Abstract
Pyoderma gangrenosum is a rare neutrophilic dermatosis that leads to exceedingly painful ulcerations of the skin. Although the exact pathogenesis is not yet fully understood, various auto-inflammatory phenomena with increased neutrophil granulocyte activity have been demonstrated. Despite the limited understanding of the pathogenesis, it is no longer a diagnosis of exclusion, as it can now be made on the basis of validated scoring systems. However, therapy remains a major multidisciplinary challenge. Various immunosuppressive and immunomodulatory therapies are available for the treatment of affected patients. In addition, concomitant topical pharmacologic therapy, wound management and pain control should always be addressed. Corticosteroids and/or cyclosporine remain the systemic therapeutics of choice for most patients. However, in recent years, there has been an increasing number of studies on the positive effects of biologic therapies such as inhibitors of tumour necrosis factor-α; interleukin-1, interleukin-17, interleukin-23 or complement factor C5a. Biologics have now become the drug of choice in certain scenarios, particularly in patients with underlying inflammatory comorbidities, and are increasingly used at an early stage in the disease rather than in therapy refractory patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Systemic therapy with corticosteroids and/or cyclosporine remains the treatment of choice for most patients with pyoderma gangrenosum. |
Based on new data, systemic therapies with biologics are gaining importance as alternative or first-line therapy in patients with inflammatory comorbidities. |
Concomitant topical therapy can be given with classic immunosuppressants, e.g. corticosteroids or calcineurin inhibitors. |
There should always be a special focus on pain management. |
1 Introduction
Pyoderma gangrenosum (PG) is an ulcerative inflammatory skin disease classified within the neutrophilic dermatoses group. It is a rare disease with a prevalence of 5.8 cases per 100,000 adults [1, 2]. PG negatively impacts quality of life and carries a three times higher mortality risk than that of the general population [3]. The classic clinical presentation are very painful skin ulcers with a predilection for the lower extremities in approximately 80% of cases [2, 4, 5] (Fig. 1). When the lower extremities are involved, it is particularly important to fully evaluate for other causes of skin ulceration. For example, venous and arterial status must be assessed, and frequent evaluation for infection is necessary. In addition, skin biopsy can rule out many other differential diagnoses. However, any hair-bearing skin on the body has the potential to develop PG. Less common presentations including bullous, verrucous and pustular variants have been described [6]. Other ulcerative variants include peristomal PG. In the initially acute inflammatory stage of classic PG, patients often have an erythematous papule or pustule that rapidly develops into a painful skin ulceration or multiple ulcerations with gunmetal grey borders and violaceous erythema. Once the acute inflammation has subsided, large wounds may also present without classic or distinctive features, making the diagnosis even more challenging [6]. The pathergy phenomenon has been reported in up to 30% of patients with PG and describes the formation of new ulcers after minor trauma (e.g. needle injury). Sometimes this is a clinical clue for the diagnosis of PG; however, it is not specific to PG and has also been described in other patients with auto-inflammatory or neutrophilic conditions [7].
PG still represents a diagnostic challenge, as it is a rare disease with several clinical mimickers. With a misdiagnosis rate of up to 39%, it is considered the prototype of misdiagnosis among other skin ulcerations where the initial diagnosis was PG [8]. The most common clinical mimickers are venous leg ulcers, vasculitis, vasculopathies and factitial ulcers (Table 1). Recent efforts from clinical researchers, including experts worldwide, have supported the implementation of diagnostic frameworks to improve this critical issue in clinical practice. Three diagnostic criteria have been proposed: the Su and Delphi consensus, and the PARACELSUS score. All these diagnostic criteria seem to be useful in clinical practice, but the PARACELSUS score has shown the highest sensitivity among comparative studies [9, 10]. However, the specificity of all of them remains relatively low. The classic histological findings in PG have been well described. In early pustules or ulcerations, diffuse neutrophilic infiltrates are found around hair follicles [11]. However, this inflammatory infiltrate evolves into a non-specific inflammatory infiltrate (e.g. lymphohistiocytic infiltrate) once the ulceration becomes chronic. In two retrospective chart reviews of patients with PG, characteristic biopsy findings of PG were reported in only 7% (8 of 103) and 11% (5 of 47), respectively [4, 9]. PG is often associated with other inflammatory disorders such as inflammatory bowel disease (IBD 13.0–22.7%) and rheumatoid arthritis (RA 9.2–16.9%) [12,13,14]. It is also important to note that PG does not reflect the clinical course of the underlying disease, and it is not uncommon for patients with PG to have well-controlled IBD or vice versa [14]. Some patients also have underlying haematologic disorders (monoclonal gammopathy of unknown significance) or malignancies (leukaemias or other neoplasia) [15, 16]. Despite these comorbidities being clinical clues in the diagnosis and workup for PG, they seem to have a more preponderant role in the prognosis and/or selection an appropriate therapeutic approach [17] (Fig. 2). Besides medical management to control the associated comorbidity and the inflammation inherent to PG with either local pharmacologic therapy and/or systemic therapy, wound care management and pain control also needs to be addressed throughout the treatment course.
2 Drug-Induced Pyoderma Gangrenosum
The occurrence of PG after taking drugs has been repeatedly reported in the literature (Table 2). Although the underlying pathomechanisms are not clearly known, it must be assumed that a dysregulated inflammatory reaction with disturbed migration and activation of neutrophil granulocytes also occurs as a result of the drugs in genetically predisposed individuals. Keratinocyte apoptosis and alteration of epigenetic mechanisms might play a role as well [18]. A direct assessment of the causal relationship of the intake of a drug and the induction of a PG is not always easy and reports with drug rechallenge are rare. Mostly, these are descriptions of single patients. Many of those patients had an underlying disease such as (haematologic) neoplasia or a chronic inflammatory disease [19]. Thus, it cannot always be said with certainty whether the drug was the trigger for the PG or whether the disease would have occurred even without the drug. Another important aspect is the temporal relationship between the start of drug therapy and the occurrence of PG. Very different time periods have been described [20, 21]. For example, if a large proportion of the world's population received appropriate vaccinations during the Covid-19 pandemic, the temporal context would include patients with PG [22]. It is then only possible to determine whether clustering has occurred by analysing large patient collectives. In the context of rarely occurring PG, however, these are exclusively individual case descriptions, which in principle, could also be random. However, other immune-mediated clinical diseases were also found significantly more frequently in association with the Covid-19 vaccinations, so a connection could certainly exist here [23].
