A are: (1) suppression of the peripheral and central release of transport neurotransmitters (such as glutamate, calcitonin gene related peptide (CGRP), and substance P (SP)) to sensory regions of the trigeminal ganglia; (2) regulation of the pain modulation system by influencing the gamma-aminobutyric acid (GABA) and opioid-ergic systems; (3) reduction of microglia activation; and (4) modulation of ion channels [transient receptor potential vanilloid 1 (TRPV1), calcium (C+), and sodium (Na+)] [6, 9,10,11,12]. Notwithstanding, there is no evidence of the role and importance of these mechanisms. Thus, due to its analgesic properties, BoNT-A is used as a treatment approach for chronic pain conditions such as chronic migraine (on-label), but also other pain conditions such as neuropathic, back, pelvic, and myogenous temporomandibular disorder (TMD) pain (M-TMD) (off-label) [13,14,15,16].
M-TMD is the most common (45%) diagnosis among the TMD diagnoses and is characterized by regional pain and increased tenderness in the masticatory muscles, diminished masticatory performance, and restricted jaw movements [17]. Although several treatment approaches have been shown to be successful in the management of M-TMD [17,18,19,20,21], persistence of pain in the masticatory muscles is common [22]. Results from well conducted randomized placebo-controlled clinical trials (RCTs) on the effects of BoNT-A on persistent M-TMD differ, but those showing positive effects of BoNT-A indicate improvements in pain levels, somatosensory alterations, muscle tenderness, jaw mobility, and psychological well-being [23,24,25,26,27,28,29]. More recently, a large number of animal and clinical studies have shown that injections of BoNT-A into the masticatory muscles could produce several adverse events, such as muscle atrophy, alterations of the muscle’s histological composition, replacement of contractile tissue with fatty tissue [30,31,32], muscle weakness [33], reduction in maximum bite force, decrease in masticatory performance [34], and reduction in mandibular bone volume and other bony structural changes mainly in the mandible’s head and alveolar region [35, 36]. Taken together, the lack of consistent evidence regarding the benefits of BoNT-A for persistent M-TMD and its high probability of causing adverse events makes the clinical decision process challenging.
Since use of BoNT-A for M-TMDs is of great interest, many systematic reviews (SRs) have been performed over the last 15 years aiming to summarize the available literature, and to reach pertinent conclusions for the use of BoNT-A for treatment of patients with M-TMD. However, due to the inconclusive data, and to shortcomings in data curation and presentation of some SRs, no treatment protocols have yet been published and no definite conclusions have been drawn regarding the efficacy and safety (adverse events) of BoNT-A in the management of M-TMD. Formulating such conclusions are necessary since the use of BoNT-A as a primary treatment approach for M-TMD is increasing worldwide. Therefore, the aim of the present umbrella review was to systematically assess the findings and quality of SRs evaluating BoNT-A regarding its treatment effects on pain intensity, mandibular range of motion, and adverse events in patients with M-TMDs.
2 Material and Methods
2.1 Protocol
This umbrella review (UR) followed the protocol that was registered a priori in PROSPERO (the International Prospective Register of Systematic Reviews, registration number CRD42023468160). The UR was conducted according to the methodology published by Aromataris et al. [37], which includes the methodological development, conduct, and reporting of an UR.
2.2 Selection Criteria
The following inclusion criteria were adopted based on the PICOTS approach:
P = Population, I = Intervention, C = Comparator, O = Outcome, T = Time, S = Study
The population (P) was adult patients with mastication myalgia (M-TMD).
The intervention (I) was intramuscular injections of BoNT-A, regardless of doses and the number of injections administered.
The comparators (C) were no treatment, placebo, or other non-invasive, minimally invasive, or invasive therapeutic interventions.
The primary outcome (O) was reduction of pain intensity using a Visual Analogue Scale (VAS), Numerical Rating Scale (NRS), or other measurable pain scale. The secondary outcomes were changes in mandibular range of motion and adverse effects. Every measurable jaw-movement limitation was considered, with a focus on Maximum Mouth Opening (MMO). When available, adverse events (AEs) were collected.
The follow-up time (T) was either short term, i.e., ≤ 3 months, intermediate term, i.e., 3–5 months, or long term ≥ 6 months.
The study design (S) included only SRs that reported the outcomes of interest.
The following exclusion criteria were used: (1) studies presented in other languages than English, Spanish, Portuguese, Greek, and Scandinavian languages; (2) editorials, letters, legal cases, interviews, case-series, duplicates, observational studies, cross-sectional studies, case-control studies, non-randomized and randomized clinical trials, cohort studies, narrative review articles, and in vitro and animal studies; (3) SRs not investigating BoNT-A treatment for M-TMD.
2.3 Search Strategy
In partnership with the librarians Narcisa Hannerz (NH) and Sabrina Gillsund (SG) at the Karolinska Institutet University Library, a literature search strategy was developed to find SRs that discuss BoNT-A treatment for patients with mastication myalgia. The search was conducted electronically on 6 December 2023 and included SRs in English, Spanish, Portuguese, Greek, and Scandinavian languages without any restrictions on publication date. The search utilized eight databases including as MEDLINE, EMBASE, CINAHL, Cochrane Central Registry of Controlled Trials (CENTRAL), Web of Science, Epistemonikos, ClinicalTrials.gov, and ICTRP from the time each database was established.
In MEDLINE (Ovid), in partnership with NH and SG, the search strategy was formulated. SG reviewed the search strategies prior to NH executing the search. Each search concept involved identifying Medical Subject Headings (MeSH terms) and free text terms. The search was partly translated into other databases using the Polyglot Search Translator [38]. To eliminate duplicates, the method outlined by Bramer et al. was utilized [39]. Detailed search strategies for all databases can be found in the Online Supplementary Material (OSM) 2.
The Rayyan tool aided in screening titles and abstracts [40]. Two authors (HJ and AL) independently and blindly evaluated the titles and abstracts. All articles included by at least one of the authors were eligible for full-text assessment. Subsequently, all potentially eligible studies were obtained, and full-text articles were assessed by the same authors (HJ and AL) for meeting the inclusion criteria. Any discrepancies were resolved through discussion involving the third author (NC).
2.4 Data Extraction
For this review, a data extraction form was created and tested on two randomly chosen studies by authors MCS and ME to ensure uniformity in extraction. The form was adjusted based on a pilot test. Any discrepancies in data extraction were settled through discussion with a third author acting as an adjudicator (GDC). Extracted information encompassed study and participant details such as authors, type of SR, year of publication, objectives, diagnostic criteria, patient age, treatment groups, number and date range of database searching, date range, number and type of studies included in each review, instruments to appraise primary studies and their quality, methods of synthesis/analysis to synthesize evidence, and outcome measures.
