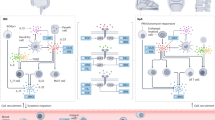
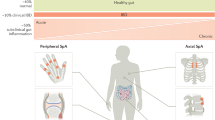
Abstract
Spondyloarthritis (SpA) represents one of the most frequent extraintestinal manifestations of inflammatory bowel disease (IBD). Evidence of shared genetic and molecular pathways underlying both diseases is emerging, which has led to rational approaches when treating patients with concomitant diseases. Clinical efficacy of tumor necrosis factor (TNF) antagonists has been ascertained over the years, and they currently represent the cornerstone of treatment in patients with IBD and SpA, but the therapeutic armamentarium in these cases has been recently expanded. Evidence for vedolizumab is controversial, as it was associated both with improvement and development of arthralgias, while ustekinumab, the first anti-interleukin 12/23 (IL-12/23) approved for IBD, has demonstrated good efficacy, especially in peripheral arthritis, and more IL-23 inhibitors are being developed in IBD. Tofacitinib was the first Janus kinase (JAK) inhibitor to be approved in IBD, and as it demonstrated efficacy in treating ankylosing spondylitis, it may represent a good choice in axial arthritis, while more selective JAK inhibitors are yet to be approved. Unexpectedly, the first anti-IL17 that was studied in IBD (secukinumab) has shown not to be effective in treating IBD, and the role of anti-IL17 drugs in these diseases needs further investigation. Therefore, as availability of biologics and small molecules is increasing, their positioning in clinical practice is becoming more and more challenging, and multidisciplinary management needs to be implemented in both research and clinical settings in order to enhance early recognition of SpA in IBD patients, optimize treatment and ultimately improve the patients’ quality of life.
Similar content being viewed by others
References
Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11(6):649–70. https://doi.org/10.1093/ecco-jcc/jjx008.
Gionchetti P, Dignass A, Danese S, et al. 3rd European evidence-based consensus on the diagnosis and management of crohn’s disease 2016: Part 2: surgical management and special situations. J Crohns Colitis. 2017;11(2):135–49. https://doi.org/10.1093/ecco-jcc/jjw169.
Bourikas LA, Papadakis KA. Musculoskeletal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2009;15(12):1915–24. https://doi.org/10.1002/ibd.20942.
Algaba A, Guerra I, Ricart E, et al. Extraintestinal manifestations in patients with inflammatory bowel disease: study based on the ENEIDA registry. Dig Dis Sci. 2021;66(6):2014–23. https://doi.org/10.1007/s10620-020-06424-x.
Di Jiang C, Raine T. IBD considerations in spondyloarthritis. Ther Adv Musculoskelet Dis. 2020. https://doi.org/10.1177/1759720X20939410.
Sieper J, Rudwaleit M, Baraliakos X, et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis. 2009;68:1–44. https://doi.org/10.1136/ard.2008.104018.
Ashrafi M, Ermann J, Weisman MH. Spondyloarthritis evolution: what is in your history? Curr Opin Rheumatol. 2020;32(4):321–9. https://doi.org/10.1097/BOR.0000000000000712.
Lipton S, Deodhar A. The new ASAS classification criteria for axial and peripheral spondyloarthritis: Promises and pitfalls. Int J Clin Rheumatol. 2012;7(6):675–82. https://doi.org/10.2217/ijr.12.61.
Robinson PC, van der Linden S, Khan MA, et al. Axial spondyloarthritis: concept, construct, classification and implications for therapy. Nat Rev Rheumatol. 2021;17:109–18. https://doi.org/10.1038/s41584-020-00552-4.
Karreman MC, Luime JJ, Hazes JMW, Weel AEAM. The prevalence and incidence of axial and peripheral spondyloarthritis in inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2017;11(5):631–42. https://doi.org/10.1093/ecco-jcc/jjw199.
Salvarani C, Fries W. Clinical features and epidemiology of spondyloarthritides associated with inflammatory bowel disease. World J Gastroenterol. 2009;15(20):2449–55. https://doi.org/10.3748/wjg.15.2449.
Orchard TR, Wordsworth BP, Jewell DP. Peripheral arthropathies in inflammatory bowel disease: their articular distribution and natural history. Gut. 1998;42(3):387–91. https://doi.org/10.1136/gut.42.3.387.
Stolwijk C, van Tubergen A, Castillo-Ortiz JD, Boonen A. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74(1):65–73. https://doi.org/10.1136/annrheumdis-2013-203582.
de Winter JJ, van Mens LJ, van der Heijde D, Landewé R, Baeten DL. Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: a meta-analysis. Arthritis Res Ther. 2016;18(1):196. https://doi.org/10.1186/s13075-016-1093-z.