In the first study summarising drug-induced PG, colony-stimulating factors (CSFs) and tyrosine kinase inhibitors (TKIs) were the most common culprits [18]. CSFs are haematopoietic growth factors used for chemotherapy-induced bone marrow suppression and have been reported as possible culprits since the 1990s [24, 25]. TKIs are small molecules used as targeted therapy in several cancers; the most common culprit in drug-induced PG within this group has been sunitinib [26]. Subsequently, in an analysis of 44 publications with 52 cases of drug-induced PG based on the Naranjo criteria [19], the most common probable culprits were cocaine and levamisole. The anthelmintic agent levamisole is often added to cocaine because it is an inexpensive additive that prolongs the effects of cocaine. Levamisole is known to alter the chemotaxis response of neutrophils. In addition, levamisole and cocaine can increase inflammation and autoimmunity [27]. Reviewing the list of drugs that have been implicated in causing PG, it is striking that these are often medicaments that have otherwise been successfully used for PG therapy. These paradoxical reactions have already been described for various other autoimmunological disease patterns, such as psoriasis, hidradenitis suppurativa or inflammatory bowel diseases [28, 29]. They begin a few weeks to months after the start of biologic therapy and resolve after discontinuation of the drugs without specific therapy [30]. In an analysis of all case reports in which PG was thought to have been triggered by the administration of biological therapy, 57 patients were identified. Here, 41 cases involved rituximab, 12 cases involved TNF-α inhibitors, 3 cases involved interleukin 17A and one case involved cytotoxic T-lymphocyte-associated protein 4 antibodies [31]. It is hypothesised that in these paradoxical reactions, there is a shift of the cytokine balance towards a molecular pattern typical of the respective induced disease. Looking more closely at the case reports, almost all published patients had systemic inflammatory diseases. Therefore, it is important to consider that the PG was possibly not induced by the biologic therapy but rather the biologic did not sufficiently suppress the induction of PG. After discontinuation of the drugs, other immunosuppressive or immunomodulatory therapies were almost always used, so the improvement of the PG could also be attributed to the newly initiated therapies.
Interestingly, some of the drugs are administered by injection. Here, it must be clarified whether the drug or a pathergy phenomenon related to the physical stimulus was causally responsible. If, for example, PG occurs at the injection site of enoxaparin [32] or erythropoietin [33], it must be assumed that the pathergy phenomenon was more likely the contributing factor. Interestingly, for azacitidine, the occurrence of a PG has been described both at the injection site [34] and independently [35]. Drug-induced PG is diagnostically challenging and often seen in the presence of other predisposing causes (e.g. underlying inflammatory conditions). Meaningful testing or other direct detection methods have not yet been developed. It is therefore very important to recognise the potential association and avoid the triggering drugs if possible.
3 Topical and Locally Administered Therapies
Once the diagnosis of PG has been established, the objective clinical features of severity for PG such as ulcer size, depth, number and location can guide the next steps. Patients with PG often present to clinicians at a late stage with ulceration, and the focus is placed on systemic therapies to gain rapid control of the inflammation. However, a subset of patients might present with early and small ulcerations, e.g. patients who are having a recurrence, and be in a position to start local treatment early in the course of the disease. Based on our experience, small PG ulcers (4 cm2 or less) without compromise of the deep structures (e.g. muscle/tendon) might be ideal for trying local or intra-lesional immunosuppressants. Since local therapy can help limit the dose and duration of systemic therapeutics, they can be useful throughout the entire treatment period.
3.1 Standard Local Therapy
The two most commonly used local therapies are super potent corticosteroids and calcineurin antagonists [36]. Corticosteroids are available in numerous formulations and whilst usually applied as either creams or ointments, they may also be delivered intra-lesionally via inhalers for eroded or mucosal surfaces, as lotions or foams, and also mixed with adhering agents such as orobase paste [37]. For calcineurin antagonists, the commercially available range of bases is more limited, with tacrolimus available as an ointment and pimecrolimus as a cream. These can also be mixed with paste such as orobase.
Local corticosteroid therapies can cause skin atrophy but have a much lower rate of other significant side effects and therefore, in our opinion, may contribute to reducing systemic immunosuppression. The largest study of topical therapies was conducted as a substudy of the STOPGAP study and recruited 66 patients who investigators felt had PG that would be amenable to local treatment [38]. The study was an observational prospective cohort study with no comparator group: 47 patients received clobetasol propionate 0.05%, 10 received tacrolimus ointment 0.1% and 8 received other treatments, mainly other topical steroids. At 6 months, 42% were completely healed with a median target lesion size of 4.4 cm2 for the clobetasol group. The majority did not heal, suggesting that additional systemic therapy may have been beneficial.
3.2 Other Topical Options
The use of intra-lesional steroid injections for PG is widely reported in case reports and case series, often in the context of peristomal disease [39, 40]. It is important to consider the steroid strength. Triamcinolone is often used as it is relatively insoluble and therefore remains in the local area to a greater extent than dexamethasone. Based on our experience, we usually inject 1 ml of 40 mg/ml on the first visit around the edge of the ulcer. In subsequent visits every 4 weeks, we consider up to 2 ml of 10 mg/ml. Caution is needed with higher concentrations or injection volumes as skin atrophy is a common side effect.
A wide variety of other treatments have been reported. These include solutions or gels with cyclosporine, dapsone, methotrexate, cromolyn sodium solution, phenytoin solution, infliximab, etanercept, benzoyl peroxide, becaplermin and cannabis [41]. Topical timolol has been also described to be effective in promoting re-epithelisation in the wound-healing stage [42]. Future alternatives will most likely include topical Janus kinase (JAK) inhibitors such as tofacitinib or ruxolitinib [43]. Many of the reports are single case articles and none of these options are commonly used.
3.3 Considerations for Special Sites
Peristomal PG presents a significant diagnostic challenge and might be overdiagnosed [44]; important differential diagnoses such as toxic and allergic contact dermatitis must be ruled out [45]. However, the need for rapid control of inflammation necessitates therapy. Topical treatment alone may be considered in mild cases where the stoma apparatus is not affected, or in more severe cases as an adjuvant treatment to minimise systemic immunosuppression. The main problem with topical therapies in peristomal PG is the effect on the adherence of the stoma apparatus to the skin. Various solutions have been suggested; intralesional steroid is one option. Steroid lotions in an alcohol base such as super potent topical corticosteroids can work as the solution will dry quite quickly. Direct application of aerosol steroid from an inhaler has been reported [46]. Mixing ointments such as clobetasol 0.05% or tacrolimus 0.03% in oral paste can allow stoma adherence. Extra-thin hydrocolloid dressings have also been used to minimise trauma. A hole for the stoma is cut into a large sheet of hydrocolloid. Following the application of topical treatments, the hydrocolloid is stuck onto the skin with enough overlap onto normal skin. The stoma apparatus is then adhered to the hydrocolloid and a good seal can often be formed. New ostomy baseplates are also being developed to manage the problems of peristomal skin complications and may be beneficial in the management of peristomal PG [47]. Steroid impregnated tapes can be used and crushed oral prednisolone tablets mixed with stomahesive protective powder has been reported [48].