2.5 Assessment of Risk of Bias and Methodological Quality of Included Systematic Reviews
Authors MCh and RP assessed the risk of bias independently using the critical appraisal checklist developed by the UR methodology working group [41]. Any discrepancies were resolved through discussion with a third author acting as an adjudicator (NC). Each of the questions posed in the checklist could be scored as being ‘met,’ ‘not met,’ ‘unclear,’ or ‘not applicable.’ The decision to include only high-quality SRs was made based on a pre-determined proportion of all criteria. The following seven questions were chosen as required to be met to get a score of high quality and low risk of bias: “Were the inclusion criteria appropriate for the review question?”, “Was the search strategy appropriate?”, “Were the sources and resources used to search for studies adequate?”, “Were the criteria for appraising studies appropriate?”, “Was critical appraisal conducted by two or more reviewers independently?”, “Was the likelihood of publication bias assessed?” and “Were recommendations for policy and/or practice supported by the reported data?” The question: “Is the review question clearly and explicitly stated?” was chosen to either be met or be unclear to get a score of high quality and low risk of bias, whereas the following two questions were chosen to not affect the methodological quality/risk of bias: “Were the methods used to combine studies appropriate?” and “Were the specific directives for new research appropriate?”
2.6 Curation and Processing of Data
Authors GDC and NC were responsible for reviewing and summarizing the evidence and data, a process that was then validated by the author EA. Subsequently, this information was organized into a table featuring a visual stop-light indicator. In this indicator, green signifies a beneficial (effective) intervention, orange represents no difference in the comparison, and red indicates that the intervention is either detrimental or less effective than the comparator. When it comes to adverse events, green indicates no adverse events, orange mild, and red indicates moderate or major/severe adverse events (Table 3).
3 Results
3.1 Literature Search Outcome
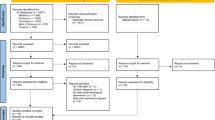
The electronic search yielded 6,514 articles from all databases. After removal of 3,205 duplicates, a total of 3,309 article titles and abstracts were screened for suitability. After reading titles and abstracts, 3,171 of the 3,309 articles were excluded because they did not meet the inclusion criteria. Thus, 138 were sought for retrieval, and three could not be retrieved. No further SRs were found from other sources, such as reference-lists, theses, etc. Finally, after reading the 135 full-text SRs, 34 met the inclusion criteria and were then evaluated regarding methodological quality/risk of bias, and data extracted. After this final assessment, 18 SRs reached the preset quality criteria and were included [18, 19, 36, 42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. Figure 1 shows the PRISMA flow diagram with the process of evaluating the SRs for inclusion.
3.2 Study Characteristics and Assessment of Risk of Bias/Methodological Quality
The initial search resulted in 34 SRs. The risk of bias/quality assessment is presented in OSM 3. Seven studies [57,58,59,60,61,62,63] were found to have some concerns (orange) and nine studies [64,65,66,67,68,69,70,71,72] a high risk of bias/low quality (red) and were excluded from the UR due to their methodological concerns. The study characteristics from the 18 SRs [18, 19, 36, 42,43,44,45,46,47,48,49,50,51,52,53,54,55,56] that were finally included are presented in detail in Table 1. These SRs were published between 2011 and 2023, and displayed a low risk of bias/high quality (green).
3.3 Summary of Findings
The majority (15 out of 18) of the included SRs investigated the pain-reducing effect of BoNT-A [18, 19, 42,43,44,45,46,47,48, 50,51,52,53, 55, 56], whereas eight investigated the effect of BoNT-A on mandibular movements [18, 19, 43,44,45,46, 52, 53]. Seven of the included SRs reported adverse events induced by treatment with BoNT-A [36, 44,45,46, 51, 52, 54]. A quantitative presentation of these outcomes is shown in Table 2. Table 3 summarizes the evidence from the quantitative research synthesis regarding the effects and adverse events after treatment with BoNT-A. Additionally, the number of times that the original RCTs were cited in each SR are presented in Table 4. Below is a brief description of each of these three outcomes reported in the included SRs.
3.3.1 Effect of BoNT-A Treatment on Pain Intensity
The effect of BoNT-A on pain intensity was reported in 15 SRs [18, 19, 42,43,44,45,46,47,48, 50,51,52,53, 55, 56], summarized in Tables 2a and 3a. In nine SRs the results pointed to an effect favoring treatment with BoNT-A compared to placebo (isotonic saline) [19, 44,45,46,47,48, 52, 53, 55]. Two SRs did not find any difference in pain reduction between BoNT-A and placebo (isotonic saline) [51, 56]. There were no significant differences between BoNT-A and standard treatments for M-TMD (occlusal splints, jaw exercises, etc.) [18, 19, 42, 44, 52, 53]. When compared with dry needling, three articles showed a significantly greater pain-reducing effect favoring dry needling compared to BoNT-A [43, 46, 48], and one showed similar results [50].
3.3.2 Effect of BoNT-A on Mandibular Movements
The effect of BoNT-A on mandibular movements was reported in eight of the included SRs [18, 19, 43,44,45,46, 52, 53] and summarized in Tables 2b and 3b. While two of the included SRs indicated that there is a favorable effect on mandibular movements for treatment with BONT-A compared to baseline (before-after) or to placebo (isotonic saline) [45, 46], four SRs did not show any significant improvements after treatment with BoNT-A compared to placebo [18, 19, 52, 53] or standard treatments [44, 52, 53]. One SR [43] concluded that dry needling therapy increased the range of motion more than wet needling with BoNT-A or other agents.
3.3.3 Reported Adverse Events Associated with Treatment with BoNT-A
With regard to possible adverse events associated with the use of BoNT-A in the masticatory muscles, seven SRs reported such outcomes [36, 44,45,46, 51, 52, 54]. The majority of these found impairment related to the reduction of masseter size with subsequent muscle weakness, leading to discomfort during chewing [44, 45]. Major adverse events reported were mostly related to injection technique and included paresthesia, eye drooping or muscle weakness, speech changes, perioral swelling, bruising, and an asymmetric smile [45]. A single study reported increased pain after injection with BoNT-A [44]. Bony changes were evaluated in two SRs, which found significant decreases in cortical thickness and volume after BoNT-A [36, 54]. Three SRs did not report any major adverse events compared to placebo or other therapies [46, 51, 52], as shown in Tables 2c and 3c.
3.3.4 Synthesis of Evidence for a Favorable Effect of BoNT-A
In summary, seven out of the 11 included SRs reporting data on the pain-reducing effect of BoNT-A versus placebo point to a favorable effect of BoNT-A [19, 44,45,46,47,48, 55]. Further, two of the 11 included SRs that reported data on the pain-reducing effect of BoNT-A versus placebo could only show a favorable effect of BoNT-A in less than half of the RCTs included in these SRs [52, 53]. However, the other two SRs did not show any favorable effect of BoNT-A when compared to placebo [51, 56]. When treatment with BoNT-A was compared to other treatments of TMD, none of the included SRs showed a favorable effect of BoNT-A. However, six of the included SRs showed similar effects between BoNT-A and other treatments of TMD [18, 19, 42, 44, 50, 53] (Table 3a).
