Abstract
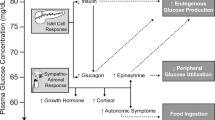
Glucagon is essential for endogenous glucose regulation along with the paired hormone, insulin. Unlike insulin, pharmaceutical use of glucagon has been limited due to the unstable nature of the peptide. Glucagon has the potential to address hypoglycemia as a major limiting factor in the treatment of diabetes, which remains very common in the type 1 and type 2 diabetes. Recent developments are poised to change this paradigm and expand the use of glucagon for people with diabetes. Glucagon emergency kits have major limitations for their use in treating severe hypoglycemia. A complicated reconstitution and injection process often results in incomplete or aborted administration. New preparations include intranasal glucagon with an easy-to-use and needle-free nasal applicator as well as two stable liquid formulations in pre-filled injection devices. These may ease the burden of severe hypoglycemia treatment. The liquid preparations may also have a role in the treatment of non-severe hypoglycemia. Despite potential benefits of expanded use of glucagon, undesirable side effects (nausea, vomiting), cost, and complexity of adding another medication may limit real-world use. Additionally, more long-term safety and outcome data are needed before widespread, frequent use of glucagon is recommended by providers.
Similar content being viewed by others
References
Draznin B, Aroda VR, Bakris G, Benson G, et al. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S83-S96.
Frier BM. Hypoglycaemia in diabetes mellitus: epidemiology and clinical implications. Nat Rev Endocrinol. 2014;10(12):711–22.
Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, DiMeglio LA, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther. 2019;21(2):66–72.
Beck RW, Riddlesworth T, Ruedy K, Ahmann A, Bergenstal R, Haller S, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND Randomized Clinical Trial. JAMA. 2017;317(4):371–8.
Lind M, Polonsky W, Hirsch IB, Heise T, Bolinder J, Dahlqvist S, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD Randomized Clinical Trial. JAMA. 2017;317(4):379–87.
U. K. Hypoglycaemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50(6):1140–7.
International Hypoglycaemia Study Group. Minimizing Hypoglycemia in Diabetes. Diabetes Care. 2015;38(8):1583–91.
Silbert R, Salcido-Montenegro A, Rodriguez-Gutierrez R, Katabi A, McCoy RG. Hypoglycemia among patients with type 2 diabetes: epidemiology, risk factors, and prevention strategies. Curr Diab Rep. 2018;18(8):53.
Brazeau AS, Rabasa-Lhoret R, Strychar I, Mircescu H. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care. 2008;31(11):2108–9.
Domgaard M, Bagger M, Rhee NA, Burton CM, Thorsteinsson B. Individual and societal consequences of hypoglycemia: a cross-sectional survey. Postgrad Med. 2015;127(5):438–45.
Zaccardi F, Davies MJ, Dhalwani NN, Webb DR, Housley G, Shaw D, et al. Trends in hospital admissions for hypoglycaemia in England: a retrospective, observational study. Lancet Diabetes Endocrinol. 2016;4(8):677–85.
Geller AI, Shehab N, Lovegrove MC, Kegler SR, Weidenbach KN, Ryan GJ, et al. National estimates of insulin-related hypoglycemia and errors leading to emergency department visits and hospitalizations. JAMA Intern Med. 2014;174(5):678–86.
Hawkes CP, De Leon DD, Rickels MR. Novel Preparations of Glucagon for the Prevention and Treatment of Hypoglycemia. Curr Diab Rep. 2019;19(10):97.
La Sala L, Pontiroli AE. New fast acting glucagon for recovery from hypoglycemia, a life-threatening situation: nasal powder and injected stable solutions. Int J Mol Sci. 2021;22(19).
Rabinovich A, Priefer R. Glucagon delivery - An overview of current and future devices. Diabetes Metab Syndr. 2021;15(4):102155.
Thieu VT, Mitchell BD, Varnado OJ, Frier BM. Treatment and prevention of severe hypoglycaemia in people with diabetes: Current and new formulations of glucagon. Diabetes Obes Metab. 2020;22(4):469–79.
Ahren B. Glucagon-Early breakthroughs and recent discoveries. Peptides. 2015;67:74–81.