4 Systemic Treatments
While the main therapeutic options for classic PG remain those listed by Maronese et al. [49], evidence from a large, multi-centre, retrospective cohort study as well as an expert survey study shows that PG patients receive an average of two different systemic agents. This underscores the importance of combination treatments [50, 51] to achieve ulcer healing [2]. Combination treatment has not been defined but based on our experience, overlapping classic immunosuppressants and corticosteroid sparing agents for at least 4 weeks throughout the course of the disease is becoming standard of care in clinical practice when patient require systemic treatments (Table 3).
4.1 Classical Immunosuppressive Drugs
4.1.1 Systemic Corticosteroids
Corticosteroids are the first-line immunosuppressant for PG worldwide. They cause immunosuppression mainly by sequestration of CD4+ T-lymphocytes in the reticuloendothelial system and by inhibiting the transcription of cytokines [52]. Treatment with systemic corticosteroids (CS) at a dosage of 0.5–1 mg/kg/day induces a clinical response in up to half of PG cases [53] but it has heterogeneous response rates [54]. Once healing has been reached, the CS dose can be tapered usually within 6 months [49, 50]; however, this decision should be guided by the clinician’s experience and patient’s input. Healing is achieved in approximately 40% of patients with multiple ulcers. It is recommended that systemic CS be combined with other immunosuppressants or immunomodulatory agents, the most common agent being cyclosporine [49]. Pulse therapy with 1 g of intravenous methylprednisolone for 3-5 consecutive days may also be considered in refractory cases [55, 56].
4.1.2 Cyclosporine
Cyclosporine is a calcineurin inhibitor that impairs the transcription of various cytokines, particularly in T-lymphocytes. A multi-centre, randomised controlled trial (Study of Treatments fOr Pyoderma GAngrenosum STOP GAP) compared oral prednisolone 0.75 mg/kg/day versus cyclosporine 4 mg/kg/day. There was no difference between the two treatments in terms of speed of healing over 6 weeks, time to healing, treatment response, inflammation resolution, pain, quality of life, treatment failures or time to recurrence. Of note, almost half of the patients healed within 6 months but almost a third of patients in both treatment groups had a recurrence after a median of 582 days. About two-thirds of patients experienced adverse effects in each group. The conclusion of the study was that the choice of prednisolone versus cyclosporine depends on patients’ comorbidities, with cyclosporine being a more cost-effective choice in patients with large PG ulcers [57]. Pre-existing conditions favouring prednisolone over cyclosporine include renal insufficiency, malignancy and hypertension, whereas patients with obesity, diabetes, osteoporosis, peptic ulcer disease or a history of mental illness may benefit from using cyclosporine rather than prednisolone [58].
4.1.3 Mycophenolate Mofetil
Mycophenolate mofetil (MMF) is an immunosuppressive drug which inhibits T- and B-cell proliferation by blocking the production of guanosine nucleotides required for DNA synthesis. In one retrospective analysis of 26 patients, 85% of patients demonstrated clinical improvement, with 13 patients (50%) healing over 1 year. All patients were on concomitant prednisolone, and a considerable proportion were also on concurrent immunomodulatory and/or immunosuppressant medications [59]. Similar findings regarding efficacy, safety and tolerability of MMF have been documented in smaller case series and case reports, even in the setting of severe, tendon-exposing, refractory PG [60, 61] or if patients declined biologic treatment [62]. In a large single-centre study, mycophenolate sodium and mofetil were used in a considerable proportion of patients (75/118) and were associated with a lower rate of adverse events than cyclosporine. The same authors combined MMF with low dose cyclosporine in some cases [63].
4.2 Classical Immunomodulation Drugs
4.2.1 Dapsone
Dapsone is a sulfone with anti-inflammatory as well as antibacterial and antibiotic properties. In a retrospective series of 27 patients with PG, oral dapsone was used as an adjuvant in conjunction with either systemic, topical or intralesional therapies. CS were the most common concurrent therapy, followed by antibiotics, cyclosporine and TNF-α inhibitors. Despite the 96.9% response rate, with an average time to initial response of 5.3 weeks, only 15.6% of patients achieved a complete response [64].
4.2.2 Intravenous Immunoglobulin
Intravenous immunoglobulin is (IVIG) a biologic agent made up of human antibodies. There are case reports, case series and systematic reviews on the treatment of PG with IVIG with doses from 0.5 to 2.0 g/kg [65, 66]. A systematic review evaluated 49 patients, reporting a healing in 53% or a partial healing in an additional 25% of patients. It should be noted that CS were additionally administered in 88% of patients [67]. In a retrospective study with 45 patients who had received IVIG in PG because of treatment resistance, 23 (51%) healed completely. When IVIG was added, patients with one ulcer were 4.1 times more likely to achieve complete healing than patients with more than one ulcer [68].
4.3 Biologics
4.3.1 Tumour Necrosis Factor (TNF)-α Inhibitors
A semi-systematic review found that 238 out of 356 (67%) patients had healed while on TNF-α inhibitors. Additionally, a review of published PG cases found 87% of patients eventually healed [69, 70]. Infliximab, adalimumab, etanercept, certolizumab pegol and golimumab have been successfully used in the treatment of PG; however, higher response rates favour adalimumab and infliximab. Response rates do not significantly vary by PG type or associated diseases, i.e. inflammatory bowel disease, haematological diseases, or other inflammatory disorders [69, 70]. To date, infliximab remains the only TNF-α inhibitor tested in a randomised, double-blind controlled trial [71] with a clinical benefit in 69% (20/29) of patients by week 6. In a phase III, open-label multi-centre study of 22 Japanese patients with refractory PG, adalimumab led to complete healing in 55% of participants by week 26 [72].
4.3.2 Interleukin (IL)-23 Inhibitors
In a semi-systematic review, ustekinumab proved effective in 71% of patients treated with this biologic [73]. In a case series, 68% (19/28) of patients treated with ustekinumab reported healing, including patients with all PG subtypes [74]. As IL-23 is implicated in the pathogenesis of PG and associated inflammatory comorbidities, newer IL-23 inhibitors targeting the p19 subunit are now being also used in the treatment of PG [75, 76].
4.3.3 Interleukin (IL)-1 Inhibitors
The IL-1 inhibitors used for the treatment of PG include anakinra (IL-1 receptor antagonist that blocks IL-1α and IL-1β) and canakinumab (IL-1β inhibitor). The latter being the only IL-1 inhibitor tested in a phase 2 open label trial [77] in which three out of five patients were completed healed by week 16. Adding reported cases of PG treated with canakinumab, 6 out of 11 patients reported complete healing and clinical improvement in 1 of 11 [73]. On the other hand, anakinra showed significant clinical improvement or complete remission in 10 of 13 patients [70]. These biologics have been particularly beneficial in patients with autosomal dominant autoinflammatory syndromes such as pyogenic arthritis, PG, acne (PAPA) spectrum disorder [78].