Fragoulis GE, Liava C, Daoussis D, Akriviadis E, Garyfallos A, Dimitroulas T. Inflammatory bowel diseases and spondyloarthropathies: From pathogenesis to treatment. World J Gastroenterol. 2019;25(18):2162–76. https://doi.org/10.3748/wjg.v25.i18.2162.
Cypers H, Varkas G, Beeckman S, et al. Elevated calprotectin levels reveal bowel inflammation in spondyloarthritis. Ann Rheum Dis. 2016;75(7):1357–62. https://doi.org/10.1136/annrheumdis-2015-208025.
Leirisalo-Repo M, Turunen U, Stenman S, Helenius P, Seppälä K. High frequency of silent inflammatory bowel disease in spondylarthropathy. Arthritis Rheum. 1994;37(1):23–31. https://doi.org/10.1002/art.1780370105.
De Vos M, Mielants H, Cuvelier C, Elewaut A, Veys E. Long-term evolution of gut inflammation in patients with spondyloarthropathy. Gastroenterology. 1996;110(6):1696–703. https://doi.org/10.1053/gast.1996.v110.pm8964393.
Ashrafi M, Kuhn KA, Weisman MH. The arthritis connection to inflammatory bowel disease (IBD): why has it taken so long to understand it? RMD Open. 2021;7(1): e001558. https://doi.org/10.1136/rmdopen-2020-001558.
Thjodleifsson B, Geirsson AJ, Björnsson S, Bjarnason I. A common genetic background for inflammatory bowel disease and ankylosing spondylitis: a genealogic study in Iceland. Arthritis Rheum. 2007;56(8):2633–9. https://doi.org/10.1002/art.22812.
De Vos M. Joint involvement associated with inflammatory bowel disease. Dig Dis. 2009;27(4):511–5. https://doi.org/10.1159/000233290.
Danoy P, Pryce K, Hadler J, et al. Association of variants at 1q32 and STAT3 with ankylosing spondylitis suggests genetic overlap with Crohn’s disease. PLoS Genet. 2011. https://doi.org/10.1371/annotation/0ee7d13b-c55e-4be6-ab3e-8e8df5bb2c97.
Yang X, Li M, Wang L, Hu Z, Zhang Y, Yang Q. Association of KIF21B genetic polymorphisms with ankylosing spondylitis in a Chinese Han population of Shandong Province. Clin Rheumatol. 2015;34(10):1729–36. https://doi.org/10.1007/s10067-014-2761-5.
Barrett JC, Hansoul S, Nicolae DL, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet. 2008;40(8):955–62. https://doi.org/10.1038/ng.175.
Ellinghaus D, Jostins L, Spain SL, et al. Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci. Nat Genet. 2016;48(5):510–8. https://doi.org/10.1038/ng.3528.
Gracey E, Vereecke L, McGovern D, et al. Revisiting the gut-joint axis: links between gut inflammation and spondyloarthritis. Nat Rev Rheumatol. 2020;16(8):415–33. https://doi.org/10.1038/s41584-020-0454-9.
Ciccia F, Guggino G, Rizzo A, et al. Dysbiosis and zonulin upregulation alter gut epithelial and vascular barriers in patients with ankylosing spondylitis. Ann Rheum Dis. 2017;76(6):1123–32. https://doi.org/10.1136/annrheumdis-2016-210000.
Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104(4):487–501. https://doi.org/10.1016/s0092-8674(01)00237-9.
MacEwan DJ. TNF ligands and receptors–a matter of life and death. Br J Pharmacol. 2002;135(4):855–75. https://doi.org/10.1038/sj.bjp.0704549.
Schulzke JD, Bojarski C, Zeissig S, Heller F, Gitter AH, Fromm M. Disrupted barrier function through epithelial cell apoptosis. Ann N Y Acad Sci. 2006;1072:288–99. https://doi.org/10.1196/annals.1326.027.
Zeissig S, Bürgel N, Günzel D, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56(1):61–72. https://doi.org/10.1136/gut.2006.094375.
Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10(3):387–98. https://doi.org/10.1016/s1074-7613(00)80038-2.
Neurath MF, Fuss I, Pasparakis M, et al. Predominant pathogenic role of tumor necrosis factor in experimental colitis in mice. Eur J Immunol. 1997;27(7):1743–50. https://doi.org/10.1002/eji.1830270722.
Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1(7):553–62. https://doi.org/10.1016/1074-7613(94)90045-0.
Ogawa E, Sato Y, Minagawa A, Okuyama R. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45(3):264–72. https://doi.org/10.1111/1346-8138.14139.
Wakefield D, Yates W, Amjadi S, McCluskey P. HLA-B27 anterior uveitis: immunology and immunopathology. Ocul Immunol Inflamm. 2016;24(4):450–9. https://doi.org/10.3109/09273948.2016.1158283.