Murlin JRC, Harry D; Gibbs, C B F; Stokes, Arthur M. Aqueous extracts of pancreas: 1. Influence on the carbohydrate metabolism of depancreatized animals. The Journal of biological chemistry. 1923 May 01, 1923;56(1):253-96.
Sutherland EW, De Duve C. Origin and distribution of the hyperglycemic-glycogenolytic factor of the pancreas. J Biol Chem. 1948;175(2):663–74.
Foa PP, Weinstein HR, Smith JA. Secretion of insulin and of a hyperglycemic substance studied by means of pancreatic-femoral cross-circulation experiments. Am J Physiol. 1949;157(2):197–204.
Bromer WW, Sinn LG, Staub A, Behrens OK. The amino acid sequence of glucagon. Diabetes. 1957;6(3):234–8.
Determination That GLUCAGON (Glucagon Hydrochloride) for Injection, Equivalent to 1 Milligram Base/Vial and Equivalent to 10 Milligram Base/Vial, Was Not Withdrawn From Sale for Reasons of Safety or Effectiveness (Docket No. FDA–2007–P–0248]). Federal Register. 2015;80(174):54294–5. https://www.govinfo.gov/content/pkg/FR-2015-09-09/pdf/2015-22673.pdf
Gerich JE, Schneider V, Dippe SE, Langlois M, Noacco C, Karam JH, et al. Characterization of the glucagon response to hypoglycemia in man. J Clin Endocrinol Metab. 1974;38(1):77–82.
Cryer PE. Glucose counterregulation in man. Diabetes. 1981;30(3):261–4.
Bolli GB, Dimitriadis GD, Pehling GB, Baker BA, Haymond MW, Cryer PE, et al. Abnormal glucose counterregulation after subcutaneous insulin in insulin-dependent diabetes mellitus. N Engl J Med. 1984;310(26):1706–11.
Bell GI, Sanchez-Pescador R, Laybourn PJ, Najarian RC. Exon duplication and divergence in the human preproglucagon gene. Nature. 1983;304(5924):368–71.
Administration USFD. HIGHLIGHTS OF PRESCRIBING INFORMATION: GlucaGen® (glucagon [rDNA origin] for injection) [cited 2022 March 24]; https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020918s030lbl.pdf
Administration USFD. INFORMATION FOR THE PHYSICIAN: GLUCAGON FOR INJECTION (rDNA ORIGIN) [cited 2022 March 24]; https://www.accessdata.fda.gov/drugsatfda_docs/label/2004/20928slr010_glucagon_lbl.pdf
Administration USFD. HIGHLIGHTS OF PRESCRIBING INFORMATION: BAQSIMI (glucagon) nasal powder. [cited 2022 March 24]; https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/210134s000lbl.pdf. Accessed 8 Feb 2022.
Administration USFD. HIGHLIGHTS OF PRESCRIBING INFORMATION: GVOKE (glucagon) injection, for subcutaneous use. [cited 2022 March 24]; https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212097s000lbl.pdf. Accessed 8 Feb 2022.
Administration USFD. HIGHLIGHTS OF PRESCRIBING INFORMATION: ZEGALOGUE (dasiglucagon) injection, for subcutaneous use [cited 2022 March 24]; https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214231s000lbl.pdf. Accessed 8 Feb 2022.
Rouillé Y, Kantengwa S, Irminger JC, Halban PA. Role of the prohormone convertase PC3 in the processing of proglucagon to glucagon-like peptide 1. J Biol Chem. 1997;272(52):32810–6.
Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–57.
Svoboda M, Tastenoy M, Vertongen P, Robberecht P. Relative quantitative analysis of glucagon receptor mRNA in rat tissues. Mol Cell Endocrinol. 1994;105(2):131–7.
Freychet L, Rizkalla SW, Desplanque N, Basdevant A, Zirinis P, Tchobroutsky G, et al. Effect of intranasal glucagon on blood glucose levels in healthy subjects and hypoglycaemic patients with insulin-dependent diabetes. Lancet. 1988;1(8599):1364–6.
Adeva-Andany MM, Funcasta-Calderon R, Fernandez-Fernandez C, Castro-Quintela E, Carneiro-Freire N. Metabolic effects of glucagon in humans. J Clin Transl Endocrinol. 2019;15:45–53.