4.3.4 Interleukin (IL)-17 Inhibitors
Sparse reports have described successful PG treatment with interleukin IL-17 inhibitors, including secukinumab (anti-IL-17A) [79], brodalumab (anti-IL-17 receptor) [80] and ixekizumab (anti-IL-17A/F) [81]. An open-label phase II trial evaluated the efficacy and safety of secukinumab monotherapy in treating PG. Although three out of seven patients worsened, the others reported pain reduction, with two experiencing clinical improvement and reduction in inflammatory markers [82]. Brodalumab either weekly or biweekly led to clinical improvements in three patients [80]. On the other hand, IL-17 inhibition has induced PG [83, 84], most likely due to paradoxical IL-23 upregulation [85]. PG induction has also been documented upon switching between anti-IL-17 agents [86].
4.3.5 Complement Factor C5a Inhibitor
Vilobelimab (IFX-1) is a chimeric monoclonal antibody that inhibits neutrophil activation, chemotaxis, reduces inflammatory signalling and complement driven tissue damage in various diseases such as hidradenitis suppurativa [87, 88]. A phase IIa open-label study testing the efficacy and safety of IFX-1 in patients with PG (NCT03971643) is now finished. A total of 19 patients were enrolled in the study. Seven patients were in the dosing cohort at 2400 mg intravenous biweekly; six of these seven patients were healed by the end of the study (ClinicalTrials.gov).
4.4 Other Systemic Therapies
Of the currently available other systemic therapies, small molecules seem to be the next most promising therapeutic alternative for PG [89,90,91]. However, there are only a few case reports and further studies are required.
5 Adjunctive Management
5.1 Wound Management
The principles of modern moist wound therapy also apply to PG. However, due to the pronounced inflammation and painfulness, special consideration is required for certain aspects of wound care in PG. First, surgical debridement should not be performed during the inflammatory phase due to the pathergy phenomenon. Here, atraumatic alternatives such as maggot therapy or autolytic (e.g. hydrogel) or enzymatic (e.g. collagenase) may be considered [92]. Because the risk of wound infection is higher in patients receiving systemic immunosuppression, we believe that the use of antimicrobial dressings, for example polihexanide- or silver-containing wound products, is reasonable [93]. Non-adhesive and hyperabsorbent wound dressings are an important aspect of this strategy as adequate exudate management must be ensured [94].
When PG occurs on the lower legs, compression bandages should be applied to support anti-inflammatory activity by reducing oedema [95]. In our experience, low resting pressures around 20 mmHg are sufficient and better tolerated [96].
5.2 Pain Management
It has been demonstrated that a PG significantly limits patients’ quality of life, mental health and ability to work [97, 98]. Domains to specifically assess the impact of PG have been established, one of which is pain [99]. Pain remains an important aspect to take into consideration when addressing the severity of PG [100]. Several instruments have been used to assess quality of life including pain, though there none are specific to PG. Improvement in pain scores by at least two points in a numeric rating scale (0–10) might be an early sign of healing and seems to be an accurate patient-reported outcome to assess the disease impact and response to therapy [101].
6 Treatment Algorithm
From our experience, local pharmacologic therapy only can be attempted when there are single small (≤ 4 cm2) PG skin lesions with or without ulceration. The most common approach is to use a high potency steroid (e.g. clobetasol) or a calcineurin inhibitor (e.g. tacrolimus). In a comparative prospective study of these two alternatives, ulcer size was an independent predictor of healing [38]. When using topical therapy for PG, it is important to closely monitor progress with objective measurements and photography, in addition to establishing a strict timeline for re-assessment of therapy. The addition of systemic treatment should be considered if progress is lacking after 2–4 weeks of therapy. In addition, given the prolonged duration needed to control the inflammation, treating PG with local therapy can contribute to bridging or tapering the systemic immunosuppression and decrease the possibility of side effects. In general, topical therapy for PG is used as an adjuvant to systemic therapy and is usually applied to the ulcer itself and/or ulcer edges [41].
Systemic therapy should be considered when an ulcer is large (> 4 cm2) or if there are numerous or PG ulcers. CS and/or cyclosporine have been used for many years due to their rapid onset of action in stopping and/or decreasing the progression of skin ulcerations. However, the healing rate is less than 50% at 6 months and classical side effects associated with each type of medication determine which patient might benefit from one or the other. Sparing agents are often added to first-line immunosuppressive medications to maintain the inhibition of the immune system. Currently, the most commonly used sparing agents are biologics. Most the data, including one randomised control trial (RCT), favours the use of TNF-α inhibitors, particularly infliximab. However, growing evidence has shown that inhibitors of IL-1, IL-12/23, IL-17 and C5a are another alternative to be considered (Fig. 3).
Biologics can already be used as first choice drugs in patients with inflammatory comorbidities such as IBD and inflammatory arthritis. Here, approval exists and several disease aspects can be treated simultaneously with one therapeutic agent.
In paraneoplastic PG, there is stronger evidence that treatment of the underlying malignancy may be helpful in treating PG. This does not always preclude the initiation of systemic immunosuppression as initial treatment when it is first required. Nevertheless, immunomodulatory therapy with options such as dapsone or IVIG can be considered initially.
It is always difficult to determine when systemic therapy should be stopped. It is important to remember that systemic therapy is intended to control inflammation. If no more inflammation can be detected, then systemic therapy can be successively reduced and does not necessarily have to be continued until the wounds are completely healed. However, this decision must always be made on an individual basis.
7 Discussion
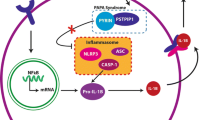
The clinical picture of PG was first described by Brocq over a hundred years ago [102]. It is still an enigmatic disease for healthcare providers and even more so for patients who often endure consultation with many physicians prior to finding one who might be familiar with the diagnosis and treatment of PG and able to provide much needed care and comfort. Sustaining progress in the management of PG relies on understanding its pathogenesis. It is still not known what stimulates neutrophil trafficking to cause skin ulcerations. It seems that abnormal activation of the innate immune system in patients with genetic predisposition provide an ideal state for PG to develop [103]. However, neutrophil-T cell crosstalk causing the activation of Th17 downstream cytokines is now supported by gene expression studies [6, 104].
Systemic therapies with CS and cyclosporine have remained the first-line options for most PG patients for several years. Despite the increased use of biologic therapy and the association of PG with inflammatory comorbidities, there is still a lack of strong evidence in support of their acceptance by regulatory agencies such as the Food and Drug Administration (FDA) and European Medicines Agency (EMA) [49]. If these patients do not have an associated comorbidity, the approval of biologics in clinical practice is even more challenging. The current evidence for the use of other, non-biologics favours MMF and dapsone (Table 3). These can be used as sparing agents, but their onset of action seems to be slower. However, the use of dapsone might confer Pneumocystis jirovecii prophylaxis (PJP) in patients treated with immunosuppressive for longer periods of time [105]. In addition, the appearance of PG ulcers on more sensitive areas (face and neck, genitalia) or the association with extracutaneous manifestations (e.g. lung involvement) might prompt the consideration of more aggressive initial treatment which could include pulses of CS or combination of CS with cyclosporine.