Braun J, Bollow M, Neure L, et al. Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum. 1995;38(4):499–505. https://doi.org/10.1002/art.1780380407.
Lata M, Hettinghouse AS, Liu CJ. Targeting tumor necrosis factor receptors in ankylosing spondylitis. Ann N Y Acad Sci. 2019;1442(1):5–16. https://doi.org/10.1111/nyas.13933.
Zeng MY, Inohara N, Nuñez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017;10(1):18–26. https://doi.org/10.1038/mi.2016.75.
Breban M, Tap J, Leboime A, et al. Faecal microbiota study reveals specific dysbiosis in spondyloarthritis. Ann Rheum Dis. 2017;76:1614–22.
Tito RY, Cypers H, Joossens M, et al. Brief report: Dialister as a microbial marker of disease activity in spondyloarthritis. Arthritis Rheumatol. 2017;69:114–21.
Taurog JD, Richardson JA, Croft JT, Simmons WA, Zhou M, et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–64.
Andoh A, Kuzuoka H, Tsujikawa T, et al. Multicenter analysis of fecal microbiota profiles in Japanese patients with Crohn’s disease. J Gastroenterol. 2012;47(12):1298–307. https://doi.org/10.1007/s00535-012-0605-0.
Huda-Faujan N, Abdulamir AS, Fatimah AB, et al. The impact of the level of the intestinal short chain Fatty acids in inflammatory bowel disease patients versus healthy subjects. Open Biochem J. 2010;4:53–8. https://doi.org/10.2174/1874091X01004010053.
Halfvarson J, Brislawn CJ, Lamendella R, et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol. 2017;13(2):17004.
Ciccia F, Guggino G, Zeng M, et al. Proinflammatory CX3CR1+CD59+ tumor necrosis factor–like molecule 1A+Interleukin-23+ monocytes are expanded in patients with ankylosing spondylitis and modulate innate lymphoid cell 3 immune functions. Arthritis Rheum. 2018;70(12):2003–13.
Sherlock JP, Cua DJ. Interleukin-23 in perspective. Rheumatology. 2021;60(4):1–3. https://doi.org/10.1093/rheumatology/keab461.
Ciccia F, Bombardieri M, Principato A, Giardina A, Tripodo C, Porcasi R, et al. Overexpression of interleukin-23, but not interleukin-17, as an immunologic signature of subclinical intestinal inflammation in ankylosing spondylitis. Arthritis Rheum. 2009;60:955–65. https://doi.org/10.1002/art.24389.
Salmi M, Jalkanen S. Human leukocyte subpopulations from inflamed gut bind to joint vasculature using distinct sets of adhesion molecules. J Immunol. 2001;166(7):4650–7. https://doi.org/10.4049/jimmunol.166.7.4650.
Norman E, Lefferts A, Kuhn K. Gut-joint T cell trafficking in a model of bacteria-driven murine IBD-SpA [abstract]. Arthritis Rheumatol. 2018;70:1828.
Evans JM, McMahon AD, Murray FE, McDevitt DG, MacDonald TM. Non-steroidal anti-inflammatory drugs are associated with emergency admission to hospital for colitis due to inflammatory bowel disease. Gut. 1997;40(5):619–22. https://doi.org/10.1136/gut.40.5.619.
Bonner GF, Fakhri A, Vennamaneni SR. A long-term cohort study of nonsteroidal anti-inflammatory drug use and disease activity in outpatients with inflammatory bowel disease. Inflamm Bowel Dis. 2004;10(6):751–7. https://doi.org/10.1097/00054725-200411000-00009.
Sandborn WJ, Stenson WF, Brynskov J, et al. Safety of celecoxib in patients with ulcerative colitis in remission: a randomized, placebo-controlled, pilot study. Clin Gastroenterol Hepatol. 2006;4(2):203–11. https://doi.org/10.1016/j.cgh.2005.12.002.
El Miedany Y, Youssef S, Ahmed I, El Gaafary M. The gastrointestinal safety and effect on disease activity of etoricoxib, a selective cox-2 inhibitor in inflammatory bowel diseases. Am J Gastroenterol. 2006;101(2):311–7. https://doi.org/10.1111/j.1572-0241.2006.00384.x.
Pouillon L, Bossuyt P, Vanderstukken J, et al. Management of patients with inflammatory bowel disease and spondyloarthritis. Expert Rev Clin Pharmacol. 2017;10(12):1363–74. https://doi.org/10.1080/17512433.2017.1377609.
van der Heijde D, Ramiro S, Landewé R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76(6):978–91. https://doi.org/10.1136/annrheumdis-2016-210770.
