Abstract
Idiopathic pulmonary fibrosis (IPF) remains a disease with poor survival. The pathogenesis is complex and encompasses multiple molecular pathways. The first-generation antifibrotics pirfenidone and nintedanib, approved more than 10 years ago, have been shown to reduce the rate of progression, increase the length of life for patients with IPF, and work for other fibrotic lung diseases. In the last two decades, most clinical trials on IPF have failed to meet the primary endpoint and an urgent unmet need remains to identify agents or treatment strategies that can stop disease progression. The pharmacotherapeutic landscape for IPF is moving forward with a number of new drugs currently in clinical development, mostly in phase I and II trials, while only a few phase III trials are running. Since our understanding of IPF pathogenesis is still limited, we should keep focusing our efforts to deeper understand the mechanisms underlying this complex disease and their reflection on clinical phenotypes. This review discusses the key pathogenetic concepts for the development of new antifibrotic agents, presents the newest data on approved therapies, and summarizes new compounds currently in clinical development. Finally, future directions in antifibrotics development are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Idiopathic pulmonary fibrosis (IPF) remains a fatal and incurable disease despite the use of approved antifibrotic drugs. |
Development of novel antifibrotics drugs has consistently increased over the last decades, but unsolved issues remain about endpoints, duration, and inclusion/exclusion criteria of future clinical trials. |
A better knowledge of mechanisms leading to IPF onset and progress is crucial to the development of new compounds. |
1 Introduction
Idiopathic pulmonary fibrosis (IPF) is an incurable chronic interstitial lung disease (ILD) characterized by irreversible fibrotic destruction of lung architecture. The prevalence of IPF ranges from 20 to 80 patients per 100,000 and the majority of affected patients are male with a positive history of cigarette smoking [1]. Median survival time is 3–5 years after diagnosis without treatment [2], but recently published observations report improving survival over the last 2 decades, may be related to earlier diagnosis, and use of antifibrotics [3, 4].
Pirfenidone and nintedanib were approved worldwide for the treatment of IPF almost 10 years ago. Although they have a different mechanism of action and safety profile, their efficacy in slowing the decline of forced vital capacity (FVC) and reducing mortality risk over time is similar. In advanced IPF stages, transplantation for selected patients and palliative care are needed. Comorbidities and complications negatively impact IPF prognosis, i.e. pulmonary hypertension, lung cancer, and, above all, acute exacerbations, which can occur in 10% of patients per year and are linked to higher mortality within 3 months [5].
The lack of curative treatment has generated research and investments in IPF, but most of the trials, especially those in phase II and III, have failed to meet the primary endpoint [2].
The aim of this review was to provide insight into the newest concepts of IPF pathogenesis and illustrate recent advances in pharmacological therapy for IPF, including new data and data on already approved agents.
2 Pathophysiology of Idiopathic Pulmonary Fibrosis (IPF) [and Selected Potential Therapeutic Targets]
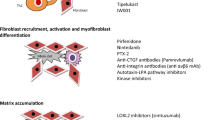
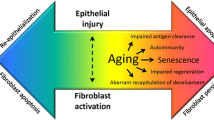
Pathologically, IPF is characterized by the excessive production and disorganized deposition of extracellular matrix (ECM) components, which, by progressively replacing the normal lung parenchyma, lead to irreversible architectural distortion and loss of organ function. Although, by definition, IPF is a disease of unknown cause, several risk factors (e.g., cigarette smoking, subclinical infection, environmental pollutants, occupational exposures, chronic microaspiration of gastric content, abnormal composition of the lung microbiota and genetic predisposition), and pathogenic mechanisms have been implicated in its development [6]. Overall, IPF is believed to occur in genetically predisposed individuals (i.e., carriers of telomerase gene mutations or short telomeres) following recurrent alveolar epithelial cell (AEC) injury [7]. In this scenario, an array of cytokines and chemokines released by damaged AECs, and including, among others, tumor necrosis factor (TNF)-α, interleukin (IL)-1, and chemokine (CC-motif) ligand 2 (CCL2), activate residential cells and recruit circulating cells, thus perpetuating alveolar damage. Dysfunctional epithelial and endothelial cells also secrete fibrogenic mediators, such as transforming growth factor (TGF)-β, which induces epithelial-to-mesenchymal transition (EMT) as well as fibroblast recruitment, proliferation, and differentiation to myofibroblasts, the main collagen-producing cells [8]. TGFβ is one of the most potent profibrotic mediators, by inducing collagen synthesis and inhibiting collagen degradation [9]. In addition, TGFβ is an inducer of a plethora of fibrogenic molecules such as connective tissue growth factor (CTGF), fibroblast growth factor (FGF), insulin-like growth factor (IGF), and platelet-derived growth factor (PDGF). TGF-β is secreted in an inactive form, with αvβ6 integrin playing a crucial role in its activation [10]. Accordingly, αvβ6 integrin is a potential therapeutic target in IPF [11].
Aging contributes to disease pathogenesis by depleting type 2 AECs, the main progenitor cells in the alveoli, thus impairing the ability of the alveoli to repair injury [12, 13]. Indeed, IPF lung tissue displays several characteristics of aging lungs, such as cellular senescence, telomere shortening, mitochondrial and lysosomal/autophagy dysfunction [14], and epigenetic changes [15].
Fibroblast foci (FF) are clusters of actively proliferating fibroblasts and myofibroblasts that lie in the subepithelial areas of damaged lung. FF are the distinguishing histologic feature of the usual interstitial pneumonia (UIP) pattern of fibrosis, and a high profusion of FF is a marker of poor prognosis in IPF [16]. Compared with normal fibroblasts, fibroblasts isolated from FF display several behavioral differences, including exuberant proliferative potential, excessive contractile capacity, resistance to apoptosis, and distinctive gene expression profile [17]. Mechanical interactions between fibroblasts and the surrounding stiffened ECM provide a positive feedback mechanism that sustains and perpetuates fibroblast activation and collagen synthesis [18]. Four different sources are hypothesized for FF fibroblasts: interstitial fibroblasts, epithelial cells via EMT, fibrocytes, and bone marrow-derived stem cells [19].
3 Therapeutic Challenges
The last decade has witnessed major advances in IPF regarding understanding of disease pathobiology, refinement of diagnostic criteria, and approval of nintedanib and pirfenidone [20]. These advances have fuelled basic translational and clinical research and have led to the identification of several potential therapeutic targets; yet, translating these advances to development of truly efficacious drugs has proven extremely challenging and, thus far, largely unsuccessful. In addition, the approval of nintedanib and pirfenidone has posed new challenges for drug developers and trialists.
3.1 Challenges in Identifying the Right Target
Despite substantial advances in our understanding of disease pathogenesis, the mechanisms involved in IPF development and progression remain elusive and controversial. In addition, although basic and clinical research has identified several potential therapeutic targets, there is no strategy for prioritizing them. Excessive collagen production remains one of the most logical targets in IPF. Indeed, in IPF, progressive fibrosis results from an imbalance between (excessive) synthesis and (reduced) degradation of collagen. Lysyl oxidase (LOX) and LOX-like (LOXL) proteins play a crucial role in ECM remodeling and wound healing by promoting cross-linking and assembly of collagens [21, 22]. Excessive LOXL-2 activity increases the risk for IPF development [23] and progression [24], but allosteric inhibition of LOXL-2 with the monoclonal antibody simtuzumab failed to improve progression-free survival in patients with IPF [25], likely because LOXL-2 is only one of several LOXL enzymes involved in collagen cross-linking. Alternatively, LOXL-2 inhibition may induce paradoxical hyperexpression of other components of collagen cross-linking or may be ineffective when given in late phases of lung fibrogenesis. Cellular senescence and premature lung aging, telomere shortening, oxidative stress, and mitochondrial dysfunction are additional plausible targets for novel drugs.