With regard to mandibular movements, one of the included SRs showed a favorable effect with increased mandibular movements after treatment with BoNT-A when compared to baseline or placebo [45], while another SR only showed a favorable effect with increased mandibular movements after treatment with BoNT-A when compared to placebo in half of the RCTs included in this SR [46] (Table 3b).
4 Discussion
Presently, there is no unanimous agreement on the use of BoNT-A for treatment of M-TMDs. The main results of this UR show that BoNT-A is more effective than placebo in reducing pain intensity levels in M-TMD, but not more effective than standard therapies. In addition, treatment with BoNT-A does not seem to result in any significant improvement in jaw mobility compared to placebo or standard treatments of M-TMD. Moreover, this UR indicates that BoNT-A may not be entirely risk free for treatment of M-TMD due to its potential side effects like muscle atrophy and weakness, injection-related complications, and even alterations in the jaw-bone structure.
The pain-relieving effects by local BoNT-A injection are unequivocal, with numerous animal studies show-casing its ability to reduce pain through various mechanisms [6]. Recent research suggests that BoNT-A not only acts locally on sensory nerve endings, but is also transported to the nerve cell bodies in the trigeminal and dorsal root ganglia and to the caudal trigeminal nucleus and spinal nerve terminals within the central nervous system, where it influences the pain modulation system [9, 10]. This dual action on peripheral and central sensitization makes BoNT-A a candidate for treatment of M-TMDs. The consistent effectiveness of BoNT-A over placebos in most SRs included in this study supports this notion. This is further reinforced by a recent RCT on persistent M-TMD patients, showing that BoNT-A outperformed placebo in diminishing pain and improving somatosensory alterations by increasing the pressure pain threshold and improving conditioned pain modulation [29]. However, the crucial question remains about whether this superiority over placebo holds when compared to standard treatments. Our findings suggest that BoNT-A is as effective as standard treatments for M-TMDs. One of the major problems when comparing BoNT-A with other treatments, also discussed in a previous article from our group [45], is that there is not a validated protocol for BoNT-A, and RCTs used different doses and injection protocols, injecting the substance in different muscles, which are factors that certainly influenced the results. Another difficulty is that even though we only included SRs of low risk of bias/high quality, most RCTs included in these SRs present methodological drawbacks, which influences their conclusions. In addition, the SRs are based on a limited number of RCTs and, even if, to the authors’ knowledge, there are no studies concerning the cost-effectiveness of BoNT treatment, the yearly treatment cost is high, which is why we question if it can be cost-effective. Thus, in line with a recently published clinical practical guideline, we recommend that healthcare providers reassess the use of BoNT-A as a treatment for M-TMDs and limit its application only to specific cases with persistent M-TMD where standard treatments are not sufficient to relieve pain [73].
Regarding the secondary outcomes, our results show that there are no beneficial effects of BoNT-A on mandibular movements, when compared to either placebo or standard treatments [18, 19, 43, 52, 53]. Only two SRs showed a positive effect of BoNT-A [45, 46], but only in half of the included RCTs in one of the reviews [46]. Although mandibular movements often are reduced in patients with M-TMD [17] as a consequence to the pain, BoNT-A does not seem to improve the range of mandibular movements, in accordance with a previous network-meta analysis showing that neither dry needling or wet needling with BoNT has a positive effect on mandibular movements [18]. One can only speculate as to why the effect on mandibular movements did not follow the pain-reducing effects shown in these SRs. Several studies show that the sensitivity of evaluating mandibular functioning is low since there is a large variability in mandibular movements among different individuals, between different ages, and also between genders [74,75,76,77]. Other studies have shown that mandibular movements correlate with body height and facial morphology, and that these variations among individuals themselves affect any possible assessment on a group level [78,79,80]. Further, reduction in mandibular movement is often associated with temporomandibular joint problems, like internal derangements and inflammatory conditions. Since these often co-exist with M-TMD, it could be difficult to assess any possible treatment effect on mandibular movements when just treating M-TMD. Finally, recent studies have shown that patients with M-TMD also suffer from kinesiophobia, i.e., an irrational and restrictive fear of movement. Thus, since they have heightened anxiety and apprehension about re-encountering painful episodes, they actively avoid situations that could trigger such painful episodes, in this case opening the mouth wide [81]. Thus, when treatment—like BoNT-A or any other treatment—reduces pain, the patients will be less anxious and more confident about opening their mouth wide. However, that does not per se mean that BoNT-A itself improved the mandibular movements; it could just be a result of decreased levels of kinesiophobia making the patients more confident and therefore feeling less anxious about opening their mouth wider [82]. Taken together, to assess treatment efficacy on mandibular movements could be misleading, resulting in inconclusive results in this patient group suffering from M-TMDs.
Although this UR indicates that BoNT-A in most cases only displays minor or mild adverse events, treatment with BoNT-A cannot be considered a safe and risk-free treatment approach for M-TMDs. Though most studies reported a spontaneous resolution of mild or minor adverse events, one has to consider that most of them did not investigate adverse events of BoNT-A treatment, but included that as a secondary variable from patient self-reports [45]. However, recent studies indicate that potential moderate and severe adverse events such as muscle atrophy [83,84,85,86,87,88], muscle weakness with significantly reduced occlusal and bite forces, as well as masticatory performance [34, 89], injection-related complications [45], and even alterations in the jaw-bone structure [36, 54] are evident after BoNT-A injection. Previous animal studies and some human studies indicate that this could be a result of incomplete re-innervation of the injected area [88], fatty infiltration [31], fibrosis [90], and increased genetic expression of bone resorption markers (Rankl/18S) [35]. Even atrophy due to necrosis of muscle fibers has been reported in mice [91] and humans [92]. However, it is important to highlight that a single injection of a low dose of BoNT-A resulted in an improvement in pain intensity but was not shown to cause adverse events such as muscular and bone changes [28].
To summarize the adverse events associated with treatment with BoNT-A on muscle thickness, muscle activity, masticatory performance, reduction in mandibular bone density and cortical thickness in relation to the moderate pain-reducing effect in patients with M-TMD, we recommend dentists and other medical care providers carefully evaluate the beneficial aspects of BoNT-A and the possible or potential side effects of BoNT-A for treatment of pain or even when used for aesthetic purposes [89]. Further, we also recommend that dentists and other medical care providers use a single injection of a low dose of BoNT-A for treatment of pain or for aesthetic purposes in masticatory muscles. This is based on the fact that a single injection of low-dose BoNT-A has been shown to be equally effective as repeated injections or higher doses [28, 55].