Braun J, Zochling J, Baraliakos X, et al. Efficacy of sulfasalazine in patients with inflammatory back pain due to undifferentiated spondyloarthritis and early ankylosing spondylitis: a multicentre randomised controlled trial. Ann Rheum Dis. 2006;65(9):1147–53. https://doi.org/10.1136/ard.2006.052878.
Chen J, Lin S, Liu C. Sulfasalazine for ankylosing spondylitis. Cochrane Database Syst Rev. 2014;11:4800. https://doi.org/10.1002/14651858.CD004800.pub3.
Dougados M, Vam der Linden S, Leirisalo-Repo M, et al. Sulfasalazine in the treatment of spondylarthropathy. A randomized, multicenter, double-blind, placebo-controlled study. Arthritis Rheum. 1995;38:618–27. https://doi.org/10.1002/art.1780380507.
Baron JH, Connell AM, Lennard-Jones JE, et al. Sulphasalazine and salicylazosulphadimidine in ulcerative colitis. Lancet. 1962;1:1094–6.
Dick AP, Carpenter RB, Petrie A. Controlled trial of sulphasalazine in the treatment of ulcerative colitis. Br Med J. 1964;5:437–42.
Dissanayake AS, Truelove SC. A controlled therapeutic trial of long-term maintenance treatment of ulcerative colitis with sulphazalazine (Salazopyrin). Gut. 1973;14:923–6.
Misiewitz LJJ, Connell AM, Baron JH, et al. Controlled trial of sulfasalazine in maintenance therapy for ulcerative colitis. Lancet. 1965;1:185–8.
Ko CW, Singh S, Feuerstein JD, Falck-Ytter C, Falck-Ytter Y, Cross RK. American Gastroenterological Association Institute Clinical Guidelines Committee AGA Clinical Practice Guidelines on the Management of Mild-to-Moderate Ulcerative Colitis. Gastroenterology. 2019;156(3):748–64.
Feagan BG, Rochon J, Fedorak RN, Irvine EJ, Wild G, Sutherland L, Steinhart AH, Greenberg GR, Gillies R, Hopkins M, et al. Methotrexate for the treatment of Crohn’s disease The North American Crohn’s Study Group Investigators. N Engl J Med. 1995;332(5):292–7. https://doi.org/10.1056/NEJM199502023320503.
Herfarth H, Barnes EL, Valentine JF, et al. Methotrexate is not superior to placebo in maintaining steroid-free response or remission in ulcerative colitis. Gastroenterology. 2018;155(4):1098–108. https://doi.org/10.1053/j.gastro.2018.06.046.
Macaluso FS, Renna S, Cottone M, Orlando A. The METEOR trial: the burial of methotrexate in ulcerative colitis? Gastroenterology. 2016;151(1):211–2. https://doi.org/10.1053/j.gastro.2016.02.085.
Olivieri I, Cantini F, Castiglione F, et al. Italian Expert Panel on the management of patients with coexisting spondyloarthritis and inflammatory bowel disease. Autoimmun Rev. 2014;13(8):822–30. https://doi.org/10.1016/j.autrev.2014.04.003.
Brandt J, Haibel H, Reddig J, et al. Successful short term treatment of severe undifferentiated spondyloarthropathy with the anti-tumor necrosis factor-alpha monoclonal antibody infliximab. J Rheumatol. 2002;29:118–22.
Raun J, Baraliakos X, Brandt J, Listing J, Zink A, Alten R, Burmester G, Gromnica-Ihle E, Kellner H, Schneider M, Sörensen H, Zeidler H, Sieper J. Persistent clinical response to the anti-TNF-alpha antibody infliximab in patients with ankylosing spondylitis over 3 years. Rheumatology (Oxford). 2005;44:670–6. https://doi.org/10.1093/rheumatology/keh584.
Baeten D, Van den BF, Kruithof E, Mielants H, Veys EM. Infliximab in patients who have spondyloarthropathy: clinical efficacy, safety, and biological immunomodulation. Rheum Dis Clin North Am. 2003;29:463–79.
Brandt J, Khariouzov A, Listing J, Haibel H, Sorensen H, Rudwaleit M, Sieper J, Braun J. Successful short term treatment of patients with severe undifferentiated spondyloarthritis with the anti-tumor necrosis factor-alpha fusion receptor protein etanercept. J Rheumatol. 2004;31:531–8.
Billmeier U, Dieterich W, Neurath MF, Atreya R. Molecular mechanism of action of anti-tumor necrosis factor antibodies in inflammatory bowel diseases. World J Gastroenterol. 2016;22(42):9300–13. https://doi.org/10.3748/wjg.v22.i42.9300.
Perrier C, de Hertogh G, Cremer J, et al. Neutralization of membrane TNF, but not soluble TNF, is crucial for the treatment of experimental colitis. Inflamm Bowel Dis. 2013;19(2):246–53. https://doi.org/10.1002/ibd.23023.
