Abstract
Axial symptoms (i.e., back pain) are common in the general population. At the same time 25–70% of patients with psoriatic arthritis (PsA) exhibit signs of inflammatory axial involvement (axial PsA). The presence of unexplained chronic (duration ≥ 3 months) back pain in a patient with psoriasis or PsA should trigger evaluation of the presence of axial involvement. Evaluation of axial involvement normally involves imaging of the axial skeleton (sacroiliac joints and/or spine) in addition to clinical and laboratory evaluation. Symptomatic patients with confirmed axial PsA are treated with a combination of non-pharmacologic and pharmacologic methods including the use of non-steroidal anti-inflammatory drugs, tumour necrosis factor, interleukin 17, and Janus kinase inhibitors. Interleukin 23 blockade might also be effective in the axial domain of PsA; a dedicated clinical study is ongoing at present. Safety considerations, patient preference, as well as the presence of other disease manifestations (especially of extra-musculoskeletal manifestations—clinically relevant psoriasis, acute anterior uveitis, inflammatory bowel disease), define the choice of a specific drug or drug class.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Axial symptoms (first of all, chronic back pain) in patients with psoriasis/psoriatic arthritis, especially if started at young age should raise a suspicion of the presence of axial involvement. |
The diagnostic evaluation of axial symptoms should normally include imaging for the detection of active inflammatory and structural changes indicative of axial involvement. |
Symptomatic patients with confirmed axial involvement should be treated with a combination of non-pharmacologic and pharmacologic methods including the use of non-steroidal anti-inflammatory drugs, tumour necrosis factor, interleukin 17, and Janus kinase inhibitors. |
1 Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory musculoskeletal disease that might manifest with peripheral arthritis, enthesitis, and dactylitis, as well as with axial involvement including inflammatory affection of sacroiliac joints and/or spine.
Involvement of the axial skeleton is considered a relatively frequent manifestation of PsA, most often along with peripheral manifestations (peripheral arthritis, enthesitis, dactylitis). Positivity for the human leucocyte antigen B27 (HLA-B27), structural damage in the peripheral joints (meaning severe peripheral arthritis), elevated acute phase reactants, nail involvement and periostitis are known factors associated with axial disease in PsA [1]. The presence of axial disease is associated with worse clinical outcomes in PsA compared with patients without axial involvement, with higher activity of PsA, poorer functional status and quality of life [2].
Depending upon the definition used and on the duration of the underlying psoriasis/PsA, the prevalence of axial involvement varies from 25 to 70% of patients with PsA [3,4,5,6,7]. As of today, there are currently no widely accepted criteria for axial involvement in PsA. A joint, currently running project of the Assessment of SpondyloArthritis international Society (ASAS) and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) named Axial Involvement in Psoriatic Arthritis (AXIS) seeks to systematically evaluate clinical and imaging manifestations indicative of axial involvement in patients with PsA and to develop classification criteria and unified nomenclature for axial involvement in PsA [8]. Despite the lack of criteria, definitions, and even unified terminology (axial PsA is in common use, but other terms can be used such as PsA with axial involvement, psoriatic spondyloarthritis, and psoriatic spondylitis), international guidelines for the management of PsA give specific guidance on the management of patients with axial disease with most of the evidence coming from the primary axial spondyloarthritis (axSpA).
This review will discuss practical aspects of the management of patients with PsA (and/or psoriasis) presenting with axial symptoms.
2 Is There a Difference Between Axial Symptoms and Axial Involvement in PsA?
The term ‘axial symptoms’ is not well defined and can include heterogeneous manifestations related to the axial skeleton (sacroiliac joints and spine). Probably the most common axial symptom is back pain or spinal pain that might involve cervical, thoracic, and lumbar spine areas as well as buttock pain. Of note, the term "hip pain" is used interchangeably with lumbar or low back pain in some settings. Morning stiffness in the spine is another common axial symptom that is characterised by reversible limitations of spinal mobility or a feeling of being stiff in the spine, which is especially present in the early morning hours upon getting up—but can improve with exercise. In contrast to morning stiffness, permanent spinal stiffness or limitations of spinal mobility are limitations related to irreversible structural changes and do not improve substantially with exercise. In addition to the “spinal” symptoms, symptoms associated with the involvement of the anterior chest wall (enthesitis, costo-sternal inflammation, inflammation of sternoclavicular joints) are often counted as axial manifestations of PsA.
In the context of inflammatory disease, spinal and buttock pain as well as morning stiffness are considered to be related to the presence of active inflammation in the spine (spondylitis, arthritis of facet, costovertebral or costo-transversal joints, enthesitis) and/or in the sacroiliac joints. In contrast, limitations of spinal mobility are usually considered to be a sign of structural damage—new bone formation/ankylosis in the spine as a result of the preceding inflammation. However, back pain (spinal pain) of non-inflammatory origin (so-called non-specific back pain, back pain related to mechanical/degenerative changes in the spine) is extremely common in the general population. A recent population-based study in Germany showed that 22.5% of examined subjects across all age strata reported the experience of chronic (duration of 3 months and longer) back pain [9], while a lifetime prevalence of any back pain is substantially higher. Degenerative spine disease might also be associated with structural damage resulting in functional impairment and spinal mobility limitations.