Notwithstanding, even though the long-lasting effects of BoNT-A in M-TMD were reported in only one study [93], it is recommended that future RCTs assess the durability of the positive effects and adverse events of BoNT-A injections and the effects of long-term repeated injections in patients with M-TMD. Also, another aspect to investigate in future RCTs is the effect of BoNT-A on centrally mediated muscle pain. To our knowledge, one study has indicated that treatment with BoNT-A could have a positive effect on centrally mediated pain, but that the effect was significantly better for localized M-TMD when compared to centrally mediated muscle pain [94].
4.1 Study Strengths and Limitations
The main strength of this UR is that it systematically reviews existing SRs. A SR itself offers a comprehensive evaluation of available information on a particular subject. However, an UR can provide insightful conclusions through thorough analysis by integrating prior SRs and/or meta-analyses. Further, URs like this one can expedite the assessment of extensive evidence and facilitate comparison with findings from previous SRs. In contrast to SRs on the use of BoNT-A where the results from different SRs vary and are inconclusive, this UR adds an additional step by synthesizing the existing high-quality reviews establishing an overall coherence [95]. It can serve as a valuable resource for immediate clinical decision making and the development of future guidelines concerning the utilization of BoNT-A in dental practice for M-TMD patients. Additionally, it streamlines the process for decision makers by presenting a consolidated view rather than requiring review of multiple individual SRs.
In the same way that this UR systematically reviews existing outcomes from SRs, it is highly dependent on the quality as well as the data from the SRs that are included for the conclusions. Another strength of this UR is therefore the strict inclusion criteria regarding high methodological quality, and that the criteria were set a priori to the start of the quality assessment. However, this can be seen as a limitation since several SRs were excluded due to methodological considerations (16 out of 34 possibly eligible SRs). Another limitation could be the fact that the included RCTs also display methodological drawbacks, etc.
4.2 Conclusion
The synthesis in this UR provides the highest level of evidence currently available. Taken together, there are indications of effectiveness of BoNT-A for treatment of M-TMDs, supported by moderate evidence. However, considering the risk of causing serious adverse events, treatment with BoNT-A is recommended to be the last treatment alternative.
References
Rossetto O, Pirazzini M, Montecucco C. Botulinum neurotoxins: genetic, structural and mechanistic insights. Nat Rev Microbiol. 2014;12(8):535–49. https://doi.org/10.1038/nrmicro3295. (Epub 2014/07/01. PubMed PMID: 24975322).
Pirazzini M, Rossetto O, Eleopra R, Montecucco C. Botulinum neurotoxins: biology, pharmacology, and toxicology. Pharmacol Rev. 2017;69(2):200–35. https://doi.org/10.1124/pr.116.012658. (Epub 2017/03/31. PubMed PMID: 28356439; PubMed Central PMCID: PMCPMC5394922).
Zhang S, Masuyer G, Zhang J, Shen Y, Lundin D, Henriksson L, et al. Identification and characterization of a novel botulinum neurotoxin. Nat Commun. 2017;8:14130. https://doi.org/10.1038/ncomms14130. (Epub 2017/08/05. PubMed PMID: 28770820; PubMed Central PMCID: PMCPMC5543303 62/360,239) for medical use of BoNT/X, with P.S., S.Z. and M.D. as inventors).
Alimohammadi M, Punga AR. Neurophysiological Measures of Efficacy and Safety for Botulinum Toxin Injection in Facial and Bulbar Muscles: Special Considerations. Toxins (Basel). 2017;9(11). Epub 2017/10/31. https://doi.org/10.3390/toxins9110352. PubMed PMID: 29084148; PubMed Central PMCID: PMCPMC5705967.
Kim DW, Lee SK, Ahnn J. Botulinum toxin as a pain killer: players and actions in antinociception. Toxins (Basel). 2015;7(7):2435–53. https://doi.org/10.3390/toxins7072435. (Epub 2015/07/03. PubMed PMID: 26134255; PubMed Central PMCID: PMCPMC4516922).
Matak I, Bolcskei K, Bach-Rojecky L, Helyes Z. Mechanisms of botulinum toxin type A action on pain. Toxins (Basel). 2019. https://doi.org/10.3390/toxins11080459. (Epub 2019/08/08. PubMed PMID: 31387301; PubMed Central PMCID: PMCPMC6723487).
Cui M, Khanijou S, Rubino J, Aoki KR. Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain. 2004;107(1–2):125–33. https://doi.org/10.1016/j.pain.2003.10.008. (Epub 2004/01/13 PubMed PMID: 14715398).
Arezzo JC. Possible mechanisms for the effects of botulinum toxin on pain. Clin J Pain. 2002;18(6 Suppl):S125–32. https://doi.org/10.1097/00002508-200211001-00003. (Epub 2003/02/07 PubMed PMID: 12569959).
Bach-Rojecky L, Lackovic Z. Central origin of the antinociceptive action of botulinum toxin type A. Pharmacol Biochem Behav. 2009;94(2):234–8. https://doi.org/10.1016/j.pbb.2009.08.012. (Epub 2009/09/08 PubMed PMID: 19732788).
Matak I, Bach-Rojecky L, Filipovic B, Lackovic Z. Behavioral and immunohistochemical evidence for central antinociceptive activity of botulinum toxin A. Neuroscience. 2011;186:201–7. https://doi.org/10.1016/j.neuroscience.2011.04.026. (Epub 2011/05/05 PubMed PMID: 21539899).
Drinovac V, Bach-Rojecky L, Lackovic Z. Association of antinociceptive action of botulinum toxin type A with GABA-A receptor. J Neural Transm (Vienna). 2014;121(6):665–9. https://doi.org/10.1007/s00702-013-1150-6. (Epub 2014/01/15 PubMed PMID: 24420081).
Manuel Munoz-Lora VR, Abdalla HB, Del Bel Cury AA, Clemente-Napimoga JT. Modulatory effect of botulinum toxin type A on the microglial P2X7/CatS/FKN activated-pathway in antigen-induced arthritis of the temporomandibular joint of rats. Toxicon. 2020;187:116–21. https://doi.org/10.1016/j.toxicon.2020.08.027. (Epub 2020/09/04 PubMed PMID: 32882256).
Foster L, Clapp L, Erickson M, Jabbari B. Botulinum toxin A and chronic low back pain: a randomized, double-blind study. Neurology. 2001;56(10):1290–3. https://doi.org/10.1212/wnl.56.10.1290. (Epub 2001/05/29 PubMed PMID: 11376175).
Diener HC, Dodick DW, Aurora SK, Turkel CC, DeGryse RE, Lipton RB, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30(7):804–14. https://doi.org/10.1177/0333102410364677. (Epub 2010/07/22 PubMed PMID: 20647171).
Attal N, de Andrade DC, Adam F, Ranoux D, Teixeira MJ, Galhardoni R, et al. Safety and efficacy of repeated injections of botulinum toxin A in peripheral neuropathic pain (BOTNEP): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2016;15(6):555–65. https://doi.org/10.1016/S1474-4422(16)00017-X. (Epub 2016/03/08 PubMed PMID: 26947719).