With all forms of therapy, it is very important to clarify in advance whether these treatments are at all feasible for patients who often have underlying illnesses. This involves clarifying side effects, but also the type of therapy to be carried out and whether the implicit costs will be covered by the insurer. Especially in the inflammatory phase, patients must be monitored closely. With control of the inflammation, the pain should decrease and the livid border around the wound should recede. If clinical symptoms progress despite 2–4 weeks of therapy, therapy should be extended or changed. Moreover, in the event of unsuccessful therapy, the PG diagnosis should also be critically questioned and, if necessary, re-examined. Misdiagnosis is still the main issue with PG [8]. Despite the proposal of new diagnostic frameworks, the impact on the misdiagnosis rate is yet to be seen.
As clinical guidelines are in development, healthcare providers rely on their knowledge of PG pathophysiology combined with their experience in wound care principles and training in analgaesic strategies to determine the most appropriate approach to treat these patients. Although more and more drugs are available for the medical treatment of PG, most are still considered off label and access is an issue for patients worldwide. When patient does not respond appropriately to immunosuppressive therapy, misdiagnosis is a consideration. However superimposed cellulitis or other concomitant ulcer aetiologies (e.g. chronic venous insufficiency) have to be addressed to assure treatment response. Conversely, high doses or levels of immunosuppressants can also negatively affect healing throughout the course of the disease.
In the future, we need to deepen our understanding of the pathophysiology of this disease, search for specific markers to aid in the enrolment of patients in clinical studies and possibly to distinguish PG from other ulcerative skin diseases, and ultimately develop targeted therapies that can feasible be studied in RCTs.
8 Conclusions
PG is a rare inflammatory dermatological disease that leads to very painful ulcerations and causes a significantly reduced quality of life. Once the diagnosis is confirmed with validated scores, numerous immunosuppressive and immunomodulatory therapies are now available. In particular, biologics will play an increasingly important role in the treatment regimens of patients with PG.
References
Xu A, Balgobind A, Strunk A, Garg A, Alloo A. Prevalence estimates for pyoderma gangrenosum in the United States: an age- and sex-adjusted population analysis. J Am Acad Dermatol. 2020;83(2):425–9.
Orfaly VE, Reese AM, Friedman M, Latour E, Ortega-Loayza AG. Pyoderma gangrenosum study pilot registry: the first step to a better understanding. Wound Repair Regen. 2022;30(3):334–7.
Langan SM, Groves RW, Card TR, Gulliford MC. Incidence, mortality, and disease associations of pyoderma gangrenosum in the United Kingdom: a retrospective cohort study. J Invest Dermatol. 2012;132(9):2166–70.
Binus AM, Qureshi AA, Li VW, Winterfield LS. Pyoderma gangrenosum: a retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol. 2011;165(6):1244–50.
Bennett ML, Jackson JM, Jorizzo JL, Fleischer AB Jr, White WL, Callen JP. Pyoderma gangrenosum. A comparison of typical and atypical forms with an emphasis on time to remission. Case review of 86 patients from 2 institutions. Medicine. 2000;79(1):37–46.
Maverakis E, Marzano AV, Le ST, Callen JP, Brüggen MC, Guenova E, Dissemond J, Shinkai K, Langan SM. Pyoderma gangrenosum. Nat Rev Dis Primers. 2020;6(1):81.
Ergun T. Pathergy phenomenon. Front Med. 2021;8: 639404.
Weenig RH, Davis MD, Dahl PR, Su WP. Skin ulcers misdiagnosed as pyoderma gangrenosum. N Engl J Med. 2002;347(18):1412–8.
Haag C, Hansen T, Hajar T, Latour E, Keller J, Shinkai K, Ortega-Loayza AG. Comparison of three diagnostic frameworks for pyoderma gangrenosum. J Invest Dermatol. 2021;141(1):59–63.
Min MS, Kus K, Wei N, Kassamali B, Faletsky A, Mostaghimi A, Lebwohl MG. Evaluating the role of histopathology in diagnosing pyoderma gangrenosum using Delphi and PARACELSUS criteria: a multicentre, retrospective cohort study. Br J Dermatol. 2022;186(6):1035–7.
Weedon D. The vasculopathic reaction pattern. In: Weedon D, editor. Weedon’s skin pathology. 3rd ed. London: Churchill Livingstone; 2010. p. 196–244.
Kridin K, Cohen AD, Amber KT. Underlying systemic diseases in pyoderma gangrenosum: a systematic review and meta-analysis. Am J Clin Dermatol. 2018;19(4):479–87.
Kridin K, Damiani G, Cohen AD. Rheumatoid arthritis and pyoderma gangrenosum: a population-based case-control study. Clin Rheumatol. 2021;40(2):521–8.
Sawka E, Zhou A, Latour E, Friedman M, Ortega-Loayza AG. Inflammatory arthritis-associated pyoderma gangrenosum: a systematic review. Clin Rheumatol. 2021;40(10):3963–9.
Montagnon CM, Fracica EA, Patel AA, Camilleri MJ, Murad MH, Dingli D, Wetter DA, Tolkachjov SN. Pyoderma gangrenosum in hematologic malignancies: a systematic review. J Am Acad Dermatol. 2020;82(6):1346–59.
Gupta AS, Ortega-Loayza AG. Pyoderma gangrenosum: a too often overlooked facultative paraneoplastic disease. Ann Hematol. 2019;98(9):2247–8.
Ravi M, Trinidad J, Spaccarelli N, Kaffenberger B. The impact of comorbidity identification on outcomes in patients with pyoderma gangrenosum: a retrospective cohort study of previously hospitalized patients. J Am Acad Dermatol. 2022;87(1):194–7.
Wu BC, Patel ED, Ortega-Loayza AG. Drug-induced pyoderma gangrenosum: a model to understand the pathogenesis of pyoderma gangrenosum. Br J Dermatol. 2017;177(1):72–83.
Wang JY, French LE, Shear NH, Amiri A, Alavi A. Drug-induced pyoderma gangrenosum: a review. Am J Clin Dermatol. 2018;19(1):67–77.
Barry M, AlRajhi A, Aljerian K. Pyoderma gangrenosum induced by BNT162b2 COVID-19 vaccine in a healthy adult. Vaccines. 2022;10(1):87.
Aggarwal P. Pyoderma gangrenosum adverse event with rituximab use: a postmarketing pharmacovigilance analysis. Dermatol Ther. 2020;33(2):13221.
Franceschi J, Darrigade AS, Sanchez-Pena P, Legrain-Lifermann V, Milpied B. Pyoderma gangrenosum after mRNA-based SARS-CoV-2 vaccine. J Eur Acad Dermatol Venereol. 2022;36(12):969–70.
Gambichler T, Boms S, Susok L, Dickel H, Finis C, Abu Rached N, Barras M, Stücker M, Kasakovski D. Cutaneous findings following COVID-19 vaccination: review of world literature and own experience. J Eur Acad Dermatol Venereol. 2022;36(2):172–80.