Gerich JE, Lorenzi M, Hane S, Gustafson G, Guillemin R, Forsham PH. Evidence for a physiologic role of pancreatic glucagon in human glucose homeostasis: studies with somatostatin. Metabolism. 1975;24(2):175–82.
Hvidberg A, Djurup R, Hilsted J. Glucose recovery after intranasal glucagon during hypoglycaemia in man. Eur J Clin Pharmacol. 1994;46(1):15–7.
Lins PE, Wajngot A, Adamson U, Vranic M, Efendić S. Minimal increases in glucagon levels enhance glucose production in man with partial hypoinsulinemia. Diabetes. 1983;32(7):633–6.
Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev. 2007;28(1):84–116.
Hartig SM, Cox AR. Paracrine signaling in islet function and survival. J Mol Med (Berl). 2020;98(4):451–67.
Taborsky GJ Jr. The physiology of glucagon. J Diabetes Sci Technol. 2010;4(6):1338–44.
Kaneko K, Shirotani T, Araki E, Matsumoto K, Taguchi T, Motoshima H, et al. Insulin inhibits glucagon secretion by the activation of PI3-kinase in In-R1-G9 cells. Diabetes Res Clin Pract. 1999;44(2):83–92.
Wendt A, Birnir B, Buschard K, Gromada J, Salehi A, Sewing S, et al. Glucose inhibition of glucagon secretion from rat alpha-cells is mediated by GABA released from neighboring beta-cells. Diabetes. 2004;53(4):1038–45.
Gedulin BR, Rink TJ, Young AA. Dose-response for glucagonostatic effect of amylin in rats. Metabolism. 1997;46(1):67–70.
Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB. Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Nat Cell Biol. 2003;5(4):330–5.
Zhou H, Zhang T, Harmon JS, Bryan J, Robertson RP. Zinc, not insulin, regulates the rat alpha-cell response to hypoglycemia in vivo. Diabetes. 2007;56(4):1107–12.
Gromada J, Høy M, Buschard K, Salehi A, Rorsman P. Somatostatin inhibits exocytosis in rat pancreatic alpha-cells by G(i2)-dependent activation of calcineurin and depriming of secretory granules. J Physiol. 2001;535(Pt 2):519–32.
Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20(3):157–98.
Yoshimoto Y, Fukuyama Y, Horio Y, Inanobe A, Gotoh M, Kurachi Y. Somatostatin induces hyperpolarization in pancreatic islet alpha cells by activating a G protein-gated K+ channel. FEBS Lett. 1999;444(2–3):265–9.
Beuers U, Jungermann K. Relative contribution of glycogenolysis and gluconeogenesis to basal, glucagon- and nerve stimulation-dependent glucose output in the perfused liver from fed and fasted rats. Biochem Int. 1990;21(3):405–15.
Doi Y, Iwai M, Matsuura B, Onji M. Glucagon attenuates the action of insulin on glucose output in the liver of the Goto-Kakizaki rat perfused in situ. Pflugers Arch. 2001;442(4):537–41.
Ikeda T, Hoshino T, Honda M, Takeuchi T, Mokuda O, Tominaga M, et al. Effect of glucagon on glucose output from bivascularly perfused rat liver. Exp Clin Endocrinol. 1989;94(3):383–6.
Band G, Jones CT. Activation by glucagon of glucose 6-phosphatase activity in the liver of the foetal guinea pig. Biochem Soc Trans. 1984;8(5):586–7.
Band GC, Jones CT. Functional activation by glucagon of glucose 6-phosphatase and gluconeogenesis in the perfused liver of the fetal guinea pig. FEBS Lett. 1980;119(1):190–4.
Jiang G, Zhang BB. Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab. 2003;284(4):E671–8.
Striffler JS, Garfield SA, Cardell EL, Cardell RR. Effects of glucagon on hepatic microsomal glucose-6-phosphatase in vivo. Diabete Metab. 1984;10(2):91–7.
Akatsuka A, Singh TJ, Nakabayashi H, Lin MC, Huang KP. Glucagon-stimulated phosphorylation of rat liver glycogen synthase in isolated hepatocytes. J Biol Chem. 1985;260(6):3239–42.