Thus, it is obvious that axial symptoms in patients with psoriasis/PsA do not necessarily mean axial involvement as a manifestation of the psoriatic disease (a true axial PsA). But the presence of axial symptoms in patients with psoriasis/psoriatic arthritis should be a trigger for the evaluation of potential axial involvement.
3 Does the Presence of Inflammatory Back Pain Mean the Presence of Inflammatory Involvement of the Axial Skeleton?
The short answer is no. Inflammatory back pain can be a manifestation of a non-inflammatory spinal disease (such as degenerative disk disease, osteitis condensans ilii, etc. [10]), so the term is misleading since it suggests the presence of inflammation, that is not always the case. A study in primary axSpA showed good sensitivity (up to 80%) but a poor specificity (below 50%) of inflammatory back pain for the diagnosis of this condition [11]. In axial PsA, one can expect a similar situation, maybe with even lower sensitivity of inflammatory back pain as shown in several studies [12].
Inflammatory back pain is in fact a syndrome including the following symptoms:
-
Slow onset within several days
-
Improvement of back pain with movement/exercise
-
No improvement with rest
-
Back pain in the night especially in the second part of the night
-
Alternating buttock pain.
Inflammatory back pain is usually chronic back pain lasting for more than 3 months and as a manifestation of axSpA it usually starts before the age of 45 years; however, that does not necessarily apply to PsA due to a generally older age of symptom onset.
There are several sets of inflammatory back criteria (Calin, Berlin, ASAS experts), which were developed for classification purposes (i.e., for clinical studies) and should not be applied in the diagnostic approach.
In daily clinical practice, inflammatory back pain could be considered as one of the potential triggers of evaluation for the presence of axial PsA, but as already mentioned above, that is true for any chronic back pain (or general axial symptoms that last more than 3 months) in patients with psoriasis/PsA.
4 Can Patients with a High Probability of Axial PsA be Identified Among Patients with Psoriasis?
Several screening tools/questionnaires for PsA in general (including the Psoriatic Arthritis Screening Evaluation [PASE] [13], the Toronto Psoriatic Arthritis Screen [ToPAS] [14], the Psoriasis Epidemiology Screening Tool [PEST] [15], and the Early Psoriatic Arthritis Screening Questionnaire [EARP] [16]) have been developed and validated in the past decades—all relying mostly on symptoms reported by a patient. In a recent study, a screening strategy focussing on axial manifestations has been tested [17]. Adult patients with psoriasis who had chronic back pain (≥ 3 months), onset < 45 years, and had not been treated with any biologic or targeted synthetic disease-modifying anti-rheumatic drug in the 12 weeks before screening, were referred to a specialised rheumatology clinic. A rheumatologic investigation that included clinical and laboratory assessments as well as imaging with conventional radiography and magnetic resonance imaging (MRI) of the sacroiliac joints and spine was performed. Of 100 evaluated patients, 14 patients (including 3 with both axial and peripheral involvement) were diagnosed with axial PsA and 5 were diagnosed with peripheral PsA solely [17]. This study indicated that checking the presence of axial symptoms (especially chronic back pain) in addition to peripheral manifestations in patients with psoriasis is an important step to select patients, who will undergo further examination to detect axial involvement.
5 How Can the Presence of Axial Involvement in Patients with Psoriasis/PsA be Confirmed or Ruled Out?
As mentioned above, the presence of axial symptoms should normally trigger an evaluation for the presence of axial involvement in patients with psoriasis/PsA if the symptoms are not clearly explained otherwise.
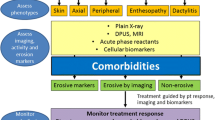
As of today, there is no single clinical or laboratory test that could help to differentiate inflammatory from non-inflammatory causes of back pain. For instance, inflammatory back pain is neither sensitive nor specific in the discussed population, elevated acute phase reactants could be related to peripheral involvement, and even HLA-B27 has a substantially lower diagnostic value in axial PsA (with lower sensitivity and specificity) as compared to primary axSpA [12]. Therefore, imaging of the axial skeleton (with radiography and MRI are the most commonly applied imaging methods) remains the key element of the diagnostic approach in patients with suspected axial PsA. Nonetheless, the final conclusion on the presence or absence of axial involvement is usually made based on a careful evaluation of clinical, laboratory, and imaging parameters (Fig. 1).