Animal models of experimentally induced lung fibrosis are a key element of preclinical drug development [26, 27]. Indeed, a drug that is not effective in animal models is unlikely to move to human studies. However, although animal models have provided important insights into the pathogenesis of pulmonary fibrosis, none of the models developed thus far fully reproduces the progressive nature of IPF or its histologic defining pattern of UIP [28]. Further research on animal models that more closely mimic human disease is warranted.
3.2 Challenges in Patient Selection
The trajectory of IPF progression is variable and unpredictable, with patients displaying slow functional decline over time and others experiencing rapid decline or even episodes of acute clinical worsening [29]. In addition, a significant minority of patients show stable disease, with as many as 15% of patients randomized to placebo in the INPULSIS trial of nintedanib experiencing either no decline or an improvement in FVC percent predicted at the end of the study [30]. Identifying patients at higher risk for rapid decline is critical for prediction of prognosis, management decision making, and design and conductance of clinical trials. In a recent prospective observational cohort, Fainberg and colleagues identified clusters of IPF patients based on lung function (FVC) trajectories by using a two-stage machine learning approach [31]. Specifically, they identified four discrete clusters that were associated with distinct biochemical and clinical features, such as forced expiratory volume in 1 s (FEV1)/FVC ratio and surfactant protein D (SPD) serum levels. While the existence of clusters of functional decline complicates the interpretation of the study endpoints (particularly if more patients likely to remain stable are randomly assigned to the placebo arm), enriching a trial for patients more likely to progress will greatly improve its efficacy.
3.3 Challenges in Clinical Trial Design
Following the approval of nintedanib and pirfenidone as standard of care (SoC) for IPF, it is unethical to compare new drug candidates with true placebo. An agent could still be tested against placebo in clinical trials restricted to the significant minority of patients who discontinue SoC due to tolerability issues. However, results obtained with this approach may not be generalizable to the broader population of IPF patients, as individuals intolerant to antifibrotic therapy may represent a biologically distinct subset. Currently, novel IPF therapies are evaluated as add-on to background antifibrotic therapy, the rationale being the possibility to target multiple coactivated profibrotic pathways. Intuitively, the best partner drugs are those with complementary, alternative, or synergistic mechanisms of action to SoC. One such example is the preferential phosphodiesterase-4 (PDE-4) inhibitor BI 1015550, which acts synergistically with nintedanib to inhibit mitogen-induced fibroblast proliferation [32].
A debated issue related to clinical trial design is about trial duration. In the last years, the duration of phase II trials in IPF has become 3–6 months, with a limited number of patients per arm. This might be an explanation for the failure of the most recent phase III trials, but the existence of sub-phenotypes of IPF patients and the influence of previous treatments cannot be excluded. Utilization of historical control data or data of the trial population before entering the trial, for instance by looking at functional decline, would be of benefit for the identification of potential predictors of response to antifibrotic treatment [33]
3.4 Challenges in Translating Clinical Trials to Patient Care
With SoC halving the rate of FVC decline compared with placebo, the window to show a further reduction in lung function decline is narrow. In addition, neither nintedanib nor pirfenidone is associated with a consistent improvement in patient-centered outcomes such as symptoms, 6-min walk distance, day-to-day functioning, and fatigue. A recent real-world study has shown that patients with IPF have similar magnitude of response and completion rates to pulmonary rehabilitation compared with patients with COPD [34]. Conversely, in IPF, nonresponse to, and noncompletion of, pulmonary rehabilitation are associated with increased all-cause mortality. These data reinforce both the benefits of pulmonary rehabilitation in patients with IPF and the need for clinical trials assessing a comprehensive therapeutic approach of antifibrotic therapy and pulmonary rehabilitation.
Traditionally, clinical trials of IPF have enrolled patients with mild-to-moderate disease. Although post hoc analyses have suggested that nintedanib [35] and pirfenidone [36] have a similar effect on FVC decline in IPF patients with more versus less severe functional impairment, patients with FVC < 50% or with significant comorbidities such as lung cancer and cardiovascular disease, which are more common in advanced disease [37], are generally excluded from clinical trials. In this regard, the short-term (i.e., 1 year) mortality in clinical trials of IPF is lower compared with the general clinical cohorts [38]. In the future, data from real-life observations and registries should be systematically used to counterbalance the findings from clinical trials, which generally include overselected patient populations, in an effort to test significant benefits for the primary outcome.
4 Efficacy of the Currently Approved Antifibrotics
IPF has been the subject of many notable clinical trials over the last 2 decades prior to the current era of approved therapies, including both pirfenidone and nintedanib. These included trials of interferon-γ [39] the endothelin receptor antagonists bosentan and macitentan [40,41,42], warfarin [43], acetylcysteine [44], sildenafil [45], and various immunosuppressive medications [46]. Despite these and other clinical trials failing to identify a viable treatment of IPF, these studies have improved our understanding of IPF biology and clinical trial endpoints. This knowledge was critical for the future success of the currently approved antifibrotics.
4.1 Pirfenidone
Pirfenidone is a small molecule that inhibits fibroblast proliferation and collagen synthesis, likely primarily through regulation of TGFβ [47]. The trials that led to the approval of pirfenidone in IPF showed a benefit on the primary endpoint FVC decline over 1 year [47, 48]. A relative benefit of pirfenidone on the composite endpoint of death or disease progression (driven primarily by FVC), but not on dyspnea or mortality, was observed. Pirfenidone was most frequently associated with gastrointestinal symptoms (primarily nausea) and skin-related events (rash and photosensitivity), which were generally two- to sixfold more common in patients treated with pirfenidone compared with placebo.
Several additional post hoc analyses have suggested consistent effects of pirfenidone in various patient subgroups [49,50,51]. Efficacy and adverse-effect profiles have further been confirmed in subsequent post hoc analyses and meta-analyses of the major clinical trials [52,53,54,55,56,57,58], as well as longer-term, open-label extension studies [36, 59, 60]. Dose reduction, dietary modifications, and skin protection are widely used strategies to manage adverse effects [61, 62].
Pirfenidone has been approved for the treatment of IPF in many countries and is recommended in current clinical practice guidelines for the treatment of mild-to-moderate IPF [63]. Subsequent studies have suggested a similar magnitude of benefit in advanced IPF [64] and a variety of other ILDs when recent progression has been documented [65, 66].
4.2 Nintedanib
Nintedanib is an intracellular tyrosine kinase inhibitor (TKI) with multiple targets involved in lung fibrosis [67]. In the trials that led to the approval of nintedanib in IPF [68], the treatment group showed approximately 50% less decline in FVC compared with placebo. Acute exacerbation was reduced in one trial but not the other, and a trend toward improvement in patients’ quality of life, as measured by the St. George’s Respiratory Questionnaire (SGRQ), was observed. The INPULSIS trials further confirmed diarrhea as the major adverse effect of nintedanib, affecting about two-thirds of patients, followed by nausea, vomiting, and weight loss. TKIs are likely to induce diarrhea by causing dysfunction in water absorption and secretion in the intestinal lumen, which might be partially mediated by increased activity of the chloride channel CaCC in the luminal membrane of enterocytes [69]. Although colonic CaCC inhibitors have been proposed as a potential therapeutic target for epidermal growth factor receptor (EGFR)-TKI-induced diarrhea [70], nintedanib-associated diarrhea can be effectively controlled with loperamide, an opioid-receptor agonist [71].