Ciudad C, Camici M, Ahmad Z, Wang Y, DePaoli-Roach AA, Roach PJ. Control of glycogen synthase phosphorylation in isolated rat hepatocytes by epinephrine, vasopressin and glucagon. Eur J Biochem. 1984;142(3):511–20.
Ramachandran C, Angelos KL, Walsh DA. Hormonal regulation of the phosphorylation of glycogen synthase in perfused rat heart. Effects of insulin, catecholamines, and glucagon. J Biol Chem. 1983;258(21):13377–83.
Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973;182(4108):171–3.
Müller TD, Finan B, Clemmensen C, DiMarchi RD, Tschöp MH. The New Biology and Pharmacology of Glucagon. Physiol Rev. 2017;97(2):721–66.
Reaven GM, Chen YD, Golay A, Swislocki AL, Jaspan JB. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1987;64(1):106–10.
Thorburn A, Litchfield A, Fabris S, Proietto J. Abnormal transient rise in hepatic glucose production after oral glucose in non-insulin-dependent diabetic subjects. Diabetes Res Clin Pract. 1995;28(2):127–35.
Unger RH, Aguilar-Parada E, Müller WA, Eisentraut AM. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970;49(4):837–48.
Voss TS, Vendelbo MH, Kampmann U, Pedersen SB, Nielsen TS, Johannsen M, et al. Substrate metabolism, hormone and cytokine levels and adipose tissue signalling in individuals with type 1 diabetes after insulin withdrawal and subsequent insulin therapy to model the initiating steps of ketoacidosis. Diabetologia. 2019;62(3):494–503.
Hare KJ, Vilsbøll T, Holst JJ, Knop FK. Inappropriate glucagon response after oral compared with isoglycemic intravenous glucose administration in patients with type 1 diabetes. Am J Physiol Endocrinol Metab. 2010;298(4):E832–7.
Sherr J, Tsalikian E, Fox L, Buckingham B, Weinzimer S, Tamborlane WV, et al. Evolution of abnormal plasma glucagon responses to mixed-meal feedings in youth with type 1 diabetes during the first 2 years after diagnosis. Diabetes Care. 2014;37(6):1741–4.
Bengtsen MB, Støy J, Rittig NF, Voss TS, Magnusson NE, Svart MV, et al. A human randomized controlled trial comparing metabolic responses to single and repeated hypoglycemia in type 1 diabetes. J Clin Endocrinol Metab. 2020;105(12):e4699–e4711.
Gerich JE, Langlois M, Noacco C, Lorenzi M, Karam JH, Korsham PH. Comparison of the suppressive effects of elevated plasma glucose and free fatty acid levels on glucagon secretion in normal and insulin-dependent diabetic subjects. Evidence for selective alpha-cell insensitivity to glucose in diabetes mellitus. J Clin Invest. 1976;58(2):320–5.
Bolli G, de Feo P, Compagnucci P, Cartechini MG, Angeletti G, Santeusanio F, et al. Abnormal glucose counterregulation in insulin-dependent diabetes mellitus. Interaction of anti-insulin antibodies and impaired glucagon and epinephrine secretion. Diabetes. 1983;32(2):134–41.
Siafarikas A, Johnston RJ, Bulsara MK, O’Leary P, Jones TW, Davis EA. Early loss of the glucagon response to hypoglycemia in adolescents with type 1 diabetes. Diabetes Care. 2012;35(8):1757–62.
Segel SA, Paramore DS, Cryer PE. Hypoglycemia-associated autonomic failure in advanced type 2 diabetes. Diabetes. 2002;51(3):724–33.
Guettet C, Rostaqui N, Navarro N, Lecuyer B, Mathe D. Effect of chronic glucagon administration on the metabolism of triacylglycerol-rich lipoproteins in rats fed a high sucrose diet. J Nutr. 1991;121(1):24–30.
Rothfeld B, Margolis S, Varady A Jr, Karmen A. Effects of glucagon on cholesterol and triglyceride deposition in tissues. Biochem Med. 1974;10(2):122–5.
Sanchez A, Hubbard RW, Smit E, Hilton GF. Testing a mechanism of control in human cholesterol metabolism: relation of arginine and glycine to insulin and glucagon. Atherosclerosis. 1988;71(1):87–92.