Eder P, Korybalska K, Łykowska-Szuber L, et al. An increase in serum tumour necrosis factor-α during anti-tumour necrosis factor-α therapy for Crohn’s disease - A paradox or a predictive index? Dig Liver Dis. 2016;48(10):1168–71. https://doi.org/10.1016/j.dld.2016.06.038.
Braun J, van der Horst-Bruinsma IE, Huang F, Burgos-Vargas R, Vlahos B, Koenig AS, Freundlich B. Clinical efficacy and safety of etanercept versus sulfasalazine in patients with ankylosing spondylitis: a randomized, double-blind trial. Arthritis Rheum. 2011;63:1543–51.
Sandborn WJ, Hanauer SB, Katz S, et al. Etanercept for active Crohn’s disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2001;121:1088–94.
Toussirot E, Houvenagel E, Goeb V, et al. Development of inflammatory bowel disease during anti-TNF-alpha therapy for inflammatory rheumatic disease: a nationwide series. Joint Bone Spine. 2012;79:457–63.
Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541–9. https://doi.org/10.1016/S0140-6736(02)08512-4.
Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337(15):1029–35. https://doi.org/10.1056/NEJM199710093371502.
Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130(2):323–591. https://doi.org/10.1053/j.gastro.2005.11.030.
Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132(1):52–65. https://doi.org/10.1053/j.gastro.2006.11.041.
Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146(12):829–38. https://doi.org/10.7326/0003-4819-146-12-200706190-00159.
Schreiber S, Rutgeerts P, Fedorak RN, et al. A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn’s disease. Gastroenterology. 2005;129(3):807–18. https://doi.org/10.1053/j.gastro.2005.06.064.
Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353(23):2462–76. https://doi.org/10.1056/NEJMoa050516.
Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60(6):780–7. https://doi.org/10.1136/gut.2010.221127.
Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142(2):257–65. https://doi.org/10.1053/j.gastro.2011.10.032.
Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146(1):85-e15. https://doi.org/10.1053/j.gastro.2013.05.048.
Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146(1):96-109.e1. https://doi.org/10.1053/j.gastro.2013.06.010.
Braun J, Brandt J, Listing J, et al. Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet. 2002;359(9313):1187–93. https://doi.org/10.1016/s0140-6736(02)08215-6.
van der Heijde D, Kivitz A, Schiff MH, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2006;54(7):2136–46. https://doi.org/10.1002/art.21913.
Inman RD, Davis JC Jr, Heijde D, et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum. 2008;58(11):3402–12. https://doi.org/10.1002/art.23969.
Vavricka SR, Gubler M, Gantenbein C, et al. Anti-TNF treatment for extraintestinal manifestations of inflammatory bowel disease in the Swiss IBD cohort study. Inflamm Bowel Dis. 2017;23(7):1174–81. https://doi.org/10.1097/MIB.0000000000001109.
Rahier JF. Prevention and management of infectious complications in IBD. Dig Dis. 2012;30(4):408–14. https://doi.org/10.1159/000338143.
Beaugerie L, Rahier JF, Kirchgesner J. Predicting, preventing, and managing treatment-related complications in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2020;18(6):1324-1335.e2. https://doi.org/10.1016/j.cgh.2020.02.009.
Tillack C, Ehmann LM, Friedrich M, et al. Anti-TNF antibody-induced psoriasiform skin lesions in patients with inflammatory bowel disease are characterised by interferon-gamma-expressing Th1 cells and IL-17A/ IL-22-expressing Th17 cells and respond to anti-IL12/IL-23 antibody treatment. Gut. 2014;63:567–77.
Vermeire S, Loftus EV Jr, Colombel JF, et al. Long-term efficacy of vedolizumab for crohn’s disease. J Crohns Colitis. 2017;11(4):412–24.
Loftus EV Jr, Colombel JF, Feagan BG, et al. Long-term efficacy of vedolizumab for ulcerative colitis. J Crohns Colitis. 2017;11(4):400–11.
Tadbiri S, et al. Impact of vedolizumab therapy on extra-intestinal manifestations in patients with inflammatory bowel disease: a multicentre cohort study nested in the OBSERV-IBD cohort. Aliment Pharmacol Ther. 2018;47:485–93.
Macaluso FS, Orlando R, Fries W, Scolaro M, Magnano A, Pluchino D, Cappello M, Morreale GC, Siringo S, Privitera AC, Ferracane C, Belluardo N, Alberghina N, Ventimiglia M, Rizzuto G, Renna S, Cottone M, Orlando A. The real-world effectiveness of vedolizumab on intestinal and articular outcomes in inflammatory bowel diseases. Dig Liver Dis. 2018;50(7):675–81. https://doi.org/10.1016/j.dld.2018.02.013.
