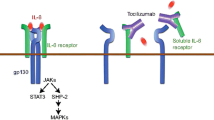
Abstract
Ozoralizumab (Nanozora®), a trivalent anti-tumour necrosis factor alpha (TNFα) NANOBODYⓇ compound, has been developed by Taisho Pharmaceutical Co. Ltd (under license from Ablynx, an affiliate of Sanofi) for the treatment of rheumatoid arthritis (RA). In September 2022, ozoralizumab was approved in Japan for the treatment of RA that is inadequately managed by current available treatments. This article summarizes the milestones in the development of ozoralizumab leading to this first approval.
Similar content being viewed by others
References
McInnes IB, Buckley CD, Isaacs JD. Cytokines in rheumatoid arthritis: shaping the immunological landscape. Nat Rev Rheumatol. 2016;12(1):63–8.
Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2021;73(7):1108–23.
Kawahito Y, Morinobu A, Kaneko Y, et al. Drug treatment algorithm and recommendations from the 2020 update of the Japan College of Rheumatology clinical practice guidelines for the management of rheumatoid arthritis: secondary publication. Mod Rheumatol. 2022. https://doi.org/10.1093/mr/roac017.
Smolen JS, Landewe RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–99.
Taisho Pharmaceutical Company Ltd. Ozoralizumab (Nanozora®): Japanese prescribing information. 2022. http://medical.taisho.co.jp/di/brand/nz/product.php?bdname=nz&brand=nz. Accessed 20 Oct 2022
Jovcevska I, Muyldermans S. The therapeutic potential of nanobodies. BioDrugs. 2020;34(1):11–26.
Sanofi. NANOBODY® Technology Platform. 2021. https://www.sanofi.com/en/science-and-innovation/research-and-development/technology-platforms/nanobody-technology-platform. Accessed 4 Oct 2022.
Fleischmann R, Nayiager S, Louw I, et al. A multiple ascending dose proof of concept study of ATN-103 (ozoralizumab) in rheumatoid arthritis subjects on a background of methotrexate [abstract no. 2630]. In: ACR/ARHP Annual Scientific Meeting. 2011.
Fleischmann RM, De Bruyn S, Duby C, et al. A novel individualized treatment approach in open-label extension study of ozoralizumab (ATN-103) in subjects with rheumatoid arthritis on a background of methotrexate [abstract no. 1311]. Arthritis Rheum. 2012;64(10 Suppl):S563.
Taisho Pharmaceutical Company Ltd. Notification of approval to manufacture and market Nanozora® 30mg syringes for S.C. injection, a therapy for rheumatoid arthritis, in Japan: Japan's first NANOBODY® therapeutic [media release]. 26 Sep 2022. https://www.taisho.co.jp/global/.
Taisho Pharmaceutical Co Ltd. Notification of application for approval to manufacture and market rheumatoid arthritis therapy Nanozora® 30mg Autoinjectors for S.C. injection in Japan [media release]. 28 Sep 2022. https://www.taisho.co.jp/global/.
Sanofi, Ablynx. Sanofi completes its acquisition of Ablynx following the expiration of the squeeze-out procedure [media release]. 19 Jun 2018. http://www.sanofi.com.
Taisho Pharmaceutical Company Ltd. Annual Report 2015. 2015. http://www.taisho.co.jp. Accessed 2 Dec 2022.
Ablynx. Ablynx signs exclusive license agreement with Eddingpharm to develop and commercialise its anti-TNFa nanobody in greater China [media release]. 1 Sep 2018. https://www.globenewswire.com.
Ishiwatari-Ogata C, Kyuuma M, Ogata H, et al. Ozoralizumab, a humanized anti-TNFα NANOBODY® compound, exhibits efficacy not only at the onset of arthritis in a human TNF transgenic mouse but also during secondary failure of administration of an anti-TNFα IgG. Front Immunol. 2022. https://doi.org/10.3389/fimmu.2022.853008.
Takeuchi T, Kawanishi M, Nakanishi M, et al. Phase II/III results of the anti-TNF multivalent NANOBODY® compound ozoralizumab in patient with rheumatoid arthritis (OHZORA trial). Arthritis Rheumatol. 2022. https://doi.org/10.1002/art.42273.
Tanaka Y, Kawanishi M, Nakanishi M, et al. Efficacy and safety of the anti-TNF multivalent NANOBODYⓇ compound ozoralizumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: a 52-week result of a phase II/III study (OHZORA trial). Mod Rheumatol. 2022. https://doi.org/10.1093/mr/roac119.
Tanaka Y, Kawanishi M, Nakanishi M, et al. Efficacy and safety of anti-TNF multivalent NANOBODYⓇ compound ozoralizumab without methotrexate co-administration in patients with active rheumatoid arthritis: a 52-week result of phase III, randomised, open-label trial (NATSUZORA trial). Mod Rheumatol. 2022. https://doi.org/10.1093/mr/roac126.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Susan J. Keam is a contracted employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Keam, S.J. Ozoralizumab: First Approval. Drugs 83, 87–92 (2023). https://doi.org/10.1007/s40265-022-01821-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01821-0