Richter WO, Robl H, Schwandt P. Human glucagon and vasoactive intestinal polypeptide (VIP) stimulate free fatty acid release from human adipose tissue in vitro. Peptides. 1989;10(2):333–5.
Vons C, Pegorier JP, Girard J, Kohl C, Ivanov MA, Franco D. Regulation of fatty-acid metabolism by pancreatic hormones in cultured human hepatocytes. Hepatology. 1991;13(6):1126–30.
Billington CJ, Bartness TJ, Briggs J, Levine AS, Morley JE. Glucagon stimulation of brown adipose tissue growth and thermogenesis. Am J Physiol. 1987;252(1 Pt 2):R160–5.
Calles-Escandón J. Insulin dissociates hepatic glucose cycling and glucagon-induced thermogenesis in man. Metabolism. 1994;43(8):1000–5.
Nair KS. Hyperglucagonemia increases resting metabolic rate in man during insulin deficiency. J Clin Endocrinol Metab. 1987;64(5):896–901.
Filali-Zegzouti Y, Abdelmelek H, Rouanet JL, Cottet-Emard JM, Pequignot JM, Barré H. Role of catecholamines in glucagon-induced thermogenesis. J Neural Transm (Vienna). 2005;112(4):481–9.
Heim T, Hull D. The effect of propranalol on the calorigenic response in brown adipose tissue of new-born rabbits to catecholamines, glucagon, corticotrophin and cold exposure. J Physiol. 1966;187(2):271–83.
Geary N, Le Sauter J, Noh U. Glucagon acts in the liver to control spontaneous meal size in rats. Am J Physiol. 1993;264(1 Pt 2):R116–22.
Geary N, Smith GP. Selective hepatic vagotomy blocks pancreatic glucagon’s satiety effect. Physiol Behav. 1983;31(3):391–4.
Martin JR, Novin D, Vanderweele DA. Loss of glucagon suppression of feeding after vagotomy in rats. Am J Physiol. 1978;234(3):E314–8.
Bagger JI. Physiological and pathophysiological aspects of incretin hormones and glucagon. Dan Med J. 2017;64(1).
Habegger KM, Heppner KM, Geary N, Bartness TJ, DiMarchi R, Tschöp MH. The metabolic actions of glucagon revisited. Nat Rev Endocrinol. 2010;6(12):689–97.
Penick SB, Hinkle LE Jr. Depression of food intake induced in healthy subjects by glucagon. N Engl J Med. 1961;4(264):893–7.
Stunkard AJ, Van Itallie TB, Reis BB. The mechanism of satiety: effect of glucagon on gastric hunger contractions in man. Proc Soc Exp Biol Med. 1955;89(2):258–61.
Valentine V, Newswanger B, Prestrelski S, Andre AD, Garibaldi M. Human factors usability and validation studies of a glucagon autoinjector in a simulated severe hypoglycemia rescue situation. diabetes Technol Ther. 2019;21(9):522–30.
Harris GAD, Sulway M, Wilkinson M. Glucagon administration—undervalued and undertaught. Practical Diabetes Int. 2001;18(1):22–5.
Yale JF, Dulude H, Egeth M, Piche CA, Lafontaine M, Carballo D, et al. Faster use and fewer failures with needle-free nasal glucagon versus injectable glucagon in severe hypoglycemia rescue: a simulation study. Diabetes Technol Ther. 2017;19(7):423–32.
Steiner SS, Li M, Hauser R, Pohl R. Stabilized glucagon formulation for bihormonal pump use. J Diabetes Sci Technol. 2010;4(6):1332–7.
Haymond MW, Liu J, Bispham J, Hickey A, McAuliffe-Fogarty AH. Use of glucagon in patients with type 1 diabetes. Clin Diabetes. 2019;37(2):162–6.
Chung ST, Haymond MW. Minimizing morbidity of hypoglycemia in diabetes: a review of mini-dose glucagon. J Diabetes Sci Technol. 2015;9(1):44–51.
Hasan KS, Kabbani M. Mini-dose glucagon is effective at diabetes camp. J Pediatr. 2004;144(6):834.
Haymond MW, DuBose SN, Rickels MR, Wolpert H, Shah VN, Sherr JL, et al. Efficacy and safety of mini-dose glucagon for treatment of nonsevere hypoglycemia in adults with type 1 diabetes. J Clin Endocrinol Metab. 2017;102(8):2994–3001.