The proposed management algorithm for patients with psoriatic arthritis/psoriasis presenting with axial symptoms. bDMARD biologic disease-modifying anti-rheumatic drug, IL-17i interleukin 17 inhibitor, JAKi Janus kinase inhibitor, MRI magnetic resonance imaging, NSAIDs non-steroidal anti-inflammatory drugs, PsA psoriatic arthritis, TNFi tumour necrosis factor inhibitor, tsDMARD targeted synthetic disease-modifying anti-rheumatic drug
Plain radiography of sacroiliac joints and spine can detect structural post-inflammatory changes, which exhibit similarities but also differences as compared to findings observed in primary axial SpA. Radiographic sacroiliitis is described as a common feature of axial PsA, occurring in 25–50% of patients with PsA, and is more frequently (as compared to axial spondyloarthritis) asymmetrical (in up to 70% of patients) [18,19,20,21]. Also in the spine, both axSpA-typical (i.e., marginal syndesmophytes arising from the bone next to the annulus fibrosis detachment) and rather atypical (e.g., asymmetrical coarse thorn-like non-marginal syndesmophytes, paravertebral ossification) structural changes might occur. Structural changes in the spine related to psoriasis/PsA seem to have more asymmetry, might occur in any part of the spine, and could be present even without affection of sacroiliac joints, which is unusual in primary axSpA (Table 1) [12, 22, 23]. Of note, differentiation between inflammation-related structural changes (as a manifestation of axial PsA) and degenerative, non-inflammatory changes (osteophytes, ossifications as a manifestation of diffuse idiopathic skeletal hyperostosis [DISH]) in the spine might be challenging, especially in older patients and in the absence of sacroiliac joint affections. The morphology of radiographic changes does not always allow for a clear-cut differentiation; in such cases, cross-sectional imaging (MRI, CT) might be helpful.
In general, in the presence of HLA-B27 (up to 50% of axial PsA patients) the radiographic phenotype resembles that of axSpA, while in HLA-B27-negative axSpA patients (in which other HLA loci, such as HLA-B08—found to be associated with less severe radiographic sacroiliitis and asymmetry [24, 25])—might play a role. Importantly, radiography is a method of detection of structural damage and is not able to detect active inflammatory changes that are especially relevant for early diagnosis. Furthermore, a recent study showed that radiography of sacroiliac joints is neither sensitive nor specific for the diagnosis of axSpA and is clearly inferior in terms of the diagnostic value as compared to MRI or computed tomography (CT) [26] that is also likely to be true for axial PsA. In the spine, the differentiation between post-inflammatory (syndesmophytes) and degenerative (osteophytes) structural changes might be challenging and often demands cross-sectional imaging such as MRI.
Magnetic resonance imaging can detect both active inflammatory and structural changes associated with axial PsA in sacroiliac joints and spine [27, 28]. It is assumed that MRI changes occurring as a manifestation of axial PsA are similar to those observed in primary axSpA (e.g., bone marrow oedema in the sacroiliac joints [sacroiliitis] and spine [spondylitis, inflammation of facet, costovertebral, and costotransverse joints] as well as enthesitis as signs of active inflammation, erosions, fat lesions, sclerosis and ankylosis as post-inflammatory structural changes [29, 30]), although axial PsA patients might exhibit some particular features in terms of localisation and symmetry as discussed above (Table 1). As of today, we have less data on MRI manifestations of axial PsA as compared to X-rays discussed above. Analysis of MRIs from the already mentioned referral study [17] showed a high frequency (in about one-third of the patients) of isolated spinal involvement in patients diagnosed with axial PsA. It is important to mention that active inflammatory changes in the sacroiliac joints and spine could be a result of mechanical stress/degenerative changes and are present frequently in subjects with no inflammatory condition [31]. Therefore, the presence of degenerative changes (i.e., degenerated disk in the spine, capsule ossification, subchondral sclerosis in the anterior portion of the joint in the sacroiliac joints), decreases the diagnostic value of active inflammatory changes (i.e., bone marrow oedema) in the axial skeleton. At the same time, the presence of typical post-inflammatory changes such as erosions in the sacroiliac joints would increase the probability of the inflammatory origin of bone marrow oedema.
In the field of axial SpA, there is an ongoing debate regarding the use of the term "non-radiographic axial SpA" to describe patients with axial SpA who do not show any visible structural changes in their sacroiliac joints on radiography. The term was initially developed when it became evident that clinicians could diagnose axial SpA before the onset of radiographic sacroiliitis (which had been the most critical imaging feature of AS in the past) through the detection of active inflammatory changes in the sacroiliac joints using MRI in patients with corresponding clinical presentation. However, this terminology is not relevant in everyday clinical practice and should be used exclusively for research classification purposes.
Although active inflammatory changes in the axial skeleton can be detected in PsA before the development of structural changes visible on radiographs, there is currently no need to apply similar classification terminology (non-radiographic/radiographic) to axial PsA. This is due to the different developmental path of axial PsA (which is currently being defined as a whole) and the specific imaging features unique to axial PsA.