Feagan BG, Sandborn WJ, Colombel JF, et al. Incidence of arthritis/arthralgia in inflammatory bowel disease with long-term vedolizumab treatment: post hoc analyses of the GEMINI trials. J Crohns Colitis. 2019;13(1):50–7. https://doi.org/10.1093/ecco-jcc/jjy125.
Feagan BG, Sandborn WJ, Danese S, et al. Ozanimod induction therapy for patients with moderate to severe Crohn’s Disease: a single-arm, phase 2, prospective observer-blinded endpoint study. Lancet Gastroenterol Hepatol. 2020;5:819–28.
Sandborn WJ, Feagan BG, Dhaens G, et al. True North Study Group. Ozanimod as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2021;385(14):1280–91. https://doi.org/10.1056/NEJMoa2033617.
Vermeire S, Chiorean M, Panés J, et al. Long-term safety and efficacy of etrasimod for ulcerative colitis: results from the open-label extension of the OASIS study. J Crohns Colitis. 2021;15(6):950–9. https://doi.org/10.1093/ecco-jcc/jjab016.
Tsunemi S, Iwasaki T, Kitano S, Imado T, Miyazawa K, Sano H. Effects of the novel immunosuppressant FTY720 in a murine rheumatoid arthritis model. Clin Immunol. 2010;136(2):197–204.
Jin J, Ji M, Fu R, et al. Sphingosine-1-phosphate receptor subtype 1 (S1P1) modulator IMMH001 regulates adjuvant- and collagen-induced arthritis. Front Pharmacol. 2019;10:1085.
Feagan BG, Sandborn WJ, Gasink C, et al. UNITI–IM-UNITI Study Group. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375(20):1946–60. https://doi.org/10.1056/NEJMoa1602773.
Sands BE, Sandborn WJ, Panaccione R, O’Brien CD, Zhang H, Johanns J, Adedokun OJ, Li K, Peyrin-Biroulet L, Van Assche G, Danese S, Targan S, Abreu MT, Hisamatsu T, Szapary P, Marano C. UNIFI Study Group Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381(13):1201–14. https://doi.org/10.1056/NEJMoa1900750.
Guillo L, D’Amico F, Danese S, Peyrin-Biroulet L. Ustekinumab for extra-intestinal manifestations of inflammatory bowel disease: a systematic literature review. J Crohns Colitis. 2021;15(7):1236–43. https://doi.org/10.1093/ecco-jcc/jjaa260.
Macaluso FS, Fries W, Viola A, Costantino G, Muscianisi M, Cappello M, Guida L, Giuffrida E, Magnano A, Pluchino D, Ferracane C, Magrì G, Di Mitri R, Mocciaro F, Privitera AC, Camilleri S, Garufi S, Renna S, Casà A, Scrivo B, Ventimiglia M, Orlando A. Effectiveness of ustekinumab on crohn’s disease associated spondyloarthropathy: real-world data from the sicilian network for inflammatory bowel diseases (SN-IBD). Expert Opin Biol Ther. 2020;20(11):1381–4. https://doi.org/10.1080/14712598.2020.1830057.
Macaluso FS, Orlando A, Cottone M. Anti-interleukin-12 and anti-interleukin-23 agents in Crohn’s disease. Expert Opin Biol Ther. 2019;19(2):89–98.
Bowman EP, Chackerian AA, Cua DJ. Rationale and safety of anti-interleukin-23 and anti interleukin-17A therapy. Curr Opin Infect Dis. 2006;19(245–52):529.
Fieschi C, Casanova JL. The role of interleukin-12 in human infectious diseases: only a faint signature. Eur J Immunol. 2003;33(1461–4):531.
Meeran SM, Mantena SK, Meleth S, Elmets CA, Katiyar SK. Interleukin-12-deficient mice are 532 at greater risk of UV radiation-induced skin tumors and malignant transformation of papillomas to 533 carcinomas. Mol Cancer Ther. 2006;5:825–32.
Mease PJ, Rahman P, Gottlieb AB, Kollmeier AP, Hsia EC, Xu XL, Sheng S, Agarwal P, Zhou B, Zhuang Y, van der Heijde D, McInnes IB. DISCOVER-2 Study Group Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1126–36. https://doi.org/10.1016/S0140-6736(20)30263-4.
Deodhar A, Helliwell PS, Boehncke WH, Kollmeier AP, Hsia EC, Subramanian RA, Xu XL, Sheng S, Agarwal P, Zhou B, Zhuang Y, Ritchlin CT. DISCOVER-1 Study Group Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1115–25. https://doi.org/10.1016/S0140-6736(20)30265-8.