Ross HJ, Moy LA, Kaplan R, Figlin RA. Bullous pyoderma gangrenosum after granulocyte colony-stimulating factor treatment. Cancer. 1991;68(2):441–3.
Takagi S, Ohsaka A, Taguchi H, Kusama H, Matsuoka T. Pyoderma gangrenosum following cytosine arabinoside, aclarubicin and granulocyte colony-stimulating factor combination therapy in myelodysplastic syndrome. Intern Med. 1998;37(3):316–9.
Khoshnam-Rad N, Gheymati A, Jahangard-Rafsanjani Z. Tyrosine kinase inhibitors-associated pyoderma gangrenosum, a systematic review of published case reports. Anticancer Drugs. 2022;33(1):1–8.
Jeong HS, Layher H, Cao L, Vandergriff T, Dominguez AR. Pyoderma gangrenosum (PG) associated with levamisole-adulterated cocaine: clinical, serologic, and histopathologic findings in a cohort of patients. J Am Acad Dermatol. 2016;74(5):892–8.
Murphy MJ, Cohen JM, Vesely MD, Damsky W. Paradoxical eruptions to targeted therapies in dermatology: a systematic review and analysis. J Am Acad Dermatol. 2022;86(5):1080–91.
Garcovich S, De Simone C, Genovese G, Berti E, Cugno M, Marzano AV. Paradoxical skin reactions to biologics in patients with rheumatologic disorders. Front Pharmacol. 2019;10:282.
Camela E, Potestio L, Fabbrocini G, Megna M. Paradoxical reactions to biologicals for psoriasis. Expert Opin Biol Ther. 2022;22(12):1435–7.
Lytvyn Y, Mufti A, Maliyar K, Sachdeva M, Yeung J. Onset of pyoderma gangrenosum in patients on biologic therapies: a systematic review. Adv Skin Wound Care. 2022;35(8):454–60.
Sobieszczańska M, Tubek S, Kura I. Pyoderma gangrenosum-like skin changes after subcutaneous administration of low molecular weight heparin. Hum Vaccin Immunother. 2014;10(4):968–9.
Park CW, Shin YS, Shin MJ, Koh SH, Chang KU, Ahn YB, Chang YS, Bang BK. Pyoderma gangrenosum and spinal epidural abscess after subcutaneous administration of recombinant human erythropoietin. Nephrol Dial Transplant. 1997;12(7):1506–8.
Roy C, Adam JP, Morin F, Lemieux-Blanchard É, Doucet S, Friedmann D, Belisle A, Charpentier D. Azacitidine-induced pyoderma gangrenosum at injection sites in a patient with myelodysplastic syndrome. Curr Oncol. 2018;25(1):103–5.
Tseng E, Alhusayen R, Sade S, Buckstein R, Prica A. Pyoderma gangrenosum secondary to azacitidine in myelodysplastic syndrome. Br J Haematol. 2015;169(4):461.
Feldman SR, Lacy FA, Huang WW. The safety of treatments used in pyoderma gangrenosum. Expert Opin Drug Saf. 2018;17(1):55–61.
Lyon CC, Stapleton M, Smith AJ, Mendelsohn S, Beck MH, Griffiths CE. Topical tacrolimus in the management of peristomal pyoderma gangrenosum. J Dermatolog Treat. 2001;12(1):13–7.
Thomas KS, Ormerod AD, Craig FE, Greenlaw N, Norrie J, Mitchell E, Mason JM, Johnston GA, Wahie S, Williams HC. UK Dermatology Clinical Trials Network’s STOP GAP Team. Clinical outcomes and response of patients applying topical therapy for pyoderma gangrenosum: a prospective cohort study. J Am Acad Dermatol. 2016;75(5):940–9.
Goldstein F, Krain R, Thornton JJ. Intralesional steroid therapy of pyoderma gangrenosum. J Clin Gastroenterol. 1985;7(6):499–501.
Jennings JL. Pyoderma gangrenosum: successful treatment with intralesional steroids. J Am Acad Dermatol. 1983;9(4):575–80.
Baltazar D, Haag C, Gupta AS, Marzano AM, Ortega Loayza AG. A comprehensive review of local pharmacologic therapy for pyoderma gangrenosum. Wounds. 2019;31(6):151–7.
Moreira C, Lopes S, Cruz MJ, Azevedo F. Topical timolol for the treatment of pyoderma gangrenosum. BMJ Case Rep. 2017;2017:20162.
Alves de Medeiros AK, Speeckaert R, Desmet E, Van Gele M, De Schepper S, Lambert J. JAK3 as an emerging target for topical treatment of inflammatory skin diseases. PLoS ONE. 2016;11(10): 164080.
Barton VR, Le ST, Wang JZ, Toussi A, Sood A, Maverakis E. Peristomal ulcers misdiagnosed as pyoderma gangrenosum: a common error. J Eur Acad Dermatol Venereol. 2020;34(2):108–10.
Dissemond J, Assenheimer B, Gerber V, Hintner M, Puntigam MJ, Kolbig N, Koller S, Kurz P, Läuchli S, Probst S, Protz K, Steiniger A, Strohal R, Traber J, Kottner J. Moisture-associated skin damage (MASD): a best practice recommendation from Wund-D.A.CH. J Dtsch Dermatol Ges. 2021;19(6):815–25.
Chriba M, Skellett AM, Levell NJ. Beclometasone inhaler used to treat pyoderma gangrenosum. Clin Exp Dermatol. 2010;35(3):337–8.
Swift T. Peristomal skin complications: new materials needed to ease the ostomy care market. Br J Dermatol. 2023;188(4):455–6.
Pearson WA, Prentice DA, Sinclair DL, Lim LY, Carville KJ. A novel topical therapy for resistant and early peristomal pyoderma gangrenosum. Int Wound J. 2019;16(5):1136–43.
Maronese CA, Pimentel MA, Li MM, Genovese G, Ortega-Loayza AG, Marzano AV. Pyoderma gangrenosum: an updated literature review on established and emerging pharmacological treatments. Am J Clin Dermatol. 2022;23(5):615–34.
Herberger K, Dissemond J, Hohaus K, Schaller J, Anastasiadou Z, Augustin M. Treatment of pyoderma gangrenosum: retrospective multicentre analysis of 121 patients. Br J Dermatol. 2016;175(5):1070–2.
Afifi L, Ortega-Loayza AG, Shinkai K. Management of classic ulcerative pyoderma gangrenosum. Cutis. 2020;106(3):119–23.
Barshes NR, Goodpastor SE, Goss JA. Pharmacologic immunosuppression. Front Biosci. 2004;9:411–20.
Kolios AGA, Gübeli A, Meier B, Maul JT, Kündig T, Nilsson J, Hafner J, Guenova E, Kerl K, Anliker M, Kempf W, Navarini AA, French LE, Cozzio A. Clinical disease patterns in a regional swiss cohort of 34 pyoderma gangrenosum patients. Dermatology. 2017;233(4):268–76.