Chen CL, Meng E. Can botulinum toxin A play a role in treatment of chronic pelvic pain syndrome in female patients?-Clinical and animal evidence. Toxins (Basel). 2020. https://doi.org/10.3390/toxins12020110. (Epub 2020/02/14. PubMed PMID: 32050685; PubMed Central PMCID: PMCPMC7076794).
Barjandi G, Svedenlof J, Jasim H, Collin M, Hedenberg-Magnusson B, Christidis N, et al. Clinical aspects of mastication myalgia-an overview. Front Pain Res (Lausanne). 2023;4:1306475. https://doi.org/10.3389/fpain.2023.1306475. (PubMed PMID: 38264542; PubMed Central PMCID: PMCPMC10803665).
Al-Moraissi EA, Alradom J, Aladashi O, Goddard G, Christidis N. Needling therapies in the management of myofascial pain of the masticatory muscles: a network meta-analysis of randomised clinical trials. J Oral Rehabil. 2020;47(7):910–22. https://doi.org/10.1111/joor.12960. (Epub 2020/03/12 PubMed PMID: 32159870).
Al-Moraissi EA, Conti PCR, Alyahya A, Alkebsi K, Elsharkawy A, Christidis N. The hierarchy of different treatments for myogenous temporomandibular disorders: a systematic review and network meta-analysis of randomized clinical trials. Oral Maxillofac Surg. 2021;21:21. https://doi.org/10.1007/s10006-021-01009-y. (PubMed PMID: 34674093).
Alkhutari AS, Alyahya A, Conti PCR, Christidis N, Al-Moraissi EA. Is the therapeutic effect of occlusal stabilization appliances more than just placebo effect in the management of painful temporomandibular disorders? A network meta-analysis of randomized clinical trials. J Prosthet Dent. 2021;126(1):24–32. https://doi.org/10.1016/j.prosdent.2020.08.015. (PubMedPMID:WOS:000670295900006).
Christidis N, Al-Moraissi EA, Barjandi G, Svedenlof J, Jasim H, Christidis M, et al. Pharmacological Treatments of Temporomandibular Disorders: A Systematic Review Including a Network Meta-Analysis. Drugs. 2024;84(1):59–81. https://doi.org/10.1007/s40265-023-01971-9. (Epub 2023/12/16 PubMed PMID: 38103150; PubMed Central PMCID: PMCPMC10789663 Svedenlof, Hajer Jasim, Maria Christidis, and Malin Collin declare that they have no conflicts of interest that might be relevant to the contents of this manuscript).
Ohrbach R, Dworkin SF. Five-year outcomes in TMD: relationship of changes in pain to changes in physical and psychological variables. Pain. 1998;74(2–3):315–26. https://doi.org/10.1016/s0304-3959(97)00194-2. (Epub 1998/03/31 PubMed PMID: 9520246).
von Lindern JJ. Type A botulinum toxin in the treatment of chronic facial pain associated with temporo-mandibular dysfunction. Acta Neurol Belg. 2001;101(1):39–41 (Epub 2001/05/31 PubMed PMID: 11379274).
Nixdorf DR, Heo G, Major PW. Randomized controlled trial of botulinum toxin A for chronic myogenous orofacial pain. Pain. 2002;99(3):465–73. https://doi.org/10.1016/S0304-3959(02)00240-3. (Epub 2002/10/31 PubMed PMID: 12406522).
Kurtoglu C, Gur OH, Kurkcu M, Sertdemir Y, Guler-Uysal F, Uysal H. Effect of botulinum toxin-A in myofascial pain patients with or without functional disc displacement. J Oral Maxillofac Surg. 2008;66(8):1644–51. https://doi.org/10.1016/j.joms.2008.03.008. (Epub 2008/07/19 PubMed PMID: 18634953).
Ernberg M, Hedenberg-Magnusson B, List T, Svensson P. Efficacy of botulinum toxin type A for treatment of persistent myofascial TMD pain: a randomized, controlled, double-blind multicenter study. Pain. 2011;152(9):1988–96. https://doi.org/10.1016/j.pain.2011.03.036. (Epub 2011/04/26 PubMed PMID: 21514731).
Patel AA, Lerner MZ, Blitzer A. IncobotulinumtoxinA injection for temporomandibular joint disorder. Ann Otol Rhinol Laryngol. 2017;126(4):328–33. https://doi.org/10.1177/0003489417693013. (Epub 2017/03/16. PubMed PMID: 28290229).
De la Torre Canales G, Alvarez-Pinzon N, Muñoz-Lora VRM, Vieira Peroni L, Farias Gomes A, Sánchez-Ayala A, et al. Efficacy and safety of botulinum toxin type a on persistent myofascial pain: a randomized clinical trial. Toxins (Basel). 2020. https://doi.org/10.3390/toxins12060395. (Epub 2020/06/19. PubMed PMID: 32549196; PubMed Central PMCID: PMCPMC7354430).
De la Torre Canales G, Poluha RL, Bonjardim LR, Ernberg M, Conti PCR. Botulinum toxin-A effects on pain, somatosensory and psychosocial features of patients with refractory masticatory myofascial pain: a randomized double-blind clinical trial. Sci Rep. 2024;14(1):4201. https://doi.org/10.1038/s41598-024-54906-z. (Epub 2024/02/21. PubMed PMID: 38378855; PubMed Central PMCID: PMCPMC10879180).
Balanta-Melo J, Torres-Quintana MA, Bemmann M, Vega C, Gonzalez C, Kupczik K, et al. Masseter muscle atrophy impairs bone quality of the mandibular condyle but not the alveolar process early after induction. J Oral Rehabil. 2019;46(3):233–41. https://doi.org/10.1111/joor.12747. (Epub 2018/11/24. PubMed PMID: 30468522).
Fortuna R, Vaz MA, Youssef AR, Longino D, Herzog W. Changes in contractile properties of muscles receiving repeat injections of botulinum toxin (Botox). J Biomech. 2011;44(1):39–44.
Gedrange T, Gredes T, Spassov A, Mai R, Kuhn DU, Dominiak M, et al. Histological changes and changes in the myosin mRNA content of the porcine masticatory muscles after masseter treatment with botulinum toxin A. Clin Oral Investig. 2013;17(3):887–96. https://doi.org/10.1007/s00784-012-0750-0. (Epub 2012/06/16. PubMed PMID: 22699659).
Ahn KY, Kim ST. The change of maximum bite force after botulinum toxin type a injection for treating masseteric hypertrophy. Plast Reconstr Surg. 2007;120(6):1662–6. https://doi.org/10.1097/01.prs.0000282309.94147.22. (Epub 2007/11/28. PubMed PMID: 18040203).
Kim KS, Byun YS, Kim YJ, Kim ST. Muscle weakness after repeated injection of botulinum toxin type A evaluated according to bite force measurement of human masseter muscle. Dermatol Surg. 2009;35(12):1902–7.