Elevated liver enzymes occurred in approximately 5% of nintedanib-treated patients compared with <1% of placebo-treated patients. Overall, approximately 20–25% of patients appeared unable to tolerate nintedanib [71]. Dose reduction to 100 mg twice daily, dietary modification, temporary discontinuation, and rechallenging with a lower dose are widely used strategies to successfully manage adverse effects [71, 72].
Efficacy and adverse-effect profiles have further been confirmed in subsequent post hoc analyses and meta-analyses of the major clinical trials [30, 35, 73,74,75,76,77,78,79,80,81], as well as longer-term open-label extension studies [82,83,84].
Based primarily on data from the INPULSIS trials, nintedanib has been approved for the treatment of IPF in many countries and has received a positive recommendation in current clinical practice guidelines for the treatment of mild-to-moderate IPF [63].
4.3 Antifibrotics for Patients with Progressive Pulmonary Fibrosis
Progressive pulmonary fibrosis (PPF) has recently been proposed as a clinical phenotype of patients with ILDs other than IPF who develop a decline in pulmonary function tests (FVC or diffusing lung capacity for carbon monoxide [DLCO]), a worsening of fibrosis at high-resolution computed tomography (HRCT), or symptoms within 1 year [85].
The INBUILD trial explored and successfully proved the efficacy and safety of nintedanib in this patient population [86]. This study and additional post hoc analyses were the basis for the recommendation of nintedanib in this expanded population that includes both IPF and non-IPF forms of PPF [85].
Although pirfenidone showed similar effects as nintedanib on FVC decline over 1 year in the two trials on progressive non-IPF ILD [65, 66], the primary endpoint of the study of pirfenidone in unclassifiable ILD was only met when calculated with FVC values measured at the study center, but not with FVC self-measured by daily home spirometry. In the case of the RELIEF trial, prematurely stopped, only 127 patients were recruited, and, in front of the positive sensitivity analyses, this was the main reason why the recent international clinical practice guidelines did not provide a recommendation for or against pirfenidone in the treatment of PPF [85]. However, after the publication of new meta-analyses [87], showing the consistency of the effect of pirfenidone on FVC decline, national guidelines provided a weak recommendation for the use of pirfenidone in this clinical phenotype [88, 89].
4.4 Combination of Antifibrotics in IPF
An additional uncertainty is the potential combination of nintedanib and pirfenidone given their distinct mechanisms of action and the lack of clear pharmacokinetic interactions [90, 91]. This has been studied in two clinical trials, including one study in which nintedanib was added to pirfenidone [92], and a second study in which pirfenidone was added to nintedanib [93]. Both studies were not powered for efficacy but a potential role for this combination was suggested. Definitive studies are needed to address this question. Based on the paucity of safety and efficacy data and reimbursement issues in most countries, international and national guidelines on IPF treatment recommend the use of antifibrotics combination only within clinical studies [85, 88].
5 New Agents in Clinical Development and Future Perspectives
The majority of past IPF trials did not meet the primary endpoint. Nevertheless, a number of compounds are now under investigation in different trial settings, as add-on therapy or versus true placebo (Table 1).
5.1 Targeting Alveolar Macrophages
5.1.1 Recombinant Human Pentraxin-2 (PRM 151)
Pentraxin-2 (PTX2), a member of the pentraxin protein family, is an endogenous regulator of tissue repair [94]. PTX2 inhibits the differentiation of monocytes into pro-fibrotic macrophages, and fibrocytes inhibit the expression of TGFβ [95, 96]. Circulating levels are decreased in pulmonary, liver, and renal fibrosis. A multicenter, randomized, phase II, double-blind study investigated recombinant human PTX2 (zinpentraxin alfa or PRM-151) in IPF patients [97].
PRM-151 10 mg/kg was administered intravenously every 4 weeks following a three-dose loading regimen. In the treatment group, there was a lesser decline in FVC percent predicted (−2.5) compared with placebo (−4.8) and persisted for up to 52 weeks [97, 98]. About 28% of patients experienced an adverse event, and the cough rate was higher in the treatment arm than placebo [98]. The phase III trial (NCT04552899) evaluating the efficacy and safety of PRM-151 compared with placebo in IPF which has enrolled 665 participants, was prematurely stopped in February 2023 due to futility.
5.2 Targeting Fibroblasts
5.2.1 GLPG 1690 (Ziritaxestat)
At present, there are several new compounds targeting autotaxin (ATX), an ecto-enzyme [98] that catalyses the hydrolysis of lysophospholipids to the lipid mediator lysophosphatidic acid (LPA; PMID: 3541519). ATX and LPA are elevated in several fibrotic and inflammatory conditions, particularly in serum and bronchoalveolar fluid of patients with IPF (PMID: 3541519). GLPG 1690 is a selective ATX inhibitor [99], and a phase IIa randomized placebo-controlled, 12-week trial (FLORA) showed an improvement in FVC in the treatment group versus placebo. The two identically designed, phase III, randomized clinical trials ISABELA 1 and ISABELA 2 were conducted in 26 countries and recruited a total of 1306 patients with IPF. Patients were randomized 1:1:1 to receive 600 mg of oral ziritaxestat, 200 mg of ziritaxestat, or placebo once daily in addition to SoC for at least 52 weeks [99]. Due to the benefit-to-risk profile of ziritaxestat, the trials were terminated early. Ziritaxestat did not improve the annual rate of FVC decline versus placebo in either study and no benefit for the key secondary outcomes was observed. Moreover, a slightly increased all-cause mortality rate was observed with ziritaxestat compared with placebo in both trials (8–9% vs. 5%).
5.2.2 BMS-986278 and BMS-986020
BMS-986278 is an LPA receptor 1 (LPA1) antagonist currently in phase II development in patients with IPF and progressing fibrosing non-IPF ILD [100]. Patients in both cohorts will be randomized 1:1:1 to receive 30 or 60 mg of BMS-986278, or placebo, administered orally twice daily for 26 weeks in the placebo-controlled treatment period. The primary endpoint is rate of change in FVC percent predicted from baseline to week 26.
A phase II trial with another LPA1 antagonist (BMS-986020), despite a significant reduction in the rate of FVC decline between the placebo and twice-daily dosage group, was terminated early due to hepatobiliary toxicity [101].
5.2.3 Anti-Connective Tissue Growth Factor-Monoclonal Antibody (Pamrevlumab)
CTGF modulates myofibroblast activation, ECM deposition, and fibrotic remodeling via TGFβ downstream signaling [102, 103]. In the PRAISE phase II trial, the recombinant human antibody pamrevlumab administered intravenously consistently reduced the decline in the percentage of predicted FVC by 60.3% at week 48 (mean change from baseline −2.9% with pamrevlumab vs. −7.2% with placebo, with a between-group difference of 4.3%; p = 0.033) [104]. The treatment effect was corroborated by the improvement of radiology (quantitative lung fibrosis score at HRCT) and symptoms (SGRQ score). Due to the relatively low number of patients included (N = 103), and since background antifibrotics were not permitted, the results should be treated with caution. A phase III program consisting of two identical trials (ZEPHYRUS I and II), (NCT03955146 and NCT04419558) was prematurely stopped in June 2023 since the ZEPHYRUS I study did not meet the primary endpoint (press release link: https://investor.fibrogen.com/news-releases/news-release-details/fibrogen-announces-topline-results-phase-3-zephyrus-1-study).