Vacas AS, Torre AC, Bollea-Garlatti ML, Warley F, Galimberti RL. Pyoderma gangrenosum: clinical characteristics, associated diseases, and responses to treatment in a retrospective cohort study of 31 patients. Int J Dermatol. 2017;56(4):386–91.
Wollina U. Clinical management of pyoderma gangrenosum. Am J Clin Dermatol. 2002;3(3):149–58.
Ehling A, Karrer S, Klebl F, Schäffler A, Müller-Ladner U. Therapeutic management of pyoderma gangrenosum. Arthritis Rheum. 2004;50(10):3076–84.
Mason JM, Thomas KS, Ormerod AD, Craig FE, Mitchell E, Norrie J, Williams HCUK. Dermatology Clinical Trials Network’s STOP GAP team. Ciclosporin compared with prednisolone therapy for patients with pyoderma gangrenosum: cost-effectiveness analysis of the STOP GAP trial. Br J Dermatol. 2017;177(6):1527–36.
Ormerod AD, Thomas KS, Craig FE, Mitchell E, Greenlaw N, Norrie J, Mason JM, Walton S, Johnston GA, Williams HC. UK Dermatology Clinical Trials Network’s STOP GAP Team. Comparison of the two most commonly used treatments for pyoderma gangrenosum: results of the STOP GAP randomised controlled trial. BMJ. 2015;350:2958.
Li J, Kelly R. Treatment of pyoderma gangrenosum with mycophenolate mofetil as a steroid-sparing agent. J Am Acad Dermatol. 2013;69(4):565–9.
Lee MR, Cooper AJ. Mycophenolate mofetil in pyoderma gangrenosum. J Dermatolog Treat. 2004;15(5):303–7.
Husein-ElAhmed H, Callejas-Rubio JL, Ríos Fernandez R, Ortego CN. Effectiveness of mycophenolic acid in refractory pyoderma gangrenosum. J Clin Rheumatol. 2010;16(7):346–7.
Young KZ, Guan LL, Friedman BJ, Rambhatla PV. Recalcitrant vulvar pyoderma gangrenosum successfully treated with mycophenolate mofetil. Int J Womens Dermatol. 2022;8(3):061.
Honigman A, Kelly RI. Pyoderma gangrenosum: a review of patient’s demographics, disease and treatment in 118 patients. Austr J Dermatol. 2022;63(2):267–9.
Din RS, Tsiaras WG, Li DG, Mostaghimi A. Efficacy of systemic dapsone treatment for pyoderma gangrenosum: a retrospective review. J Drugs Dermatol. 2018;17(10):1058–60.
Herberger K, Dissemond J, Brüggestrat S, Sorbe C, Augustin M. Biologics and immunoglobulins in the treatment of pyoderma gangrenosum - analysis of 52 patients. J Dtsch Dermatol Ges. 2019;17(1):32–41.
de Zwaan SE, Iland HJ, Damian DL. Treatment of refractory pyoderma gangrenosum with intravenous immunoglobulin. Australas J Dermatol. 2009;50(1):56–9.
Song H, Lahood N, Mostaghimi A. Intravenous immunoglobulin as adjunct therapy for refractory pyoderma gangrenosum: systematic review of cases and case series. Br J Dermatol. 2018;178(2):363–8.
Haag CK, Ortega-Loayza AG, Latour E, Keller JJ, Fett NM. Clinical factors influencing the response to intravenous immunoglobulin treatment in cases of treatment-resistant pyoderma gangrenosum. J Dermatolog Treat. 2020;31(7):723–6.
Ben Abdallah H, Fogh K, Bech R. Pyoderma gangrenosum and tumour necrosis factor alpha inhibitors: a semi-systematic review. Int Wound J. 2019;16(2):511–21.
McKenzie F, Cash D, Gupta A, Cummings LW, Ortega-Loayza AG. Biologic and small-molecule medications in the management of pyoderma gangrenosum. J Dermatolog Treat. 2019;30(3):264–76.
Brooklyn TN, Dunnill MG, Shetty A, Bowden JJ, Williams JD, Griffiths CE, Forbes A, Greenwood R, Probert CS. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55(4):505–9.
Yamasaki K, Yamanaka K, Zhao Y, Iwano S, Takei K, Suzuki K, Yamamoto T. Adalimumab in Japanese patients with active ulcers of pyoderma gangrenosum: final analysis of a 52-week phase 3 open-label study. J Dermatol. 2022;49(5):479–87.
Ben Abdallah H, Fogh K, Vestergaard C, Bech R. Pyoderma gangrenosum and interleukin inhibitors: a semi-systematic review. Dermatology. 2022;238(4):785–92.
Westerdahl JS, Nusbaum KB, Chung CG, Kaffenberger BH, Ortega-Loayza AG. Ustekinumab as adjuvant treatment for all pyoderma gangrenosum subtypes. J Dermatolog Treat. 2022;33(4):2386–90.
Burgdorf B, Schlott S, Ivanov IH, Dissemond J. Successful treatment of a refractory pyoderma gangrenosum with risankizumab. Int Wound J. 2020;17(4):1086–8.
Reese AM, Erickson K, Reed KB, Ortega-Loayza AG. Modified dose of guselkumab for treatment of pyoderma gangrenosum. JAAD Case Rep. 2022;21:38–42.
Kolios AG, Maul JT, Meier B, Kerl K, Traidl-Hoffmann C, Hertl M, Zillikens D, Röcken M, Ring J, Facchiano A, Mondino C, Yawalkar N, Contassot E, Navarini AA, French LE. Canakinumab in adults with steroid-refractory pyoderma gangrenosum. Br J Dermatol. 2015;173(5):1216–23.
Genovese G, Moltrasio C, Garcovich S, Marzano AV. PAPA spectrum disorders. G Ital Dermatol Venereol. 2020;155(5):542–50.
McPhie ML, Kirchhof MG. Pyoderma gangrenosum treated with secukinumab: a case report. SAGE Open Med Case Rep. 2020;8:430.
Tee MW, Avarbock AB, Ungar J, Frew JW. Rapid resolution of pyoderma gangrenosum with brodalumab therapy. JAAD Case Rep. 2020;6(11):1167–9.
Kao AS, King AD, Daveluy S. Successful treatment of cabozantinib-induced pyoderma gangrenosum with ixekizumab therapy: a case report. Dermatol Ther. 2022;35(9):15716.
Lauffer F, Seiringer P, Böhmer D, Oesterlin C, Eyerich K. Safety and efficacy of anti-IL-17 (secukinumab) for the treatment of pyoderma gangrenosum. J Investig Dermatol. 2021;141(10):156.
Wollina U, Schönlebe J, Fürll C. Pyoderma gangrenosum induced by secukinumab—A late paradoxical drug reaction. Dermatol Ther. 2020;33(1):13161.