In general, CT of sacroiliac joints is less sensitive but quite specific for the detection of inflammatory affection of the axial skeleton, especially of sacroiliac joints [26], since this method is not able to detect active inflammation but depicts structural post-inflammatory changes. Computed tomography is usually not recommended routinely but can be applied in situations when MRI (with or without radiography) does not provide conclusive results on the presence of structural damage (especially erosion in the sacroiliac joints).
Thus, diagnosis and differential diagnosis of axial PsA relies on a careful interpretation of imaging findings in the clinical context. Objective confirmation of inflammatory affection of the axial skeleton increases the diagnostic confidence in patients with suspected axial involvement as a manifestation of psoriatic disease.
6 What is the Optimal Treatment Approach in Patients with Confirmed Axial PsA?
There are three major international guidelines addressing the treatment of patients with PsA: the 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis [32], the 2019 European Alliance of Associations for Rheumatology (EULAR) recommendations for the pharmacological treatment of PsA [33], and the most recent 2021 Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) updated treatment recommendations for PsA [34]. In the following we will be largely referring to the latter recommendation set that aggregated the most recent evidence in the field; the evidence in axial PsA has also been summarised in a separately published manuscript [35].
It is important to mention that there are very few interventional studies addressing patients with axial PsA; therefore, the evidence and guidelines in primary axSpA [36] also play a role in axial PsA.
6.1 Treatment Goal and Outcome Measures
According to the treat-to-target recommendations for SpA, the major treatment goal in all SpA patients (including PsA and irrespectively of axial involvement), is the achievement of remission, defined as the absence of clinical and laboratory signs of inflammatory activity [37]. For PsA, two validated instruments (Disease Activity Index for Psoriatic Arthritis [DAPSA] and Minimal Disease Activity [MDA]) are recommended in this context. For axial PsA, no specific instruments have been developed. It is reasonable, however, to evaluate leading manifestations reflecting axial symptoms—spinal pain and morning stiffness, e.g., on a 0–10 numeric rating scale or visual analogue scale. These measures are also incorporated in two instruments widely used in primary axSpA—the Ankylosing Spondylitis Disease Activity Score (ASDAS) and the Bath Ankylosing Spondylitis Activity Disease Activity Index (BASDAI).
In general, most cases and peripheral manifestations coexist in patients with PsA, although the relative importance of a manifestation based on the intensity of symptoms in the respective domain might be different. In any case, the presence of axial manifestations could have an impact on the choice of a specific treatment method or a drug class as discussed below.
6.2 Non-pharmacological Treatment
In patients with PsA and axial involvement, regular exercises/active physiotherapy seem to play a similarly important role in the management approach as in patients with primary axial SpA [38], although there are no studies specifically addressing this patient population. Active supervised or unsupervised physiotherapy/exercises aim to improve and preserve the function and spinal mobility in this patient group. Other non-pharmacological treatment modalities (i.e., education, smoking cessation, etc.) are not specific for axial involvement.
6.3 Pharmacological Treatment
Nonsteroidal anti-inflammatory drugs (NSAIDs) are usually considered the first-line pharmacologic treatment in patients presenting with axial involvement. These drugs are usually recommended “on-demand” meaning that the duration of intake and the dose can be adjusted depending on the symptom intensity and taking tolerability and side effects as well as the maximal recommended dose into account. Nonsteroidal anti-inflammatory drugs can usually be combined with other drug classes, which might be applied due to the presence of other psoriatic manifestations.
So-called conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs), such as methotrexate, are not effective in axSpA and most likely not effective in axial disease in PsA. Also, systemic steroids are not recommended in patients with axial PsA, especially a long-term treatment, while a short-term treatment (up to 2 weeks) can be used as a bridging therapy or in a case of a flare.
Biological (b-) and targeted synthetic (ts-) DMARDs are usually the next treatment step for patients with axial PsA not responding to the first-line treatment with NSAIDs. While it is postulated that there is no major difference in efficacy of currently available b- and tsDMARDs against peripheral manifestations, there might be some relevant differences in the axial domain that could affect the choice of a drug class in patients with active axial disease.
As of today, there is no evidence for the efficacy of the CTLA4-immunoglobulin fusion protein abatacept and of the phosphodiesterase-4 inhibitor apremilast with negative studies in axSpA [39, 40] and no positive data in axial PsA.