5.2.4 Preferential Phosphodiesterase-4B Inhibitor (BI 1015550)
Phosphodiesterases (PDEs) are the principal superfamily of enzymes responsible for degrading the secondary messengers 3′,5′-cyclic nucleotides cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP). Inhibiting this pathway leads to a decrease in levels of prostaglandin E2, which regulates essential functions of fibroblasts [105]. BI 1015550 appears to also inhibit TGFβ1-induced myofibroblast transformation and ECM deposition [32, 106]. Based on preclinical models, the selectivity for PDE-4B is associated with fewer gastrointestinal adverse effects [106]. In a double-blind, placebo-controlled, parallel-design, phase II trial, patients were randomly assigned in a 2:1 ratio to receive either BI 1015550 18 mg twice daily or placebo, administered orally, for 12 weeks. The primary endpoint was a change in baseline FVC at week 12 and patients were stratified according to background antifibrotic treatment (PMID: 35569036). Bayesian analysis was used to calculate the probabilities that BI 1015550 was superior to placebo in each group. Patients in the BI 1015550 arm, both with and without pre-existing antifibrotic use, had a lower median change, or even a slight improvement, in FVC at 12 weeks compared with those in the placebo group (median difference 62–84 mL). A mixed model with repeated measures (MMRM) analysis provided results that were consistent with those of the Bayesian analysis. Diarrhea was the most common adverse effect leading to discontinuation in 13 patients, almost all of whom were taking antifibrotics [107]. FIBRONEER™ is a currently recruiting phase III program initiated globally to evaluate BI 1015550 in IPF and other progressive fibrosing ILDs (NCT05321069 and NCT05321082, respectively).
5.2.5 PBI-4050
By binding G protein-coupled receptors GPR40 and GPR84, PBI-4050, an orally active synthetic analog of a medium-chain fatty acid, reduces fibrosis via the regulation of multiple antifibrotic pathways [108]. PBI-4050 inhibits the differentiation of fibroblasts to myofibroblasts, and reduces accumulation of ECM protein deposition and fibrosis.
In the phase II, open-label trial of PBI-4050 in IPF, besides a good safety profile, no significant changes in FVC, either in percent predicted or milliliters, from baseline to week 12 were observed for PBI-4050 alone or PBI-4050 + nintedanib, but not in combination with pirfenidone [109]. The results should be interpreted with caution since the trial had no placebo control group.
5.3 Targeting Epithelial Cells
PLN-74809, an oral small molecule inhibitor of integrins αvβ6 and αvβ1, suppresses TGFβ in fibrotic lung tissue and potentially reduces systemic adverse effects in the treatment of IPF [110]. PLN-74809 INTEGRIS-IPF is an ongoing, phase IIa, open, randomized, double-blind, dose-ranging (60–320 mg), placebo-controlled study evaluating the safety, tolerability, and pharmacokinetics of this compound in IPF patients. The average decline in FVC for patients receiving placebo was 74.1 mL, and 15.1 mL for all patients taking PLN-74809, with a dose-dependent effect. A similar trend was observed for the quantitative HRCT lung fibrosis score [111].
5.4 Senotherapy for IPF
Senolytics can selectively induce the death of senescent cells by selective apoptosis in senescent cells, with the potential to prevent onset of age-related diseases [112]. Since senescence is driven by chronic oxidative stress, antioxidants, including novel molecules and mammalian target of rapamycin (mTOR) signaling inhibitors, might be used as senostatics [113, 114]. The flexible nature of senolytics could have great potential to promote healthy immune function in aging populations.
An open-label pilot study investigated the combination of dasatinib, a TKI, and quercetin, a flavonoid, both having senolytic effects in vitro in human and murine cells [115,116,117], in patients with IPF. The study had a small sample size (N = 14) and demonstrated a significant improvement in the 6-min walk distance (p < 0.05), but no improvements in the FVC, frailty index (FI-LAB), and reported health. The most common adverse effects were skin irritation and gastrointestinal discomfort [118]. Further studies with senolytics in pulmonary fibrosis are warranted.
5.5 New Perspectives on Inhaled Treatment
Delivering drugs directly to the alveolar space has the potential to achieve higher concentrations in the lung and to reduce systemic effects.
A phase I, randomized, double-blinded, placebo-controlled, dose-escalation study investigated the safety and pharmacokinetics of a single administration of an aqueous formulation of pirfenidone delivered by a high-efficiency vibrating plate nebulizer [119]. Aerosolized pirfenidone was well tolerated in healthy volunteers, smokers, and IPF patients. Interestingly, the nebulizer dose averaged a 15-fold lower systemic pirfenidone exposure than reported with oral administration of the licensed oral dose [119]. A phase I/II clinical study of two dose regimens of AP01 (a formulation of pirfenidone optimized for delivery via inhalation) recruited 91 IPF patients, randomly assigned to 50 mg once daily or 100 mg twice daily. Adverse effects, with cough being the most frequent adverse effect, were less frequent with AP01 than with oral pirfenidone in other clinical trials. Mean FVC percent predicted remained stable in the 100 mg twice daily group [120]. A phase III program in IPF and PPF is planned.
Inhaled N-acetylcysteine, combined with oral pirfenidone, did not result in substantial benefits for IPF patients in a recently published phase II trial [121].
The efficacy of inhalational TD139, a small molecule inhibiting Gal-3, a member of the β-galactoside-binding lectins family, which regulates fibrotic processes and is overexpressed in the BAL fluid of patients with IPF, was evaluated through a randomized controlled, phase I/IIa dose-ascending trial. Sixty participants were recruited, 24 of whom were diagnosed with IPF [122]. TD139 was well-tolerated by both healthy and IPF patients: taste disturbance (36.1%) and cough (11.1%) were the most common adverse effects [122]. A phase II trial in IPF for efficacy evaluation is ongoing (NCT03832946).
TRK250, previously known as BNC-1021, inhibits the transcription of TGFβ1 by producing silencing RNA (siRNA) targeting TGFβ1 messenger RNA (mRNA). TRK250 has demonstrated its ability to reduce the expression of TGFβ1 and collagen production in the lungs in animal models [123]. A phase I, placebo-controlled, double-blind, randomized study assessing the safety and tolerability of single and multiple inhaled doses of TRK250 in subjects with IPF for 4 weeks was completed in April 2022 (NCT03727802). The results of that study are yet to be released.
Older formulations of sodium cromoglycate are approved for the treatment of asthma. PA101 is a novel formulation that was tested in IPF patients for 2 weeks and compared with placebo. There was a mean reduction in daytime cough frequency at day 14 (31.1%) when adjusted for the placebo [124]. Another phase IIb trial (SCENIC) that investigated varying doses of inhaled sodium cromoglycate over 12 weeks in IPF patients found no benefit in reducing cough [125].
In the last two decades, PDE-5 inhibitors have raised interest as a potential treatment for IPF, whereas discordant effects were observed for sildenafil, an oral PDE-5 inhibitor and pulmonary vasodilator, in patients with IPF [45, 126,127,128].
The INCREASE trial, which investigated treprostinil, an inhaled form of PDE-5 inhibitor, in IPF and non-IPF ILD with pulmonary hypertension, found a significant improvement in the mean FVC at 16 weeks compared with placebo [129]. A post hoc analysis of 326 patients (inhaled treprostinil, n = 163; placebo, n = 163) assessed the effect of continued treatment with inhaled treprostinil on multiple disease progression events, defined as a 15% or more decline in 6-min walk distance, a 10% or more decline in FVC, acute exacerbation, cardiopulmonary hospitalization, lung transplantation, or death. Patients who received inhaled treprostinil were significantly less likely to experience further disease progression events after an initial event, compared with patients receiving placebo [130].
6 Conclusion
Thus far, most clinical trials in IPF have failed to meet the primary endpoint. Aside from reasons related to clinical trial design, duration, and heterogeneity of included populations, we must consider that we are examining short-term intervention effects of new drugs. Moreover, our understanding of IPF pathogenesis is still limited and we should keep focusing our efforts to deeper understand the mechanisms underlying this complex disease and their reflection on clinical phenotypes.