Pollack IR, Wolner ZJ, Hammett J, Swerlick RA. Pyoderma gangrenosum in a patient on ixekizumab. JAAD Case Rep. 2021;16:152–4.
Petty AJ, Whitley MJ, Balaban A, Ellington K, Marano AL. Pyoderma gangrenosum induced by secukinumab in a patient with psoriasis successfully treated with ustekinumab. JAAD Case Rep. 2020;6(8):731–3.
Sadik CD, Thieme M, Zillikens D, Terheyden P. First emergence of pyoderma gangraenosum, palmoplantar pustulosis and sacroiliitis in a psoriasis patient associated with switching from secukinumab to brodalumab. J Eur Acad Dermatol Venereol. 2019;33(11):406–7.
Giamarellos-Bourboulis EJ, Argyropoulou M, Kanni T, Spyridopoulos T, Otto I, Zenker O, Guo R, Riedemann NC. Clinical efficacy of complement C5a inhibition by IFX-1 in hidradenitis suppurativa: an open-label single-arm trial in patients not eligible for adalimumab. Br J Dermatol. 2020;183(1):176–8.
Lu JD, Milakovic M, Ortega-Loayza AG, Marzano AV, Alavi A. Pyoderma gangrenosum: proposed pathogenesis and current use of biologics with an emphasis on complement C5a inhibitor IFX-1. Expert Opin Investig Drugs. 2020;29(11):1179–85.
Kochar B, Herfarth N, Mamie C, Navarini AA, Scharl M, Herfarth HH. Tofacitinib for the treatment of pyoderma gangrenosum. Clin Gastroenterol Hepatol. 2019;17(5):991–3.
Orfaly VE, Kovalenko I, Tolkachjov SN, Ortega-Loayza AG, Nunley JR. Tofacitinib for the treatment of refractory pyoderma gangrenosum. Clin Exp Dermatol. 2021;46(6):1082–5.
Bordeaux ZA, Kwatra SG, West CE. Treatment of pyoderma gangrenosum with apremilast monotherapy. JAAD Case Rep. 2022;30:8–10.
Strohal R, Dissemond J, Jordan O’Brien J, Piaggesi A, Rimdeika R, Young T, Apelqvist J. An updated overview and clarification of the principle role of debridement. J Wound Care. 2013;22:1–52.
Kramer A, Dissemond J, Kim S, Willy C, Mayer D, Papke R, Tuchmann F, Assadian O. Consensus on wound antisepsis: update 2018. Skin Pharmacol Physiol. 2018;31(1):28–58.
Strunck JL, Cutler B, Latour E, Seminario-Vidal L, Ortega-Loayza AG. Wound care dressings for pyoderma gangrenosum. J Am Acad Dermatol. 2022;86(2):458–60.
Beidler SK, Douillet CD, Berndt DF, Keagy BA, Rich PB, Marston WA. Inflammatory cytokine levels in chronic venous insufficiency ulcer tissue before and after compression therapy. J Vasc Surg. 2009;49(4):1013–20.
Partsch H, Stücker M, Vanscheidt W, Läuchli S, Eder S, Protz K, Dissemond J. Importance of adequate pressure in compression therapy: basis for successful treatment. Hautarzt. 2019;70(9):707–14.
Hobbs MM, Byler R, Latour E, Bonomo L, Hennessy K, Cruz-Diaz CN, Shinohara MM, Seminario-Vidal L, Shinkai K, Ortega-Loayza AG. Treatment of pyoderma gangrenosum: a multicenter survey-based study assessing satisfaction and quality of life. Dermatol Ther. 2021;34(2):14736.
McPhie ML, Fletcher J, Machado MO, Carvalho AF, Piguet V, Alavi A. A systematic review of depression and anxiety in adults with pyoderma gangrenosum. Adv Skin Wound Care. 2021;34(8):432–6.
Nusbaum KB, Ortega-Loayza AG, Kaffenberger BH. Health-related domains of quality of life in pyoderma gangrenosum: a qualitative analysis. J Am Acad Dermatol. 2022;86(6):1382–5.
Pimentel MA, Li MM, Noe MH, Latour E, Seminario-Vidal L, Greiling T, Shinkai K, Hamilton A, Alavi A, Bolognia JL, Cowen EW, Dominguez A, Fernandez AP, Fivenson D, Huang WW, Madigan LM, Mauskar M, Means AD, Nelson CA, Patsatsi A, Pugliese D, Rojek NW, Rosenbach M, Smith GP, Swerlick RA, Heffernan MP, Mostaghimi A, Ortega-Loayza AG. Features that define clinical severity of ulcerative pyoderma gangrenosum: a Delphi consensus study of experts and patients on behalf of the US Medical Dermatology Society. Br J Dermatol. 2023;188(4):566–8.
Erickson KM, Kody S and Ortega-Loayza AG. Pain as a patient reported outcome measure in pyoderma gangrenosum: defining clinical significance. JAMA Dermatol 2023; accepted for publication
Hobbs MM, Ortega-Loayza AG. Pyoderma gangrenosum: from historical perspectives to emerging investigations. Int Wound J. 2020;17(5):1255–65.
Moura RR, Brandão L, Moltrasio C, Agrelli A, Tricarico PM, Maronese CA, Crovella S, Marzano AV. Different molecular pathways are disrupted in Pyoderma gangrenosum patients and are associated with the severity of the disease. Sci Rep. 2023;13(1):4919.
Flora A, Kozera E, Frew JW. Pyoderma gangrenosum: a systematic review of the molecular characteristics of disease. Exp Dermatol. 2022;31(4):498–515.
Alkeraye S, AlZamil LR, Alenazi S. Dapsone in the management of pemphigus and pemphigoid: rediscovery of its long-lost efficacy. Cureus. 2020;12(6):8805.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding enabled and organized by Projekt DEAL. None.
Conflicts of interest
Dissemond received educational and research funding and/or contributed to advisory work for Infectopharm, InflaRx, Jansen Cilag and Novartis. Marzano received educational and research funding and/or contributed to advisory work for Abbvie, Boehringer-Ingelheim, Novartis, Pfizer, Sanofi and UCB. Hampton received educational and research funding and/or contributed to advisory work for Abbvie, Almiral, UCB, Jansen and Novartis. Ortega Loayza received educational and research funding and/or contributed to advisory work for Genentech, Guidepoint, Bristol Meyer Squibb, Boehringer Ingelheim, Janssen, Lilly, Janssen and Pfizer.
Ethics approval
Not applicable
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Author contributions
JD: Planning, writing several sections, revision of the whole manuscript. AVN: Writing one section, revision of the whole manuscript. PJH: Writing one section, revision of the whole manuscript. AGOL: Planning, writing several sections, revision of the whole manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Dissemond, J., Marzano, A.V., Hampton, P.J. et al. Pyoderma Gangrenosum: Treatment Options. Drugs 83, 1255–1267 (2023). https://doi.org/10.1007/s40265-023-01931-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-023-01931-3