Danese S, Sandborn WJ, Feagan BG, et al. The effect of guselkumab induction therapy on early clinical outcome measures in patients with Moderately to Severely Active Crohn’s Disease: Results from the phase 2 GALAXI 1 study (abstract)
Hanžel J, D’Haens GR. Anti-interleukin-23 agents for the treatment of ulcerative colitis. Expert Opin Biol Ther. 2020;20(4):399–406. https://doi.org/10.1080/14712598.2020.1697227.
Sandborn WJ, Ferrante M, Bhandari BR, et al. Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with ulcerative colitis. Gastroenterology. 2020;158(3):537-549.e10. https://doi.org/10.1053/j.gastro.2019.08.043.
Sands BE, Peyrin-Biroulet L, Kierkus J, et al. Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with Crohn’s disease. Gastroenterology. 2022;162(2):495–508. https://doi.org/10.1053/j.gastro.2021.10.050.
Schreiber SW, Ferrante M, Panaccione R, Colombel JF, Hisamatsu T, et al. OP26 risankizumab induces early clinical remission and response in patients with moderate-to-severe Crohn’s disease: Results from the phase 3 ADVANCE and MOTIVATE studies. J Crohn’s Colitis. 2021;15(1):S026–7. https://doi.org/10.1093/ecco-jcc/jjab075.025.
Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376(1551–60):541.
Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650–61.
Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178(1):114–23. https://doi.org/10.1111/bjd.15750.
Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–40. https://doi.org/10.1084/jem.20041257.
Hueber W, Patel DD, Dryja T, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2(52):52–72. https://doi.org/10.1126/scitranslmed.3001107.
Hueber W, Sands BE, Lewitzky S, et al. Secukinumab in Crohn’s Disease Study Group Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61(12):1693–700.
Targan SR, Feagan B, Vermeire S, et al. A randomized, double-blind, placebo-controlled phase 2 study of brodalumab in patients with moderate-to-severe crohn’s disease. Am J Gastroenterol. 2016;111(11):1599–607. https://doi.org/10.1038/ajg.2016.298.
Mease PJ, Helliwell PS, Hjuler KF, et al. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis. 2021;80:185–93.
Kukol W, Jose LA, Marino D. P055 Development of Crohn’s disease with use of secukinumab. Am J Gastroenterol. 2019. https://doi.org/10.14309/01.ajg.0000578292.95094.87.
Colombel JF, Sendid B, Jouault T, et al. Secukinumab failure in Crohn’s disease: the yeast connection? Gut. 2013;62:800–1.
Van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.e4.
Baeten D, Sieper J, Braun J, et al. MEASURE 1 Study Group. MEASURE 2 Study Group Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015;373:2534–48.
Tang C, Kakuta S, Shimizu K, et al. Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing Treg cells through modification of the intestinal microbiota. Nat Immunol. 2018;19(7):755–65.
Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley JD, Gruben D, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370:2377–86.
Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, et al. Placebocontrolled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367:495–507.
Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin-Mola E, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2013;159:253–61.
Mease P, Hall S, FitzGerald O, van der Heijde D, Merola JF, Avila-Zapata F, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med. 2017;377:1537–50.
Gladman D, Rigby W, Azevedo VF, Behrens F, Blanco R, Kaszuba A, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med. 2017;377:1525–36.
Deodhar A, Sliwinska-Stanczyk P, Xu H, et al. Tofacitinib for the treatment of ankylosing spondylitis: a phase III, randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2021;80:1004–13.
Sandborn WJ, Su C, Panes J. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;377(5):496–7. https://doi.org/10.1056/NEJMc1707500.
Sandborn WJ, Ghosh S, Panes J, Vranic I, Wang W, Niezychowski W. Study A3921043 Investigators A phase 2 study of tofacitinib, an oral Janus kinase inhibitor, in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:1485–93.
Panés J, Sandborn WJ, Schreiber S, et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut. 2017;66:1049–59.
Ytterberg SR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316–26.
U.S. Food and Drug Administration. FDA approves Boxed Warning about increased risk of blood clots and death with higher dose of arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR). https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-boxed-warning-about-increased-risk-blood-clots-and-death-higher-dose-arthritis-and (2021).
Winthrop KL, Cohen SB. Oral surveillance and JAK inhibitor safety: the theory of relativity. Nat Rev Rheumatol. 2022. https://doi.org/10.1038/s41584-022-00767-7.
Genovese MC, Kalunian K, Gottenberg JE, et al. Effect of filgotinib vs placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease-modifying antirheumatic drug therapy: the FINCH 2 randomized clinical trial. JAMA. 2019;322:315–25.
Kavanaugh A, Kremer J, Ponce L, et al. Filgotinib (GLPG0634/GS-6034), an oral selective JAK1 inhibitor, is effective as monotherapy in patients with active rheumatoid arthritis: results from a randomised, dose-finding study (DARWIN 2). Ann Rheum Dis. 2017;76:1009–19.