References
Hilberg O, Hoffmann-Vold AM, Smith V, Bouros D, Kilpelainen M, Guiot J, et al. Epidemiology of interstitial lung diseases and their progressive-fibrosing behaviour in six European countries. ERJ Open Res. 2022;8(1):00597–2021.
Podolanczuk AJ, Thomson CC, Remy-Jardin M, Richeldi L, Martinez FJ, Kolb M, et al. Idiopathic pulmonary fibrosis: state of the art for 2023. Eur Respir J. 2023;61(4):2200957.
Guenther A, Krauss E, Tello S, Wagner J, Paul B, Kuhn S, et al. The European IPF registry (eurIPFreg): baseline characteristics and survival of patients with idiopathic pulmonary fibrosis. Respir Res. 2018;19(1):141.
Jegal Y, Park JS, Kim SY, Yoo H, Jeong SH, Song JW, et al. Clinical features, diagnosis, management, and outcomes of idiopathic pulmonary fibrosis in Korea: analysis of the Korea IPF cohort (KICO) registry. Tubercul Respir Dis. 2022;85(2):185–94.
Homma S, Suda T, Hongo Y, Yoshida M, Hiroi S, Iwasaki K, et al. Incidence and changes in treatment of acute exacerbation of idiopathic pulmonary fibrosis in Japan: a claims-based retrospective study. Respir Investig. 2022;60(6):798–805.
Zaman T, Lee JS. Risk factors for the development of idiopathic pulmonary fibrosis: a review. Curr Pulmonol Rep. 2018;7(4):118–25.
Spagnolo P, Cottin V. Genetics of idiopathic pulmonary fibrosis: from mechanistic pathways to personalised medicine. J Med Genet. 2017;54(2):93–9.
Kropski JA, Blackwell TS. Progress in understanding and treating idiopathic pulmonary fibrosis. Annu Rev Med. 2019;70:211–24.
Fernandez IE, Eickelberg O. The impact of TGF-beta on lung fibrosis: From targeting to biomarkers. Proc Am Thorac Soc. 2012;9(3):111–6.
Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96(3):319–28.
John AE, Graves RH, Pun KT, Vitulli G, Forty EJ, Mercer PF, et al. Translational pharmacology of an inhaled small molecule alphavbeta6 integrin inhibitor for idiopathic pulmonary fibrosis. Nat Commun. 2020;11(1):4659.
Mora AL, Rojas M, Pardo A, Selman M. Emerging therapies for idiopathic pulmonary fibrosis, a progressive age-related disease. Nat Rev Drug Discov. 2017;16(11):755–72.
Borok Z, Horie M, Flodby P, Wang H, Liu Y, Ganesh S, et al. Grp78 loss in epithelial progenitors reveals an age-linked role for endoplasmic reticulum stress in pulmonary fibrosis. Am J Respir Crit Care Med. 2020;201(2):198–211.
Yue YL, Zhang MY, Liu JY, Fang LJ, Qu YQ. The role of autophagy in idiopathic pulmonary fibrosis: from mechanisms to therapies. Ther Adv Respir Dis. 2022;16:17534666221140972.
Pardo A, Cabrera S, Maldonado M, Selman M. Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis. Respir Res. 2016;17:23.
Flaherty KR, Colby TV, Travis WD, Toews GB, Mumford J, Murray S, et al. Fibroblastic foci in usual interstitial pneumonia: idiopathic versus collagen vascular disease. Am J Respir Crit Care Med. 2003;167(10):1410–5.
Vuga LJ, Ben-Yehudah A, Kovkarova-Naumovski E, Oriss T, Gibson KF, Feghali-Bostwick C, et al. WNT5A is a regulator of fibroblast proliferation and resistance to apoptosis. Am J Respir Cell Mol Biol. 2009;41(5):583–9.
Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, et al. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest. 2013;123(3):1096–108.
LeBleu VS, Neilson EG. Origin and functional heterogeneity of fibroblasts. FASEB J. 2020;34(3):3519–36.
Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. 2017;3:17074.
Ikenaga N, Peng ZW, Vaid KA, Liu SB, Yoshida S, Sverdlov DY, et al. Selective targeting of lysyl oxidase-like 2 (LOXL2) suppresses hepatic fibrosis progression and accelerates its reversal. Gut. 2017;66(9):1697–708.
Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16(9):1009–17.
Aumiller V, Strobel B, Romeike M, Schuler M, Stierstorfer BE, Kreuz S. Comparative analysis of lysyl oxidase (like) family members in pulmonary fibrosis. Sci Rep. 2017;7(1):149.
Chien JW, Richards TJ, Gibson KF, Zhang Y, Lindell KO, Shao L, et al. Serum lysyl oxidase-like 2 levels and idiopathic pulmonary fibrosis disease progression. Eur Respir J. 2014;43(5):1430–8.
Raghu G, Brown KK, Collard HR, Cottin V, Gibson KF, Kaner RJ, et al. Efficacy of simtuzumab versus placebo in patients with idiopathic pulmonary fibrosis: a randomised, double-blind, controlled, phase 2 trial. Lancet Respir Med. 2017;5(1):22–32.
Xiong L, Xiong L, Ye H, Ma WL. Animal models of rheumatoid arthritis-associated interstitial lung disease. Immun Inflamm Dis. 2021;9(1):37–47.
Duerr J, Leitz DHW, Szczygiel M, Dvornikov D, Fraumann SG, Kreutz C, et al. Conditional deletion of Nedd4-2 in lung epithelial cells causes progressive pulmonary fibrosis in adult mice. Nat Commun. 2020;11(1):2012.
Moore BB, Lawson WE, Oury TD, Sisson TH, Raghavendran K, Hogaboam CM. Animal models of fibrotic lung disease. Am J Respir Cell Mol Biol. 2013;49(2):167–79.
Ley B, Collard HR, King TE Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183(4):431–40.
Brown KK, Flaherty KR, Cottin V, Raghu G, Inoue Y, Azuma A, et al. Lung function outcomes in the INPULSIS((R)) trials of nintedanib in idiopathic pulmonary fibrosis. Respir Med. 2019;146:42–8.
Fainberg HP, Oldham JM, Molyneau PL, Allen RJ, Kraven LM, Fahy WA, et al. Forced vital capacity trajectories in patients with idiopathic pulmonary fibrosis: a secondary analysis of a multicentre, prospective, observational cohort. Lancet Digit Health. 2022;4(12):e862–72.
Herrmann FE, Hesslinger C, Wollin L, Nickolaus P. BI 1015550 is a PDE4B inhibitor and a clinical drug candidate for the oral treatment of idiopathic pulmonary fibrosis. Front Pharmacol. 2022;13:838449.
Spagnolo P, Bonella F. Trial of a phosphodiesterase 4 inhibitor for idiopathic pulmonary fibrosis. N Engl J Med. 2022;387(8):761–2.
Nolan CM, Polgar O, Schofield SJ, Patel S, Barker RE, Walsh JA, et al. Pulmonary rehabilitation in idiopathic pulmonary fibrosis and COPD: a propensity-matched real-world study. Chest. 2022;161(3):728–37.
Richeldi L, Kolb M, Jouneau S, Wuyts WA, Schinzel B, Stowasser S, et al. Efficacy and safety of nintedanib in patients with advanced idiopathic pulmonary fibrosis. BMC Pulm Med. 2020;20(1):3.
Costabel U, Albera C, Glassberg MK, Lancaster LH, Wuyts WA, Petzinger U, et al. Effect of pirfenidone in patients with more advanced idiopathic pulmonary fibrosis. Respir Res. 2019;20(1):55.