Haymond MW, Redondo MJ, McKay S, Cummins MJ, Newswanger B, Kinzell J, et al. Nonaqueous, mini-dose glucagon for treatment of mild hypoglycemia in adults with type 1 diabetes: a dose-seeking study. Diabetes Care. 2016;39(3):465–8.
Wilson LM, Castle JR. Stable liquid glucagon: beyond emergency hypoglycemia rescue. J Diabetes Sci Technol. 2018;12(4):847–53.
Brink S, Laffel L, Likitmaskul S, Liu L, Maguire AM, Olsen B, et al. Sick day management in children and adolescents with diabetes. Pediatr Diabetes. 2009;10(Suppl 12):146–53.
Haymond MW, Schreiner B. Mini-dose glucagon rescue for hypoglycemia in children with type 1 diabetes. Diabetes Care. 2001;24(4):643–5.
Caputo N, Castle JR, Bergstrom CP, Carroll JM, Bakhtiani PA, Jackson MA, et al. Mechanisms of glucagon degradation at alkaline pH. Peptides. 2013;45:40–7.
Jackson MA, Caputo N, Castle JR, David LL, Roberts CT Jr, Ward WK. Stable liquid glucagon formulations for rescue treatment and bi-hormonal closed-loop pancreas. Curr Diab Rep. 2012;12(6):705–10.
Joshi AB, Rus E, Kirsch LE. The degradation pathways of glucagon in acidic solutions. Int J Pharm. 2000;203(1–2):115–25.
Pontiroli AE. Intranasal glucagon: a promising approach for treatment of severe hypoglycemia. J Diabetes Sci Technol. 2015;9(1):38–43.
Pontiroli AE, Alberetto M, Pozza G. Intranasal glucagon raises blood glucose concentrations in healthy volunteers. Br Med J (Clin Res Ed). 1983;287(6390):462–3.
Pontiroli AE, Calderara A, Pajetta E, Alberetto M, Pozza G. Intranasal glucagon as remedy for hypoglycemia. Studies in healthy subjects and type I diabetic patients. Diabetes Care. 1989;12(9):604–8.
Lilly. Lilly acquires phase III intranasal glucagon from locemia solutions. 2015 October 9, 2015 [cited 2022 August 2]; Available from: https://investor.lilly.com/news-releases/news-release-details/lilly-acquires-phase-iii-intranasal-glucagon-locemia-solutions.
Guzman CB, Dulude H, Piche C, Rufiange M, Sadoune AA, Rampakakis E, et al. Effects of common cold and concomitant administration of nasal decongestant on the pharmacokinetics and pharmacodynamics of nasal glucagon in otherwise healthy participants: a randomized clinical trial. Diabetes Obes Metab. 2018;20(3):646–53.
Rickels MR, Ruedy KJ, Foster NC, Piche CA, Dulude H, Sherr JL, et al. Intranasal glucagon for treatment of insulin-induced hypoglycemia in adults with type 1 diabetes: a randomized crossover noninferiority study. Diabetes Care. 2016;39(2):264–70.
Reno FE, Normand P, McInally K, Silo S, Stotland P, Triest M, et al. A novel nasal powder formulation of glucagon: toxicology studies in animal models. BMC Pharmacol Toxicol. 2015;26(16):29.
Blair HA. Dasiglucagon: first approval. Drugs. 2021;81(9):1115–20.
Xu B, Tang G, Chen Z. Dasiglucagon: an effective medicine for severe hypoglycemia. Eur J Clin Pharmacol. 2021;77(12):1783–90.
Pharma Z. Dasiglucagon - A novel glucagon analog, Phase II update. 2017 [cited 2022 March 24]; https://static1.squarespace.com/static/58983777d1758e28995640b4/t/5912d3251e5b6cb374772105/1494405933418/TIDES. Accessed 8 Feb 2022.
Macchi F WC, Lundholt BK. 512. Dasiglucagon is a novel stable glucagon analogue with fast glucose response following subcutaneous injection in hypoglycaemic rats. Diabetologia. 2022;63:1–485.