Balanta-Melo J, Toro-Ibacache V, Kupczik K, Buvinic S. Mandibular bone loss after masticatory muscles intervention with botulinum toxin: an approach from basic research to clinical findings. Toxins (Basel). 2019. https://doi.org/10.3390/toxins11020084. (Epub 2019/02/06. PubMed PMID: 30717172; PubMed Central PMCID: PMCPMC6409568).
Moussa MS, Bachour D, Komarova SV. Adverse effect of botulinum toxin-A injections on mandibular bone: a systematic review and meta-analysis. J Oral Rehabil. 2024;51(2):404–15. https://doi.org/10.1111/joor.13590. (Epub 2023/09/05. PubMed PMID: 37668276).
Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13(3):132–40. https://doi.org/10.1097/XEB.0000000000000055. (Epub 2015/09/12. PubMed PMID: 26360830).
Clark JM, Sanders S, Carter M, Honeyman D, Cleo G, Auld Y, et al. Improving the translation of search strategies using the Polyglot Search Translator: a randomized controlled trial. J Med Libr Assoc. 2020;108(2):195–207. https://doi.org/10.5195/jmla.2020.834. (Epub 2020/04/08. PubMed PMID: 32256231; PubMed Central PMCID: PMCPMC7069833 developing the Polyglot Search Translator from the Australian Library Information Association. All other authors declare that they have no other competing interests).
Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104(3):240–3. https://doi.org/10.3163/1536-5050.104.3.014. (Epub 2016/07/02. PubMed PMID: 27366130; PubMed Central PMCID: PMCPMC4915647).
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. https://doi.org/10.1186/s13643-016-0384-4. (Epub 2016/12/07. PubMed PMID: 27919275; PubMed Central PMCID: PMCPMC5139140).
Aromataris E, Fernandez RS, Godfrey C, Holly C, Khalil H, Tungpunkom P. Methodology for JBI umbrella reviews. Joanna Briggs Institute reviewer's manual 2014. Adelaide, Australia: Joanna Briggs Institute; 2014.
Ahmed S, Subramaniam S, Sidhu K, Khattab S, Singh D, Babineau J, et al. Effect of local anesthetic versus botulinum toxin-A injections for myofascial pain disorders: a systematic review and meta-analysis. Clin J Pain. 2019;35(4):353–67. https://doi.org/10.1097/AJP.0000000000000681. (Epub 2018/12/28. PubMed PMID: 30589660).
Arribas-Pascual M, Hernandez-Hernandez S, Jimenez-Arranz C, Grande-Alonso M, Angulo-Diaz-Parreno S, La Touche R, et al. Effects of physiotherapy on pain and mouth opening in temporomandibular disorders: an umbrella and mapping systematic review with meta-meta-analysis. J Clin Med. 2023. https://doi.org/10.3390/jcm12030788. (Epub 2023/02/12. PubMed PMID: 36769437; PubMed Central PMCID: PMCPMC9917698).
Awan KH, Patil S, Alamir AWH, Maddur N, Arakeri G, Carrozzo M, et al. Botulinum toxin in the management of myofascial pain associated with temporomandibular dysfunction. J Oral Pathol Med. 2019;48(3):192–200. https://doi.org/10.1111/jop.12822. (Epub 2019/01/04. PubMed PMID: 30604895).
De la Torre Canales G, Poluha RL, Lora VM, Araujo Ferreira DM, Stuginski-Barbosa J, Bonjardim LR, et al. Botulinum toxin type A applications for masticatory myofascial pain and trigeminal neuralgia: what is the evidence regarding adverse effects? Clin Oral Investig. 2019;23(9):3411–21. https://doi.org/10.1007/s00784-019-03026-4.
Delcanho R, Val M, Guarda Nardini L, Manfredini D. Botulinum toxin for treating temporomandibular disorders: what is the evidence? J Oral Facial Pain Headache. 2022;36(1):6–20. https://doi.org/10.11607/ofph.3023.
De la Torre Canales G, Camara-Souza MB, do Amaral CF, Garcia RC, Manfredini D. Is there enough evidence to use botulinum toxin injections for bruxism management? A systematic literature review. Clin Oral Investig. 2017;21(3):727–34. https://doi.org/10.1007/s00784-017-2092-4. (Epub 2017/03/04. PubMed PMID: 28255752).
Di Francesco F, Lanza A, Di Blasio M, Vaienti B, Cafferata EA, Cervino G, et al. Application of botulinum toxin in temporomandibular disorders: a systematic review of randomized controlled trials (RCTs). Appl Sci. 2022;12(23):12409. https://doi.org/10.3390/app122312409.
Feng J, Luo M, Ma J, Tian Y, Han X, Bai D. The treatment modalities of masticatory muscle pain a network meta-analysis. Medicine (Baltimore). 2019;98(46): e17934. https://doi.org/10.1097/MD.0000000000017934. (Epub 2019/11/15. PubMed PMID: 31725647; PubMed Central PMCID: PMCPMC6867722).
Griswold D, Learman K, Ickert E, Clewley D, Donaldson MB, Wilhelm M, et al. Comparing dry needling or local acupuncture to various wet needling injection types for musculoskeletal pain and disability. A systematic review of randomized clinical trials. Disabil Rehabil. 2024;46(3):414–28. https://doi.org/10.1080/09638288.2023.2165731. (Epub 2023/01/13. PubMed PMID: 36633385).
Khalifeh M, Mehta K, Varguise N, Suarez-Durall P, Enciso R. Botulinum toxin type A for the treatment of head and neck chronic myofascial pain syndrome: a systematic review and meta-analysis. J Am Dent Assoc. 2016;147(12):959-73e1. https://doi.org/10.1016/j.adaj.2016.08.022. (Epub 2016/10/16. PubMed PMID: 27737756).
Machado D, Martimbianco ALC, Bussadori SK, Pacheco RL, Riera R, Santos EM. Botulinum toxin type A for painful temporomandibular disorders: systematic review and meta-analysis. J Pain. 2020;21(3–4):281–93. https://doi.org/10.1016/j.jpain.2019.08.011. (Epub 2019/09/13. PubMed PMID: 31513934).
Machado E, Machado P, Wandscher VF, Marchionatti AME, Zanatta FB, Kaizer OB. A systematic review of different substance injection and dry needling for treatment of temporomandibular myofascial pain. Int J Oral Maxillofac Surg. 2018;47(11):1420–32. https://doi.org/10.1016/j.ijom.2018.05.003. (Epub 2018/05/29. PubMed PMID: 29801994).
Owen M, Gray B, Hack N, Perez L, Allard RJ, Hawkins JM. Impact of botulinum toxin injection into the masticatory muscles on mandibular bone: a systematic review. J Oral Rehabil. 2022;49(6):644–53. https://doi.org/10.1111/joor.13326. (Epub 2022/03/30. PubMed PMID: 35348239).