Kreuter M, Ehlers-Tenenbaum S, Palmowski K, Bruhwyler J, Oltmanns U, Muley T, et al. Impact of comorbidities on mortality in patients with idiopathic pulmonary fibrosis. PLoS ONE. 2016;11(3):e0151425.
Ley B, Bradford WZ, Weycker D, Vittinghoff E, du Bois RM, Collard HR. Unified baseline and longitudinal mortality prediction in idiopathic pulmonary fibrosis. Eur Respir J. 2015;45(5):1374–81.
King TE Jr, Albera C, Bradford WZ, Costabel U, Hormel P, Lancaster L, et al. Effect of interferon gamma-1b on survival in patients with idiopathic pulmonary fibrosis (INSPIRE): a multicentre, randomised, placebo-controlled trial. Lancet. 2009;374(9685):222–8.
Raghu G, Million-Rousseau R, Morganti A, Perchenet L, Behr J, Group MS. Macitentan for the treatment of idiopathic pulmonary fibrosis: the randomised controlled MUSIC trial. Eur Respir J. 2013;42(6):1622–32.
King TE Jr, Behr J, Brown KK, du Bois RM, Lancaster L, de Andrade JA, et al. BUILD-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177(1):75–81.
TE King J, Brown KK, Raghu G, du Bois RM, Lynch DA, Martinez F, et al. BUILD-3: a randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184(1):92–9.
Noth I, Anstrom KJ, Calvert SB, de Andrade J, Flaherty KR, Glazer C, et al. A placebo-controlled randomized trial of warfarin in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;186(1):88–95.
Idiopathic Pulmonary Fibrosis Clinical Research N, Martinez FJ, de Andrade JA, Anstrom KJ, King TE Jr, Raghu G. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2093–101.
Idiopathic Pulmonary Fibrosis Clinical Research Network, Zisman DA, Schwarz M, Anstrom KJ, Collard HR, Flaherty KR, et al. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med. 2010;363(7):620–8.
Idiopathic Pulmonary Fibrosis Clinical Research N, Raghu G, Anstrom KJ, King TE Jr, Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366(21):1968–77.
Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377(9779):1760–9.
King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–92.
Nathan SD, Costabel U, Albera C, Behr J, Wuyts WA, Kirchgaessler KU, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis and more advanced lung function impairment. Respir Med. 2019;153:44–51.
Glassberg MK, Wijsenbeek MS, Gilberg F, Petzinger U, Kirchgaessler KU, Albera C. Effect of pirfenidone on breathlessness in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2019;54:3.
Albera C, Costabel U, Fagan EA, Glassberg MK, Gorina E, Lancaster L, et al. Efficacy of pirfenidone in patients with idiopathic pulmonary fibrosis with more preserved lung function. Eur Respir J. 2016;48(3):843–51.
Nathan SD, Lancaster LH, Albera C, Glassberg MK, Swigris JJ, Gilberg F, et al. Dose modification and dose intensity during treatment with pirfenidone: analysis of pooled data from three multinational phase III trials. BMJ Open Respir Res. 2018;5(1):e000323.
Glassberg MK, Nathan SD, Lin CY, Morgenthien EA, Stauffer JL, Chou W, et al. Cardiovascular risks, bleeding risks, and clinical events from 3 phase III trials of pirfenidone in patients with idiopathic pulmonary fibrosis. Adv Ther. 2019;36(10):2910–26.
Wu W, Qiu L, Wu J, Liu X, Zhang G. Efficacy and safety of pirfenidone in the treatment of idiopathic pulmonary fibrosis patients: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2021;11(12):e050004.
Noble PW, Albera C, Bradford WZ, Costabel U, du Bois RM, Fagan EA, et al. Pirfenidone for idiopathic pulmonary fibrosis: analysis of pooled data from three multinational phase 3 trials. Eur Respir J. 2016;47(1):243–53.
Lancaster L, Albera C, Bradford WZ, Costabel U, du Bois RM, Fagan EA, et al. Safety of pirfenidone in patients with idiopathic pulmonary fibrosis: integrated analysis of cumulative data from 5 clinical trials. BMJ Open Respir Res. 2016;3(1):e000105.
Nathan SD, Albera C, Bradford WZ, Costabel U, Glaspole I, Glassberg MK, et al. Effect of pirfenidone on mortality: pooled analyses and meta-analyses of clinical trials in idiopathic pulmonary fibrosis. Lancet Respir Med. 2017;5(1):33–41.
Ley B, Swigris J, Day BM, Stauffer JL, Raimundo K, Chou W, et al. Pirfenidone reduces respiratory-related hospitalizations in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2017;196:756.
Costabel U, Albera C, Lancaster LH, Lin CY, Hormel P, Hulter HN, et al. An open-label study of the long-term safety of pirfenidone in patients with idiopathic pulmonary fibrosis (RECAP). Respir Int Rev Thoracic Dis. 2017;94(5):408–15.
Maher TM, Lancaster LH, Jouneau S, Morrison L, Lederer DJ, Molina-Molina M, et al. Pirfenidone treatment in individuals with idiopathic pulmonary fibrosis: impact of timing of treatment initiation. Ann Am Thorac Soc. 2019;16(7):927–30.
Bonella F, Wessendorf T, Costabel U. Clinical experience with pirfenidone for the treatment of idiopathic pulmonary fibrosis. Eur Respir J. 2013;42:P3808.
Lancaster LH, de Andrade JA, Zibrak JD, Padilla ML, Albera C, Nathan SD, et al. Pirfenidone safety and adverse event management in idiopathic pulmonary fibrosis. Eur Respir Rev. 2017;26(146):170057.
Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al. An Official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis an update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192(2):e3–19.
Chung MP, Park MS, Oh IJ, Lee HB, Kim YW, Park JS, et al. Safety and efficacy of pirfenidone in advanced idiopathic pulmonary fibrosis: a nationwide post-marketing surveillance study in korean patients. Adv Ther. 2020;37(5):2303–16.
Maher TM, Corte TJ, Fischer A, Kreuter M, Lederer DJ, Molina-Molina M, et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2020;8(2):147–57.
Behr J, Prasse A, Kreuter M, Johow J, Rabe KF, Bonella F, et al. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): a double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir Med. 2021;9(5):476–86.
Wollin L, Wex E, Pautsch A, Schnapp G, Hostettler KE, Stowasser S, et al. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J. 2015;45(5):1434–45.
Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–82.
Rugo HS, Di Palma JA, Tripathy D, Bryce R, Moran S, Olek E, et al. The characterization, management, and future considerations for ErbB-family TKI-associated diarrhea. Breast Cancer Res Treat. 2019;175(1):5–15.
Harada Y, Sekine H, Kubota K, Sadatomi D, Iizuka S, Fujitsuka N. Calcium-activated chloride channel is involved in the onset of diarrhea triggered by EGFR tyrosine kinase inhibitor treatment in rats. Biomed Pharmacother. 2021;141:111860.
Corte T, Bonella F, Crestani B, Demedts MG, Richeldi L, Coeck C, et al. Safety, tolerability and appropriate use of nintedanib in idiopathic pulmonary fibrosis. Resp Res. 2015;16:116.
Bonella F, Kreuter M, Hagmeyer L, Neurohr C, Keller C, Kohlhaeufl MJ, et al. Insights from the german compassionate use program of nintedanib for the treatment of idiopathic pulmonary fibrosis. Respiration. 2016;92(2):98–106.
Raghu G, Wells AU, Nicholson AG, Richeldi L, Flaherty KR, Le Maulf F, et al. Effect of nintedanib in subgroups of idiopathic pulmonary fibrosis by diagnostic criteria. Am J Respir Crit Care Med. 2017;195(1):78–85.