In contrast, tumor necrosis factor (TNF) inhibitors (adalimumab, certolizumab pegol, etanercept, infliximab, golimumab) are highly effective in axSpA [41] and a similar effect is assumed also in axial PsA, although there are no studies specifically addressing the latter patient population. Similarly, interleukin-17 (IL-17) inhibitors (bimekizumab—an IL-17A and F inhibitor, ixekizumab and secukinumab—both IL-17A inhibitors) showed efficacy in Phase III trials in axSpA [41, 42] and are also considered to be effective in axial PsA. However, secukinumab is, as of today, the only bDMARD, which has been investigated on the target patient population with axial PsA. In the MAXIMISE study, patients with axial PsA (that was defined based on the clinical judgement of the investigator and the BASDAI score of > 4) who had an inadequate response to NSAIDs, were randomized to receive secukinumab 300 mg, 150 mg or placebo for 12 weeks. At Week 12, 63.1% and 66.3% of patients who received secukinumab 300 mg and 150 mg, respectively, achieved the primary endpoint—ASAS20 responses compared with 31.3% on placebo [43]. In this study, no objective confirmation of axial disease was required at baseline, but approximately 60% of the patients had active inflammatory lesions in the MRIs defined by bone marrow oedema for the sacroiliac joints and spine. Clinical improvement was accompanied by the reduction of active inflammation on MRI [43].
The story is more complicated when talking about bDMARDs targeting the IL-12/23 pathway. In primary axSpA, this drug class (represented by ustekinumab—an IL-12/23 inhibitor, and Risankizumab—an IL-23 inhibitor) failed to demonstrate clinical efficacy [44, 45]. These results were especially surprising in light of positive results of IL-17 blockade, which is considered as a downstream cytokine of IL-23 (Th17 pathway) and evidence of the importance of IL-23 for the development of entheseal inflammation [46]—the postulated leading pathology in all SpA. This discrepancy might be explained by the fact that many cell types (involved in both innate and adaptive immunity) can produce IL-17 and not all of them require IL-23 as a stimulus [47]. So, in the skin, IL-17 production seems to be largely IL-23–related, which explains similar clinical results of IL-17 and IL-23 blockade in psoriasis, while in the spine of axSpA patients, IL-23–independent mechanisms of IL-17 (and to a further extent of TNF) production might prevail.
In PsA, however, several studies have suggested that IL-23 inhibitors may be effective for the treatment of axial symptoms. A post hoc analysis of the pooled PSUMMIT-1 and PSUMMIT-2 studies demonstrated that patients with PsA and physician-reported axial involvement (originally worded as “spondylitis”) who received ustekinumab had larger improvements in axial symptoms including neck/pain/hip pain than those receiving placebo [48]. In an exploratory post hoc analysis of the Phase III DISCOVER-1 and DISCOVER-2 trials, patients with PsA with imaging-confirmed sacroiliitis (according to the local clinician’s judgement on radiography and/or MRI) who received the IL-23 inhibitor guselkumab showed larger improvements in BASDAI, ASDAS and spinal pain as compared with placebo [49]. Importantly, the presence of sacroiliitis on imaging in this study was not confirmed by central evaluation, there was no follow-up imaging, and patients were included in this study based on the presence of active peripheral arthritis that improved under treatment with guselkumab. Therefore, it is difficult to judge how much of the improvement in the “axial” outcome parameters was related to the improvement of peripheral PsA manifestations and of psoriasis. Currently, a prospective controlled study with guselkumab (STAR) focusing axial involvement in PsA (confirmed on MRI by central reading) is in progress [50], to resolve the existing uncertainty with regard to the efficacy of IL-23 blockade in axial PsA.
Janus kinase (JAK) inhibitors, tofacitinib and upadacitinib, showed efficacy in Phase III studies in axSpA [51,52,53]; therefore, their efficacy is assumed in axial PsA, although confirmation in dedicated clinical trials would be highly desired. One ongoing study (PASTOR) evaluates the efficacy of tofacitinib in reducing inflammation on MRI as well as signs and symptoms in patients with axial PsA [54]. There is an ongoing discussion on the place of JAK inhibitors in the treatment algorithms of patients with inflammatory diseases related to the safety concerns (cardiovascular and malignancy risks) raised by the results of the ORAL Surveillance study with tofacitinib in rheumatoid arthritis [55, 56]. Although no comparable data have been generated in axSpA and PsA, it is likely that cardiovascular and malignancy risk factors will require a careful evaluation in all patients receiving JAK inhibitors independently of indication. The efficacy of different pharmacological treatment options in the axial domain of PsA is summarised in Table 2.
In addition to the efficacy data in the musculoskeletal domain and safety considerations, the presence of extra-musculoskeletal manifestations might affect the choice of a particular drug class in patients with axial PsA. For instance, in patients with relevant psoriatic skin involvement, IL-17 inhibitors would be more effective than TNF inhibitors, while in patients with inflammatory bowel disease or acute anterior uveitis, monoclonal antibodies against TNF should normally be preferred. Figure 1 summarises the proposed clinical approach in patients with PsA and suspected axial involvement.
7 Conclusion
The presence of unexplained chronic (duration ≥3 months) back pain in a patient with psoriasis or PsA should normally trigger evaluation of the presence of axial involvement (axial PsA), especially if back pain started before the age of 45 years. Evaluation of axial involvement normally involves imaging of the axial skeleton (sacroiliac joints and/or spine). Symptomatic patients with confirmed axial PsA are treated with a combination of non-pharmacological and pharmacological treatment modalities including use of NSAIDs, TNF, IL-17, and JAK inhibitors. Safety considerations, patient preference, as well as the presence of other disease manifestations, define the choice of a specific drug or drug class.