Westhovens R, Taylor PC, Alten R, et al. Filgotinib (GLPG0634/GS-6034), an oral JAK1 selective inhibitor, is effective in combination with methotrexate (MTX) in patients with active rheumatoid arthritis and insufficient response to MTX: results from a randomised, dose-finding study (DARWIN 1). Ann Rheum Dis. 2017;76:998–1008.
Mease P, Coates LC, Helliwell PS, et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active psoriatic arthritis (EQUATOR): results from a randomised, placebocontrolled, phase 2 trial. Lancet. 2018;392:2367–77.
van der Heijde D, Baraliakos X, Gensler LS, et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active ankylosing spondylitis (TORTUGA): results from a randomised, placebo-controlled, phase 2 trial. Lancet. 2018;392:2378–87.
Feagan BG, Danese S, Loftus EV Jr. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): a phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet. 2021;397(10292):2372–84. https://doi.org/10.1016/S0140-6736(21)00666-8.
Vermeire S, Schreiber S, Petryka R, et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet. 2017;389:266–75.
Mease PJ, Lertratanakul A, Anderson JK, Papp K, Van den Bosch F, Tsuji S, et al. Upadacitinib for psoriatic arthritis refractory to biologics: SELECT-PsA 2. Ann Rheum Dis. 2020;2:2.
McInnes IB, Anderson JK, Magrey M, Merola JF, Liu Y, Kishimoto M, et al. Trial of upadacitinib and adalimumab for psoriatic arthritis. N Engl J Med. 2021;384:1227–39.
Vermeire, S et al. OP23 Efficacy and safety of upadacitinib as induction therapy in patients with moderately to severely active ulcerative colitis: results from phase 3 U-ACCOMPLISH study. ECCO presentation 2021
Danese S. et al. OP24 Efficacy and safety of upadacitinib induction therapy in patients with Moderately to Severely Active Ulcerative Colitis: Results from the phase 3 U-ACHIEVE study. ECCO presentation 2021
AbbVie. Data on File: ABVRRTI73568.
Lasa JS, Olivera PA, Danese S, Peyrin-Biroulet L. Efficacy and safety of biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2022;7(2):161–70. https://doi.org/10.1016/S2468-1253(21)00377-0.
Felice C, Leccese P, Scudeller L, et al. Red flags for appropriate referral to the gastroenterologist and the rheumatologist of patients with inflammatory bowel disease and spondyloarthritis. Clin Exp Immunol. 2019;196(1):123–38. https://doi.org/10.1111/cei.13246.
Rizzello F, Olivieri I, Armuzzi A, et al. Multidisciplinary management of spondyloarthritis-related immune-mediated inflammatory disease. Adv Ther. 2018;35(4):545–62. https://doi.org/10.1007/s12325-018-0672-6.
Schwartzman M, Ermann J, Kuhn KA, Schwartzman S, Weisman MH. Spondyloarthritis in inflammatory bowel disease cohorts: systematic literature review and critical appraisal of study designs. RMD Open. 2022;8(1): e001777. https://doi.org/10.1136/rmdopen-2021-001777.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors received no specific funding for this work.
Conflicts of interest
FSM served as an advisory board member and/or received lecture grants from AbbVie, Biogen, Galapagos, Janssen, MSD, Pfizer, Samsung Bioepis, and Takeda Pharmaceuticals. AO served as an advisory board member for AbbVie, Galapagos, MSD, Janssen, Pfizer, Takeda Pharmaceuticals, and received lecture grants from AbbVie, MSD, Sofar, Chiesi, Janssen, Pfizer, and Takeda Pharmaceuticals. AO served as an advisory board member for AbbVie, MSD, Janssen, Pfizer, Takeda Pharmaceuticals, and received lecture grants from AbbVie, MSD, Sofar, Chiesi, Janssen, Pfizer, and Takeda Pharmaceuticals. SR served as an advisory board member for AbbVie, Janssen and MSD Pharmaceuticals, and received lecture grants from AbbVie, Janssen, MSD and Takeda Pharmaceuticals. FC, MG, EMB, NM, GR, AC declared no conflicting interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Author contributions
FC and MG drafted the article. FC, MG, EMB, NM, GR, AC were responsible for critical revision of the manuscript for important intellectual content. FSM and AO were responsible for study supervision. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Rights and permissions
About this article
Cite this article
Crispino, F., Grova, M., Bruno, E.M. et al. Spondyloarthropathy in Inflammatory Bowel Disease: From Pathophysiology to Pharmacological Targets. Drugs 82, 1151–1163 (2022). https://doi.org/10.1007/s40265-022-01750-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01750-y