Wuyts WA, Kolb M, Stowasser S, Stansen W, Huggins JT, Raghu G. First data on efficacy and safety of nintedanib in patients with idiopathic pulmonary fibrosis and forced vital capacity of ≤50% of predicted value. Lung. 2016;194(5):739–43.
Kolb M, Richeldi L, Behr J, Maher TM, Tang W, Stowasser S, et al. Nintedanib in patients with idiopathic pulmonary fibrosis and preserved lung volume. Thorax. 2017;72(4):340–6.
Costabel U, Inoue Y, Richeldi L, Collard HR, Tschoepe I, Stowasser S, et al. Efficacy of nintedanib in idiopathic pulmonary fibrosis across prespecified subgroups in INPULSIS. Am J Respir Crit Care Med. 2016;193(2):178–85.
Taniguchi H, Xu Z, Azuma A, Inoue Y, Li H, Fujimoto T, et al. Subgroup analysis of Asian patients in the INPULSIS((R)) trials of nintedanib in idiopathic pulmonary fibrosis. Respirology. 2016;21(8):1425–30.
Cottin V, Azuma A, Raghu G, Stansen W, Stowasser S, Schlenker-Herceg R, et al. Therapeutic effects of nintedanib are not influenced by emphysema in the INPULSIS trials. Eur Respir J. 2019;53:4.
Ryerson CJ, Kolb M, Richeldi L, Lee J, Wachtlin D, Stowasser S, et al. Effects of nintedanib in patients with idiopathic pulmonary fibrosis by GAP stage. ERJ Open Res. 2019;5(2):00127–2018.
Richeldi L, Cottin V, du Bois RM, Selman M, Kimura T, Bailes Z, et al. Nintedanib in patients with idiopathic pulmonary fibrosis: combined evidence from the TOMORROW and INPULSIS® trials. Respir Med. 2016;113:74–9.
Lancaster L, Crestani B, Hernandez P, Inoue Y, Wachtlin D, Loaiza L, et al. Safety and survival data in patients with idiopathic pulmonary fibrosis treated with nintedanib: pooled data from six clinical trials. BMJ Open Respir Res. 2019;6(1):e000397.
Richeldi L, Crestani B, Azuma A, Kolb M, Selman M, Stansen W, et al. Outcomes following decline in forced vital capacity in patients with idiopathic pulmonary fibrosis: results from the INPULSIS and INPULSIS-ON trials of nintedanib. Respir Med. 2019;156:20–5.
Crestani B, Huggins JT, Kaye M, Costabel U, Glaspole I, Ogura T, et al. Long-term safety and tolerability of nintedanib in patients with idiopathic pulmonary fibrosis: results from the open-label extension study, INPULSIS-ON. Lancet Respir Med. 2019;7(1):60–8.
Richeldi L, Kreuter M, Selman M, Crestani B, Kirsten AM, Wuyts WA, et al. Long-term treatment of patients with idiopathic pulmonary fibrosis with nintedanib: results from the TOMORROW trial and its open-label extension. Thorax. 2018;73(6):581–3.
Raghu G, Remy-Jardin M, Richeldi L, Thomson CC, Inoue Y, Johkoh T, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2022;205(9):e18–47.
Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381(18):1718–27.
Ghazipura M, Mammen MJ, Bissell BD, Macrea M, Herman DD, Hon SM, et al. Pirfenidone in progressive pulmonary fibrosis: a systematic review and meta-analysis. Ann Am Thorac Soc. 2022;19(6):1030–9.
Behr J, Bonella F, Frye BC, Gunther A, Hagmeyer L, Henes J, et al. Pharmacological treatment of idiopathic pulmonary fibrosis (update) and progressive pulmonary fibrosis—S2k Guideline of the German Respiratory Society. Pneumologie. 2023;77(2):94–119.
Cottin V, Bonniaud P, Cadranel J, Crestani B, Jouneau S, Marchand-Adam S, et al. French practical guidelines for the diagnosis and management of idiopathic pulmonary fibrosis - 2021 update. Full-length version. Respir Med Res. 2023;83: 100948.
Richeldi L, Fletcher S, Adamali H, Chaudhuri N, Wiebe S, Wind S, et al. No relevant pharmacokinetic drug-drug interaction between nintedanib and pirfenidone. Eur Respir J. 2019;53(1):1801060.
Ogura T, Taniguchi H, Azuma A, Inoue Y, Kondoh Y, Hasegawa Y, et al. Safety and pharmacokinetics of nintedanib and pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2015;45(5):1382–92.
Flaherty KR, Fell CD, Huggins JT, Nunes H, Sussman R, Valenzuela C, et al. Safety of nintedanib added to pirfenidone treatment for idiopathic pulmonary fibrosis. Eur Respir J. 2018;52:2.
Vancheri C, Kreuter M, Richeldi L, Ryerson CJ, Valeyre D, Grutters JC, et al. Nintedanib with add-on pirfenidone in idiopathic pulmonary fibrosis. Results of the INJOURNEY trial. Am J Respir Crit Care Med. 2018;197(3):356–63.
Cox N, Pilling D, Gomer RH. Serum amyloid P: a systemic regulator of the innate immune response. J Leukoc Biol. 2014;96(5):739–43.
Nakagawa N, Barron L, Gomez IG, Johnson BG, Roach AM, Kameoka S, et al. Pentraxin-2 suppresses c-Jun/AP-1 signaling to inhibit progressive fibrotic disease. JCI Insight. 2016;1(20):e87446.
Pilling D, Galvis-Carvajal E, Karhadkar TR, Cox N, Gomer RH. Monocyte differentiation and macrophage priming are regulated differentially by pentraxins and their ligands. BMC Immunol. 2017;18(1):30.
Raghu G, van den Blink B, Hamblin MJ, Brown AW, Golden JA, Ho LA, et al. Effect of recombinant human pentraxin 2 vs Placebo on change in forced vital capacity in patients with idiopathic pulmonary fibrosis: a randomized clinical trial. JAMA. 2018;319(22):2299–307.
Raghu G, van den Blink B, Hamblin MJ, Brown AW, Golden JA, Ho LA, et al. Long-term treatment with recombinant human pentraxin 2 protein in patients with idiopathic pulmonary fibrosis: an open-label extension study. Lancet Respir Med. 2019;7(8):657–64.
Maher TM, van der Aar EM, Van de Steen O, Allamassey L, Desrivot J, Dupont S, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of GLPG1690, a novel autotaxin inhibitor, to treat idiopathic pulmonary fibrosis (FLORA): a phase 2a randomised placebo-controlled trial. Lancet Respir Med. 2018;6(8):627–35.
Corte TJ, Lancaster L, Swigris JJ, Maher TM, Goldin JG, Palmer SM, et al. Phase 2 trial design of BMS-986278, a lysophosphatidic acid receptor 1 (LPA(1)) antagonist, in patients with idiopathic pulmonary fibrosis (IPF) or progressive fibrotic interstitial lung disease (PF-ILD). BMJ Open Respir Res. 2021;8:1.
Palmer SM, Snyder L, Todd JL, Soule B, Christian R, Anstrom K, et al. Randomized, double-blind, placebo-controlled, phase 2 trial of BMS-986020, a lysophosphatidic acid receptor antagonist for the treatment of idiopathic pulmonary fibrosis. Chest. 2018;154(5):1061–9.
Yanagihara T, Tsubouchi K, Gholiof M, Chong SG, Lipson KE, Zhou Q, et al. Connective-tissue growth factor contributes to TGF-beta1-induced lung fibrosis. Am J Respir Cell Mol Biol. 2022;66(3):260–70.
Effendi WI, Nagano T. Connective tissue growth factor in idiopathic pulmonary fibrosis: breaking the bridge. Int J Mol Sci. 2022;23(11):6064.