Ramos-Herrada RM, Arriola-Guillen LE, Atoche-Socola KJ, Bellini-Pereira SA, Castillo AA. Effects of botulinum toxin in patients with myofascial pain related to temporomandibular joint disorders: a systematic review. Dent Med Probl. 2022;59(2):271–80. https://doi.org/10.17219/dmp/145759. (Epub 2022/07/02. PubMed PMID: 35775414).
Zhang T, Adatia A, Zarin W, Moitri M, Vijenthira A, Chu R, et al. The efficacy of botulinum toxin type A in managing chronic musculoskeletal pain: a systematic review and meta analysis. Inflammopharmacology. 2011;19(1):21–34. https://doi.org/10.1007/s10787-010-0069-x. (Epub 2010/11/16. PubMed PMID: 21076878).
Almutairi FA, Almansour MM, Almansour IM, Alwazzan RA, Alrashaid HM, Baseer MA. The role of botox in the management of TMJ disorders—a systematic review. Ann Dent Spec. 2020;8(4):1–9.
Cheng Y, Yuan L, Ma L, Pang F, Qu X, Zhang A. Efficacy of botulinum-A for nocturnal bruxism pain and the occurrence of bruxism events: a meta-analysis and systematic review. Br J Oral Maxillofac Surg. 2022;60(2):174–82. https://doi.org/10.1016/j.bjoms.2021.03.005. (Epub 2021/12/28. PubMed PMID: 34955330).
Chen YW, Chiu YW, Chen CY, Chuang SK. Botulinum toxin therapy for temporomandibular joint disorders: a systematic review of randomized controlled trials. Int J Oral Maxillofac Surg. 2015;44(8):1018–26. https://doi.org/10.1016/j.ijom.2015.04.003. (Epub 2015/04/30. PubMed PMID: 25920597).
Pereira IN, Hassan H. Botulinum toxin A in dentistry and orofacial surgery: an evidence-based review - part 1: therapeutic applications. Evid Based Dent. 2022. https://doi.org/10.1038/s41432-022-0256-9. (Epub 2022/05/28. PubMed PMID: 35624296).
Fernandez-Nunez T, Amghar-Maach S, Gay-Escoda C. Efficacy of botulinum toxin in the treatment of bruxism: systematic review. Med Oral Patol Oral Cir Bucal. 2019;24(4):e416–24. https://doi.org/10.4317/medoral.22923. (Epub 2019/06/28. PubMed PMID: 31246937; PubMed Central PMCID: PMCPMC6667018 interest exist).
Rajamoorthy SN. Botulinum toxin-A injections into facial muscles for the treatment of temporomandibular disorders and bruxism: a systematic review. J Popul Ther Clin Pharmacol. 2023;30(16):428–52.
Thambar S, Kulkarni S, Armstrong S, Nikolarakos D. Botulinum toxin in the management of temporomandibular disorders: a systematic review. Br J Oral Maxillofac Surg. 2020;58(5):508–19. https://doi.org/10.1016/j.bjoms.2020.02.007. (Epub 2020/03/08. PubMed PMID: 32143934).
Chen Y, Tsai CH, Bae TH, Huang CY, Chen C, Kang YN, et al. Effectiveness of botulinum toxin injection on bruxism: a systematic review and meta-analysis of randomized controlled trials. Aesthetic Plast Surg. 2023;47(2):775–90. https://doi.org/10.1007/s00266-023-03256-8. (Epub 2023/01/25. PubMed PMID: 36694050).
Dall’Antonia M, Netto RMdO, Sanches ML, Guimarães AS. Jaw muscles myofascial pain and botulinum toxin. Revista Dor. 2013;14:52–7.
de Mello Sposito MM, Teixeira SAF. Botulinum Toxin Type A in the treatment of myofascial pain related to masticatory muscles. Acta Fisiátrica. 2014;21(3):152–7.
Nowak Z, Checinski M, Nitecka-Buchta A, Bulanda S, Ilczuk-Rypula D, Postek-Stefanska L, et al. Intramuscular injections and dry needling within masticatory muscles in management of myofascial pain. Systematic review of clinical trials. Int J Environ Res Public Health. 2021. https://doi.org/10.3390/ijerph18189552. (Epub 2021/09/29. PubMed PMID: 34574476; PubMed Central PMCID: PMCPMC8465617).
Patel J, Cardoso JA, Mehta S. A systematic review of botulinum toxin in the management of patients with temporomandibular disorders and bruxism. Br Dent J. 2019;226(9):667–72. https://doi.org/10.1038/s41415-019-0257-z. (Epub 2019/05/12. PubMed PMID: 31076698).
Ihde SK, Konstantinovic VS. The therapeutic use of botulinum toxin in cervical and maxillofacial conditions: an evidence-based review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104(2):e1-11. https://doi.org/10.1016/j.tripleo.2007.02.004. (Epub 2007/06/15. PubMed PMID: 17560141).
Linde M, Hagen K, Stovner LJ. Botulinum toxin treatment of secondary headaches and cranial neuralgias: a review of evidence. Acta Neurol Scand Suppl. 2011;191:50–5. https://doi.org/10.1111/j.1600-0404.2011.01544.x. (Epub 2011/07/08. PubMed PMID: 21711257).
Machado E, Santos LZd, Custódio LG, Cunali PA. Botulinum toxin for treating muscular temporomandibular disorders: a systematic review. Dental Press J Orthodont. 2012;17:167–71.
Ataran R, Bahramian A, Jamali Z, Pishahang V, Sadeghi Barzegani H, Sarbakhsh P, et al. The role of botulinum toxin A in treatment of temporomandibular joint disorders: a review. J Dent (Shiraz). 2017;18(3):157–64 (Epub 2017/10/17. PubMed PMID: 29034269; PubMed Central PMCID: PMCPMC5634354).
Busse JW, Casassus R, Carrasco-Labra A, Durham J, Mock D, Zakrzewska JM, et al. Management of chronic pain associated with temporomandibular disorders: a clinical practice guideline. BMJ. 2023;383: e076227. https://doi.org/10.1136/bmj-2023-076227.
Zawawi KH, Al-Badawi EA, Lobo SL, Melis M, Mehta NR. An index for the measurement of normal maximum mouth opening. J Can Dent Assoc. 2003;69(11):737–41 (Epub 2003/12/05 PubMed PMID: 14653940).
Posselt U. Studies in mobility of the human mandible. Acta Odontol Scand. 1952;10:1–160.
Travell J. Temporomandibular joint pain referred from muscles of the head and neck. J Prosthet Dent. 1960;10(4):745–63.
Agerberg G. Maximal mandibular movements in young men and women. Sven Tandlak Tidskr. 1974;67(2):81–100 (Epub 1974/03/01 PubMed PMID: 4524736).
Landtwing K. Evaluation of the normal range of vertical mandibular opening in children and adolescents with special reference to age and stature. J Maxillofac Surg. 1978;6(3):157–62 (Epub 1978/08/01 PubMed PMID: 279633).