Castle JR, Elander M. Long-term safety and tolerability of dasiglucagon, a stable-in-solution glucagon analogue. Diabetes Technol Ther. 2019;21(2):94–6.
Hovelmann U, Bysted BV, Mouritzen U, Macchi F, Lamers D, Kronshage B, et al. Pharmacokinetic and pharmacodynamic characteristics of dasiglucagon, a novel soluble and stable glucagon analog. Diabetes Care. 2018;41(3):531–7.
Hovelmann U, Olsen MB, Mouritzen U, Lamers D, Kronshage B, Heise T. Low doses of dasiglucagon consistently increase plasma glucose levels from hypoglycaemia and euglycaemia in people with type 1 diabetes mellitus. Diabetes Obes Metab. 2019;21(3):601–10.
Trial to Confirm the Clinical Efficacy and Safety of Dasiglucagon in the Treatment of Hypoglycemia in Subjects With T1DM. [cited 2022 March 24]; https://clinicaltrials.gov/ct2/show/NCT03688711
Pieber TR, Aronson R, Hovelmann U, Willard J, Plum-Morschel L, Knudsen KM, et al. Dasiglucagon-A next-generation glucagon analog for rapid and effective treatment of severe hypoglycemia: results of phase 3 randomized double-blind clinical trial. Diabetes Care. 2021;44(6):1361–7.
Battelino T, Tehranchi R, Bailey T, Dovc K, Melgaard A, Yager Stone J, et al. Dasiglucagon, a next-generation ready-to-use glucagon analog, for treatment of severe hypoglycemia in children and adolescents with type 1 diabetes: Results of a phase 3, randomized controlled trial. Pediatr Diabetes. 2021;22(5):734–41.
Newswanger B, Ammons S, Phadnis N, Ward WK, Castle J, Campbell RW, et al. Development of a highly stable, nonaqueous glucagon formulation for delivery via infusion pump systems. J Diabetes Sci Technol. 2015;9(1):24–33.
Castle JR, Youssef JE, Branigan D, Newswanger B, Strange P, Cummins M, et al. Comparative pharmacokinetic/pharmacodynamic study of liquid stable glucagon versus lyophilized glucagon in type 1 diabetes subjects. J Diabetes Sci Technol. 2016;10(5):1101–7.
Adocia. BIOCHAPERONE® [cited 2022 March 26]; https://www.adocia.com/technology/biochaperone-technology-2/#:~:text=Adocia%20designed%20the%20BioChaperone%C2%AE,degradation%20and%20enhances%20their%20performance. Accessed 8 Feb 2022.
Glezer; S, Hovelmann; U, Teng; S, Lamers; D, Odoul; M, Correia; J, et al. BioChaperone Glucagon (BCG), a stable ready-to-use liquid glucagon formulation, is well tolerated and quickly restores euglycemia after insulin-induced hypoglycemia. American Diabetes Association; 2018;67(Supplement_1).
Adocia. Pipeline. [cited 2022 March 26]; https://www.adocia.com/products-pipeline/. Accessed 8 Feb 2022.
Steineck IIK, Ranjan A, Schmidt S, Clausen TR, Holst JJ, Norgaard K. Preserved glucose response to low-dose glucagon after exercise in insulin-pump-treated individuals with type 1 diabetes: a randomised crossover study. Diabetologia. 2019;62(4):582–92.
Rickels MR, DuBose SN, Toschi E, Beck RW, Verdejo AS, Wolpert H, et al. Mini-dose glucagon as a novel approach to prevent exercise-induced hypoglycemia in type 1 diabetes. Diabetes Care. 2018;41(9):1909–16.
Wilson LM, Jacobs PG, Ramsey KL, Resalat N, Reddy R, Branigan D, et al. Dual-hormone closed-loop system using a liquid stable glucagon formulation versus insulin-only closed-loop system compared with a predictive low glucose suspend system: an open-label, outpatient, single-center, crossover, randomized controlled trial. Diabetes Care. 2020.
Bekiari E, Kitsios K, Thabit H, Tauschmann M, Athanasiadou E, Karagiannis T, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ. 2018;18(361): k1310.
Castellanos LE, Balliro CA, Sherwood JS, Jafri R, Hillard MA, Greaux E, et al. Performance of the insulin-only iLet bionic pancreas and the bihormonal iLet using dasiglucagon in adults with type 1 diabetes in a home-use setting. Diabetes Care. 2021;44(6):e118–20.