References
Chandran V, Tolusso DC, Cook RJ, Gladman DD. Risk factors for axial inflammatory arthritis in patients with psoriatic arthritis. J Rheumatol. 2010;37(4):809–15.
Mease PJ, Palmer JB, Liu M, Kavanaugh A, Pandurengan R, Ritchlin CT, et al. Influence of axial involvement on clinical characteristics of psoriatic arthritis: analysis from the corrona psoriatic arthritis/Spondyloarthritis Registry. J Rheumatol. 2018;45(10):1389–96.
Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic arthritis (PSA)—an analysis of 220 patients. Q J Med. 1987;62(238):127–41.
Gladman DD. Axial disease in psoriatic arthritis. Curr Rheumatol Rep. 2007;9(6):455–60.
Chandran V. Psoriatic spondylitis or ankylosing spondylitis with psoriasis: same or different? Curr Opin Rheumatol. 2019;31(4):329–34.
Jadon DR, Sengupta R, Nightingale A, Lindsay M, Korendowych E, Robinson G, et al. Axial disease in psoriatic arthritis study: defining the clinical and radiographic phenotype of psoriatic spondyloarthritis. Ann Rheum Dis. 2017;76(4):701–7.
Feld J, Ye JY, Chandran V, Inman RD, Haroon N, Cook R, et al. Axial disease in psoriatic arthritis: the presence and progression of unilateral grade 2 sacroiliitis in a psoriatic arthritis cohort. Semin Arthritis Rheum. 2021;51(2):464–8.
Poddubnyy D, Baraliakos X, Van den Bosch F, Braun J, Coates LC, Chandran V, et al. Axial Involvement in Psoriatic Arthritis cohort (AXIS): the protocol of a joint project of the Assessment of SpondyloArthritis international Society (ASAS) and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA). Ther Adv Musculoskelet Dis. 2021;13:1759720X211057975.
Schmidt CO, Gunther KP, Goronzy J, Albrecht K, Chenot JF, Callhoff J, et al. Frequencies of musculoskeletal symptoms and disorders in the population-based German National Cohort (GNC). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2020;63(4):415–25.
Poddubnyy D, Weineck H, Diekhoff T, Redeker I, Gobejishvili N, Llop M, et al. Clinical and imaging characteristics of osteitis condensans ilii as compared with axial spondyloarthritis. Rheumatology (Oxford). 2020;59(12):3798–806.
Poddubnyy D, Callhoff J, Spiller I, Listing J, Braun J, Sieper J, et al. Diagnostic accuracy of inflammatory back pain for axial spondyloarthritis in rheumatological care. RMD Open. 2018;4(2): e000825.
Poddubnyy D, Jadon DR, Van den Bosch F, Mease PJ, Gladman DD. Axial involvement in psoriatic arthritis: An update for rheumatologists. Semin Arthritis Rheum. 2021;51(4):880–7.
Dominguez P, Husni ME, Garg A, Qureshi AA. Psoriatic Arthritis Screening and Evaluation (PASE) questionnaire and the role of dermatologists: a report from the GRAPPA 2009 annual meeting. J Rheumatol. 2011;38(3):548–50.
Gladman DD, Schentag CT, Tom BD, Chandran V, Brockbank J, Rosen C, et al. Development and initial validation of a screening questionnaire for psoriatic arthritis: the Toronto Psoriatic Arthritis Screen (ToPAS). Ann Rheum Dis. 2009;68(4):497–501.
Ibrahim GH, Buch MH, Lawson C, Waxman R, Helliwell PS. Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: the Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin Exp Rheumatol. 2009;27(3):469–74.
Tinazzi I, Adami S, Zanolin EM, Caimmi C, Confente S, Girolomoni G, et al. The early psoriatic arthritis screening questionnaire: a simple and fast method for the identification of arthritis in patients with psoriasis. Rheumatology (Oxford). 2012;51(11):2058–63.
Proft F, Luders S, Hunter T, Luna G, Rios Rodriguez V, Protopopov M, et al. Early identification of axial psoriatic arthritis among patients with psoriasis: a prospective multicentre study. Ann Rheum Dis. 2022;81(11):1534–40.
Gladman DD. Clinical, radiological, and functional assessment in psoriatic arthritis: is it different from other inflammatory joint diseases? Ann Rheum Dis. 2006;65(Suppl 3):iii22–4.
Haroon M, Winchester R, Giles JT, Heffernan E, FitzGerald O. Clinical and genetic associations of radiographic sacroiliitis and its different patterns in psoriatic arthritis. Clin Exp Rheumatol. 2017;35(2):270–6.