Vanderas AP. Mandibular movements and their relationship to age and body height in children with or without clinical signs of craniomandibular dysfunction: part IV. A comparative study. ASDC J Dent Child. 1992;59(5):338–41 (Epub 1992/09/01 PubMed PMID: 1401404).
Agerberg G. Maximal mandibular movements in children. Acta Odontol Scand. 1974;32(3):147–59 (Epub 1974/01/01 PubMed PMID: 4531163).
Häggman-Henrikson B, Jawad N, Acuña XM, Visscher CM, Schiffman E, List T. Fear of movement and catastrophizing in participants with temporomandibular disorders. J Oral Facial Pain Headache. 2022;36(1):59–66. https://doi.org/10.11607/ofph.3060. (Epub 2022/03/18. PubMed PMID: 35298576; PubMed Central PMCID: PMCPMC10586572 authorship and/or publication of this article).
Visscher CM, Ohrbach R, van Wijk AJ, Wilkosz M, Naeije M. The Tampa Scale for Kinesiophobia for Temporomandibular Disorders (TSK-TMD). Pain. 2010;150(3):492–500. https://doi.org/10.1016/j.pain.2010.06.002. (Epub 2010/07/06. PubMed PMID: 20598804).
Kim J-H, Shin JH, Kim ST, Kim C-Y. Effects of two different units of botulinum toxin type a evaluated by computed tomography and electromyographic measurements of human masseter muscle. Plast Reconstr Surg. 2007;119(2):711–7.
Kim HJ, Yum KW, Lee SS, Heo MS, Seo K. Effects of botulinum toxin type A on bilateral masseteric hypertrophy evaluated with computed tomographic measurement. Dermatol Surg. 2003;29(5):484–9.
Park MY, Ahn KY, Jung DS. Botulinum toxin type A treatment for contouring of the lower face. Dermatol Surg. 2003;29(5):477–83.
To EW, Ho W, Wong W, Pang PC, Ahuja AT, Hui AC, et al. A prospective study of the effect of botulinum toxin A on masseteric muscle hypertrophy with ultrasonographic and electromyographic measurement. Br J Plast Surg. 2001;54(3):197–200.
Shome D, Khare S, Kapoor R. Efficacy of botulinum toxin in treating Asian Indian patients with masseter hypertrophy: a 4-year follow-up study. Plast Reconstr Surg. 2019;144(3):390e-e396.
Baldwin MC, Liu ZJ, Rafferty KL, Keith A, Tamasas B, Kaiyala K, et al. Botulinum toxin in the masseter muscle: Lingering effects of denervation. Anat Rec. 2022;305(5):1215–30.
Fedorowicz Z, van Zuuren EJ, Schoones J. Botulinum toxin for masseter hypertrophy. Cochrane Database Syst Rev. 2013;2013(9):Cd007510. https://doi.org/10.1002/14651858.CD007510.pub3. (Epub 2013/09/11. PubMed PMID: 24018587; PubMed Central PMCID: PMCPMC7207780 not have any associations with any parties who may have vested interests in the results of this review).
Carpenter S, Karpati G. Pathology of Skeletal Muscle. New York: Oxford University Press; 2001.
Duchen L, Strich SJ. The effects of botulinum toxin on the pattern of innervation of skeletal muscle in the mouse. Q J Exp Physiol Cognate Med Sci Transl Integr. 1968;53(1):84–9.
Kim N-H, Chung J-H, Park R-H, Park J-B. The use of botulinum toxin type A in aesthetic mandibular contouring. Plast Reconstr Surg. 2005;115(3):919–30.
De la Torre CG, Camara-Souza MB, Poluha RL, de Figueredo OMC, Nobre BBS, Ernberg M, et al. Long-term effects of a single application of botulinum toxin type A in temporomandibular myofascial pain patients: a controlled clinical trial. Toxins (Basel). 2022. https://doi.org/10.3390/toxins14110741. (Epub 2022/11/11. PubMed PMID: 36355991; PubMed Central PMCID: PMCPMC9721314).
Montes-Carmona J-F, Gonzalez-Perez L-M, Infante-Cossio P. Treatment of localized and referred masticatory myofascial pain with botulinum toxin injection. Toxins. 2021;13(1):6. https://doi.org/10.3390/toxins13010006.
Choi GJ, Kang H. The umbrella review: a useful strategy in the rain of evidence. Korean J Pain. 2022;35(2):127–8. https://doi.org/10.3344/kjp.2022.35.2.127. (Epub 2022/04/01. PubMed PMID: 35354675; PubMed Central PMCID: PMCPMC8977198 was reported).
Acknowledgements
The authors thank the librarians Narcisa Hannerz and Sabrina Gillsund at the Karolinska Institutet University Library for the assistance with the electronic literature search.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by Karolinska Institute.
Conflict of Interest
G. De la Torre Canales, M.B. Câmara-Souza,, M. Ernberg, E.A. Al-Moraissi, A. Grigoriadis, R.L. Poluha, M. Christidis, H. Jasim, A. Lövgren, and N. Christidis all declare that they have no conflicts of interest that might be relevant to the contents of this article.
Ethics Approval
Not applicable.
Consent (participation and publication)
Not applicable.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Nikolaos Christidis and Giancarlo De la Torre Canales conceived the main idea for the article. However, all authors contributed to the study conception and design. Hajer Jasim and Anna Lövgren performed the literature search with help from the university library at Karolinska Institutet. Selection of papers was performed by Hajer Jasim and Anna Lövgren. The protocols for data extraction and the risk of bias were developed by Giancarlo De la Torre Canales, Nikolaos Christidis, Anastasios Grigoriadis, and Essam Ahmed Al-Moraissi. Data extraction was done by Mariana Barbosa Câmara-Souza and Malin Ernberg. Analysis of risk of bias was performed by Rodrigo Lorenzi Poluha and Maria Christidis. Maria Christidis is a senior lecturer and responsible for the course “Scientific theory and methods,” and teaches specifically about different methods for risk of bias and evaluation of certainty of evidence. The synthesis of the results was performed by Giancarlo De la Torre Canales and Nikolaos Christidis and double-checked by Anastasios Grigoriadis and Essam Ahmed Al-Moraissi. Further, both Nikolaos Christidis and Giancarlo De la Torre Canales double checked all parts of the data extraction, assessment of risk of bias, and certainty of evidence. Giancarlo De la Torre Canales and Nikolaos Christidis drafted the first manuscript, which was critically revised by all authors who commented on previous versions of the manuscript. All authors read and approved the final version manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
De la Torre Canales, G., Câmara-Souza, M.B., Ernberg, M. et al. Botulinum Toxin-A for the Treatment of Myogenous Temporomandibular Disorders: An Umbrella Review of Systematic Reviews. Drugs (2024). https://doi.org/10.1007/s40265-024-02048-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s40265-024-02048-x