Richeldi L, Fernandez Perez ER, Costabel U, Albera C, Lederer DJ, Flaherty KR, et al. Pamrevlumab, an anti-connective tissue growth factor therapy, for idiopathic pulmonary fibrosis (PRAISE): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2020;8(1):25–33.
Huang S, Wettlaufer SH, Hogaboam C, Aronoff DM, Peters-Golden M. Prostaglandin E(2) inhibits collagen expression and proliferation in patient-derived normal lung fibroblasts via E prostanoid 2 receptor and cAMP signaling. Am J Physiol Lung Cell Mol Physiol. 2007;292(2):L405–13.
Kolb M, Crestani B, Maher TM. Phosphodiesterase 4B inhibition: a potential novel strategy for treating pulmonary fibrosis. Eur Respir Rev. 2023;32(167):220206.
Richeldi L, Azuma A, Cottin V, Hesslinger C, Stowasser S, Valenzuela C, et al. Trial of a preferential phosphodiesterase 4B inhibitor for idiopathic pulmonary fibrosis. N Engl J Med. 2022;386(23):2178–87.
Gagnon L, Leduc M, Thibodeau JF, Zhang MZ, Grouix B, Sarra-Bournet F, et al. A newly discovered antifibrotic pathway regulated by two fatty acid receptors: GPR40 and GPR84. Am J Pathol. 2018;188(5):1132–48.
Khalil N, Manganas H, Ryerson CJ, Shapera S, Cantin AM, Hernandez P, et al. Phase 2 clinical trial of PBI-4050 in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2019;53(3):1800663.
Decaris ML, Schaub JR, Chen C, Cha J, Lee GG, Rexhepaj M, et al. Dual inhibition of alpha(v)beta(6) and alpha(v)beta(1) reduces fibrogenesis in lung tissue explants from patients with IPF. Respir Res. 2021;22(1):265.
Mullard A. Pliant’s integrin inhibitor boosted by phase II IPF data. Nat Rev Drug Discov. 2022;21(9):626.
Chaib S, Tchkonia T, Kirkland JL. Cellular senescence and senolytics: the path to the clinic. Nat Med. 2022;28(8):1556–68.
Barnes PJ. Senotherapy for lung diseases. Adv Pharmacol. 2023;98:249–71.
Du PY, Gandhi A, Bawa M, Gromala J. The ageing immune system as a potential target of senolytics. Oxf Open Immunol. 2023;4(1):iqad004.
Ogrodnik M, Miwa S, Tchkonia T, Tiniakos D, Wilson CL, Lahat A, et al. Cellular senescence drives age-dependent hepatic steatosis. Nat Commun. 2017;8:15691.
Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532.
Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15(5):973–7.
Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–63.
Khoo JK, Montgomery AB, Otto KL, Surber M, Faggian J, Lickliter JD, et al. A randomized, double-blinded, placebo-controlled, dose-escalation phase 1 study of aerosolized pirfenidone delivered via the PARI investigational eFlow nebulizer in volunteers and patients with idiopathic pulmonary fibrosis. J Aerosol Med Pulm Drug Deliv. 2020;33(1):15–20.
West A, Chaudhuri N, Barczyk A, Wilsher ML, Hopkins P, Glaspole I, et al. Inhaled pirfenidone solution (AP01) for IPF: a randomised, open-label, dose-response trial. Thorax. 2023;78(9):882–9.
Sakamoto S, Kataoka K, Kondoh Y, Kato M, Okamoto M, Mukae H, et al. Pirfenidone plus inhaled N-acetylcysteine for idiopathic pulmonary fibrosis: a randomised trial. Eur Respir J. 2021;57(1):2000348.
Hirani N, MacKinnon AC, Nicol L, Ford P, Schambye H, Pedersen A, et al. Target inhibition of galectin-3 by inhaled TD139 in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2021;57(5):2002559.
Matsumoto T, Uchida M, Yamada M, Miyamoto Y, Inada H, Eguchi Y, et al. A novel siRNA-based oligonucleotide, TRK-250, and its efficacy for treatment of idiopathic pulmonary fibrosis (IPF). In: C64 Pulmonary Fibrosis Models and Mechanistic Insights; p. A5391. 2022.
Birring SS, Wijsenbeek MS, Agrawal S, van den Berg JWK, Stone H, Maher TM, et al. A novel formulation of inhaled sodium cromoglicate (PA101) in idiopathic pulmonary fibrosis and chronic cough: a randomised, double-blind, proof-of-concept, phase 2 trial. Lancet Respir Med. 2017;5(10):806–15.
Martinez FJ, Wijsenbeek MS, Raghu G, Flaherty KR, Maher TM, Wuyts WA, et al. Phase 2B study of inhaled RVT-1601 for chronic cough in idiopathic pulmonary fibrosis: a multicenter, randomized, placebo-controlled study (SCENIC Trial). Am J Respir Crit Care Med. 2022;205(9):1084–92.
Han MK, Bach DS, Hagan PG, Yow E, Flaherty KR, Toews GB, et al. Sildenafil preserves exercise capacity in patients with idiopathic pulmonary fibrosis and right-sided ventricular dysfunction. Chest. 2013;143(6):1699–708.
Behr J, Kolb M, Song JW, Luppi F, Schinzel B, Stowasser S, et al. Nintedanib and sildenafil in patients with idiopathic pulmonary fibrosis and right heart dysfunction. A prespecified subgroup analysis of a double-blind randomized clinical trial (INSTAGE). Am J Respir Crit Care Med. 2019;200(12):1505–12.
Kolb M, Raghu G, Wells AU, Behr J, Richeldi L, Schinzel B, et al. Nintedanib plus sildenafil in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2018;379(18):1722–31.
Nathan SD, Waxman A, Rajagopal S, Case A, Johri S, DuBrock H, et al. Inhaled treprostinil and forced vital capacity in patients with interstitial lung disease and associated pulmonary hypertension: a post-hoc analysis of the INCREASE study. Lancet Respir Med. 2021;9(11):1266–74.
Nathan SD, Tapson VF, Elwing J, Rischard F, Mehta J, Shapiro S, et al. Efficacy of inhaled treprostinil on multiple disease progression events in patients with pulmonary hypertension due to parenchymal lung disease in the INCREASE trial. Am J Respir Crit Care Med. 2022;205(2):198–207.
Waxman A, Restrepo-Jaramillo R, Thenappan T, Ravichandran A, Engel P, Bajwa A, et al. Inhaled treprostinil in pulmonary hypertension due to interstitial lung disease. N Engl J Med. 2021;384(4):325–34.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding enabled and organized by Projekt DEAL.
Conflicts of interest
Francesco Bonella reports consulting fees and non-financial support from Boehringer Ingelheim, Sanofi, Savara, and Trevi Therapeutics, outside the submitted work. Paolo Spagnolo reports institutional grants, consulting fees and non-financial support from PPM Services; institutional grants, personal fees and non-financial support from Roche and Boehringer Ingelheim; and personal fees from Chiesi, Galapagos, Lupin, Pieris and REDX Pharma, outside the submitted work. Chris Ryerson reports grants from Boehringer Ingelheim, Hoffmann-La Roche and VIDA diagnostics; and personal fees from Boehringer Ingelheim, Hoffmann-La Roche, Veracyte, Pliant Therapeutics, Astra Zeneca, Cipla Ltd and Trevi Therapeutics, outside the submitted work.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
All authors contributed to conception and drafting of the work and are responsible for all aspects of the manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bonella, F., Spagnolo, P. & Ryerson, C. Current and Future Treatment Landscape for Idiopathic Pulmonary Fibrosis. Drugs 83, 1581–1593 (2023). https://doi.org/10.1007/s40265-023-01950-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-023-01950-0