Williamson L, Dockerty JL, Dalbeth N, McNally E, Ostlere S, Wordsworth BP. Clinical assessment of sacroiliitis and HLA-B27 are poor predictors of sacroiliitis diagnosed by magnetic resonance imaging in psoriatic arthritis. Rheumatology (Oxford). 2004;43(1):85–8.
Kishimoto M, Deshpande GA, Fukuoka K, Kawakami T, Ikegaya N, Kawashima S, et al. Clinical features of psoriatic arthritis. Best Pract Res Clin Rheumatol. 2021;17: 101670.
Feld J, Chandran V, Haroon N, Inman R, Gladman D. Axial disease in psoriatic arthritis and ankylosing spondylitis: a critical comparison. Nat Rev Rheumatol. 2018;14(6):363–71.
Helliwell PS. Axial disease in psoriatic arthritis. Rheumatology (Oxford). 2020;59(6):1193–5.
Eder L, Chandran V, Gladman DD. What have we learned about genetic susceptibility in psoriasis and psoriatic arthritis? Curr Opin Rheumatol. 2015;27(1):91–8.
Chandran V, Barrett J, Schentag CT, Farewell VT, Gladman DD. Axial psoriatic arthritis: update on a longterm prospective study. J Rheumatol. 2009;36(12):2744–50.
Diekhoff T, Eshed I, Radny F, Ziegeler K, Proft F, Greese J, et al. Choose wisely: imaging for diagnosis of axial spondyloarthritis. Ann Rheum Dis. 2022;81(2):237–42.
Felbo SK, Terslev L, Østergaard M. Imaging in peripheral and axial psoriatic arthritis: contributions to diagnosis, follow-up, prognosis and knowledge of pathogenesis. Clin Exp Rheumatol. 2018;36 Suppl 114(5):24–34.
Castillo-Gallego C, Aydin SZ, Emery P, McGonagle DG, Marzo-Ortega H. Magnetic resonance imaging assessment of axial psoriatic arthritis: extent of disease relates to HLA-B27. Arthritis Rheum. 2013;65(9):2274–8.
Maksymowych WP, Lambert RG, Ostergaard M, Pedersen SJ, Machado PM, Weber U, et al. MRI lesions in the sacroiliac joints of patients with spondyloarthritis: an update of definitions and validation by the ASAS MRI working group. Ann Rheum Dis. 2019;78(11):1550–8.
Baraliakos X, Ostergaard M, Lambert RG, Eshed I, Machado PM, Pedersen SJ, et al. MRI lesions of the spine in patients with axial spondyloarthritis: an update of lesion definitions and validation by the ASAS MRI working group. Ann Rheum Dis. 2022;81:1243–51.
Baraliakos X, Richter A, Feldmann D, Ott A, Buelow R, Schmidt CO, et al. Frequency of MRI changes suggestive of axial spondyloarthritis in the axial skeleton in a large population-based cohort of individuals aged < 45 years. Ann Rheum Dis. 2020;79(2):186–92.
Singh JA, Guyatt G, Ogdie A, Gladman DD, Deal C, Deodhar A, et al. Special article: 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol. 2019;71(1):5–32.
Gossec L, Baraliakos X, Kerschbaumer A, de Wit M, McInnes I, Dougados M, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79(6):700–12.
Coates LC, Soriano ER, Corp N, Bertheussen H, Callis Duffin K, Campanholo CB, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18(8):465–79.
Lubrano E, Chan J, Queiro-Silva R, Cauli A, Goel N, Poddubnyy D, et al. Management of axial disease in patients with psoriatic arthritis: an updated literature review informing the 2021 GRAPPA treatment recommendations. J Rheumatol. 2023;50(2):279–284.
Ramiro S, Nikiphorou E, Sepriano A, Ortolan A, Webers C, Baraliakos X, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023;82(1):19–34.
Smolen JS, Schols M, Braun J, Dougados M, FitzGerald O, Gladman DD, et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis. 2018;77(1):3–17.
Ortolan A, Webers C, Sepriano A, Falzon L, Baraliakos X, Landewe RB, et al. Efficacy and safety of non-pharmacological and non-biological interventions: a systematic literature review informing the 2022 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. Ann Rheum Dis. 2023;82(1):142–52.
Song IH, Heldmann F, Rudwaleit M, Haibel H, Weiss A, Braun J, et al. Treatment of active ankylosing spondylitis with abatacept: an open-label, 24-week pilot study. Ann Rheum Dis. 2011;70(6):1108–10.
Taylor PC, van der Heijde D, Landewe R, McCue S, Cheng S, Boonen A. A phase III randomized study of apremilast, an oral phosphodiesterase 4 inhibitor, for active ankylosing spondylitis. J Rheumatol. 2021;48(8):1259–67.