Hawkes CP, Lado JJ, Givler S, De Leon DD. The effect of continuous intravenous glucagon on glucose requirements in infants with congenital hyperinsulinism. JIMD Rep. 2019;45:45–50.
Open-label Trial Evaluating Efficacy and safety of dasiglucagon in children with congenital hyperinsulinism. [cited 2022 March 26]; https://clinicaltrials.gov/ct2/show/NCT03777176
CSI-Glucagon for Prevention of Hypoglycemia in Children With Congenital Hyperinsulinism. [cited 2022 March 26]; https://clinicaltrials.gov/ct2/show/NCT02937558
Zealand Pharma announces data from the first phase 3-trial with dasiglucagon in Congenital Hyperinsulinism (CHI). 2020 December 15, 2020 [cited 2022 August 2]; Available from: https://www.globenewswire.com/news-release/2020/12/15/2145350/0/en/Zealand-Pharma-announces-data-from-the-first-phase-3-trial-with-dasiglucagon-in-Congenital-Hyperinsulinism-CHI.html.
Salehi M, Vella A, McLaughlin T, Patti ME. Hypoglycemia after gastric bypass surgery: current concepts and controversies. J Clin Endocrinol Metab. 2018;103(8):2815–26.
Sheehan A, Patti ME. Hypoglycemia after upper gastrointestinal surgery: clinical approach to assessment, diagnosis, and treatment. Diabetes Metab Syndr Obes. 2020;13:4469–82.
Fischer LE, Wolfe BM, Fino N, Elman MR, Flum DR, Mitchell JE, et al. Postbariatric hypoglycemia: symptom patterns and associated risk factors in the Longitudinal Assessment of Bariatric Surgery study. Surg Obes Relat Dis. 2021;17(10):1787–98.
Laguna Sanz AJ, Mulla CM, Fowler KM, Cloutier E, Goldfine AB, Newswanger B, et al. Design and clinical evaluation of a novel low-glucose prediction algorithm with mini-dose stable glucagon delivery in post-bariatric hypoglycemia. Diabetes Technol Ther. 2018;20(2):127–39.
Nielsen CK, Ohrstrom CC, Kielgast UL, Hansen DL, Hartmann B, Holst JJ, et al. Dasiglucagon effectively mitigates postbariatric postprandial hypoglycemia: a randomized, double-blind, placebo-controlled, crossover trial. Diabetes Care. 2022;45(6):1476–1481.
Mulla CM, Zavitsanou S, Laguna Sanz AJ, Pober D, Richardson L, Walcott P, et al. A Randomized, Placebo-Controlled Double-Blind Trial of a Closed-Loop Glucagon System for Postbariatric Hypoglycemia. J Clin Endocrinol Metab. 2020;105(4):e1260–71.
Pharma Z. Dasiglucagon for Bi-Hormonal Artificial Pancreas Systems [cited 2022 March 26]. https://www.zealandpharma.com/dasiglucagon-pump#:~:text=In%202016%2C%20Zealand%20entered%20into,world's%20first%20autonomous%20bionic%20pancreas. Accessed 8 Feb 2022.
Nielsen CK, Oehrstroem C, Kielgast U, Hansen DL, Lund A, Vilsboll T, et al. 10-LB: Dasiglucagon ameliorates postprandial hypoglycemia after Roux-en-y gastric bypass. American Diabetes Assoication; 2020: Diabetes; 2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
LMW’s time to write this manuscript was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (1R01DK129382-01) and the Oregon Health and Science University Wheels Up program.
Conflicts of Interest
LHS has nothing to disclose. LMW participated in completion and publication of a study that was sponsored by Xeris Pharmaceuticals via subaward from JDRF. LMW has received research supplies from Dexcom.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Author contribution
LHS and LMW contributed to the design of this review, literature search and writing and revision of the manuscript. All authors take responsibility for the integrity of the work and have given approval for this manuscript to be published.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Story, L.H., Wilson, L.M. New Developments in Glucagon Treatment for Hypoglycemia. Drugs 82, 1179–1191 (2022). https://doi.org/10.1007/s40265-022-01754-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01754-8