Webers C, Ortolan A, Sepriano A, Falzon L, Baraliakos X, Landewe RBM, et al. Efficacy and safety of biological DMARDs: a systematic literature review informing the 2022 update of the ASAS-EULAR recommendations for the management of axial spondyloarthritis. Ann Rheum Dis. 2023;82(1):130–41.
van der Heijde D, Deodhar A, Baraliakos X, Brown MA, Dobashi H, Dougados M, et al. Efficacy and safety of bimekizumab in axial spondyloarthritis: results of two parallel phase 3 randomised controlled trials. Ann Rheum Dis. 2023.
Baraliakos X, Coates LC, Gossec L, Sławomir J, Mera Varela A, Schulz B, et al. Secukinumab improves axial manifestations in patients with psoriatic arthritis and inadequate response to NSAIDs: primary analysis of the MAXIMISE trial. Ann Rheum Dis. 2019;78:195–6.
Baeten D, Ostergaard M, Wei JC, Sieper J, Jarvinen P, Tam LS, et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann Rheum Dis. 2018;77(9):1295–302.
Deodhar A, Gensler LS, Sieper J, Clark M, Calderon C, Wang Y, et al. Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis Rheum. 2019;71(2):258–70.
Sherlock JP, Buckley CD, Cua DJ. The critical role of interleukin-23 in spondyloarthropathy. Mol Immunol. 2014;57(1):38–43.
Sieper J, Poddubnyy D, Miossec P. The IL-23-IL-17 pathway as a therapeutic target in axial spondyloarthritis. Nat Rev Rheumatol. 2019;15(12):747–57.
Helliwell PS, Gladman DD, Chakravarty SD, Kafka S, Karyekar CS, You Y, et al. Effects of ustekinumab on spondylitis-associated endpoints in TNFi-naïve active psoriatic arthritis patients with physician-reported spondylitis: pooled results from two phase 3, randomised, controlled trials. RMD Open. 2020;6(1):e001149.
Mease P, Helliwell P, Gladman D, Poddubnyy D, Baraliakos X, Chakravarty SD, et al. Efficacy of guselkumab on axial involvement in patients with active psoriatic arthritis and sacroiliitis: a post-hoc analysis of the phase 3 DISCOVER-1 and DISCOVER-2 studies. Lancet Rheumatol. 2021;3(10):e715–23.
Gladman DD, Mease PJ, Bird P, Soriano ER, Chakravarty SD, Shawi M, et al. Efficacy and safety of guselkumab in biologic-naive patients with active axial psoriatic arthritis: study protocol for STAR, a phase 4, randomized, double-blinded, placebo-controlled trial. Trials. 2022;23(1):743.
Deodhar A, Sliwinska-Stanczyk P, Xu H, Baraliakos X, Gensler LS, Fleishaker D, et al. Tofacitinib for the treatment of ankylosing spondylitis: a phase III, randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2021;80(8):1004–13.
van der Heijde D, Song IH, Pangan AL, Deodhar A, van den Bosch F, Maksymowych WP, et al. Efficacy and safety of upadacitinib in patients with active ankylosing spondylitis (SELECT-AXIS 1): a multicentre, randomised, double-blind, placebo-controlled, phase 2/3 trial. Lancet. 2019;394(10214):2108–17.
Deodhar A, Van den Bosch F, Poddubnyy D, Maksymowych WP, van der Heijde D, Kim TH, et al. Upadacitinib for the treatment of active non-radiographic axial spondyloarthritis (SELECT-AXIS 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2022;400(10349):369–79.
Proft F, Torgutalp M, Muche B, Rios Rodriguez V, Verba M, Poddubnyy D. Efficacy of tofacitinib in reduction of inflammation detected on MRI in patients with Psoriatic ArthritiS presenTing with axial involvement (PASTOR): protocol of a randomised, double-blind, placebo-controlled, multicentre trial. BMJ Open. 2021;11(11): e048647.
Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316–26.
Curtis JR, Yamaoka K, Chen YH, Bhatt DL, Gunay LM, Sugiyama N, et al. Malignancy risk with tofacitinib versus TNF inhibitors in rheumatoid arthritis: results from the open-label, randomised controlled ORAL Surveillance trial. Ann Rheum Dis. 2023;82(3):331–343.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
DP: research support from: AbbVie, Eli Lilly, MSD, Novartis, Pfizer; consulting fees from: AbbVie, Biocad, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, MSD, Moonlake, Novartis, Pfizer, Samsung Bioepis, and UCB; speaker fees from: AbbVie, Bristol-Myers Squibb, Eli Lilly, Janssen, MSD, Medscape, Novartis, Peervoice, Pfizer, and UCB. Member of executive committee of ASAS. Member of steering committee of GRAPPA.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Author contributions
DP is the sole author of this review.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Poddubnyy, D. Managing Psoriatic Arthritis Patients Presenting with Axial Symptoms. Drugs 83, 497–505 (2023). https://doi.org/10.1007/s40265-023-01857-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-023-01857-w