Abstract
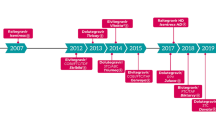
The newest class of antiretrovirals for all persons living with HIV are the integrase strand transfer inhibitors (INSTIs). Since 2007, five INSTIs have been introduced: raltegravir, elvitegravir, dolutegravir, bictegravir, and cabotegravir. The INSTIs have favorable pharmacokinetic and pharmacodynamic properties, which contribute to both their effectiveness and their ease of use. With the exception of cabotegravir, each INSTI is US Food and Drug Administration approved for treatment-naïve individuals initiating antiretroviral therapy. All of the INSTIs, except raltegravir, are approved for antiretroviral treatment simplification for virologically suppressed patients without INSTI resistance. Data also support the use of dolutegravir and raltegravir in individuals with antiretroviral resistance as part of an optimized antiretroviral regimen. INSTIs are generally well tolerated by people living with HIV compared with older classes of antiretrovirals, but emerging data suggest that some INSTIs contribute to weight gain. Due to their efficacy, safety, and ease of use, HIV treatment guidelines recommend oral INSTIs as preferred components of antiretroviral therapy for individuals initiating therapy. The newest INSTI, cabotegravir, represents an alternative to oral administration of life-long antiretroviral therapy with the availability of a long-acting injectable formulation. This review summarizes the current use of INSTIs in adults living with HIV, highlighting the similarities and differences within the class related to pharmacodynamics, pharmacokinetics, safety, dosing, and administration that contribute to their role in modern antiretroviral therapy.
Similar content being viewed by others
References
UNAIDS. Global HIV & AIDS statistics—2019 fact sheets. https://www.unaids.org/en/resources/fact-sheet Accessed 2 Apr 2020.
Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8(12):e81355. https://doi.org/10.1371/journal.pone.0081355.
INSIGHT START Study Group, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795–807. https://doi.org/10.1056/nejmoa1506816.
TEMPRANO ANRS 12136 Study Group, Danel C, Moh R, Gabillard D, Badje A, Le Carrou J et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373(9):808–22. https://doi.org/10.1056/nejmoa1507198.
Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375(9):830–9. https://doi.org/10.1056/NEJMoa1600693.
Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, van Lunzen J, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA. 2016;316(2):171–81. https://doi.org/10.1001/jama.2016.5148.
Townsend CL, Cortina-Borja M, Peckham CS, de Ruiter A, Lyall H, Tookey PA. Low rates of mother-to-child transmission of HIV following effective pregnancy interventions in the United Kingdom and Ireland, 2000-2006. AIDS. 2008;22(8):973–81. https://doi.org/10.1097/QAD.0b013e3282f9b67a.
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed 19 Aug 2020.
Update of recommendations on first- and second-line antiretroviral regimens. Geneva, Switzerland: World Health Organization; 2019 (WHO/CDS/HIV/19.15). Licence: CC BY-NC-SA 3.0 IGO.
Saag MS, Benson CA, Gandhi RT, Hoy JF, Landovitz RJ, Mugavero MJ, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the international antiviral society-USA panel. JAMA. 2018;320(4):379–96. https://doi.org/10.1001/jama.2018.8431.
Peñafiel J, De LE, Padilla M, Rojas J, Gonzalez-Cordon A, Blanco JL, et al. Tolerability of integrase inhibitors in a real-life setting. J Antimicrob Chemother. 2017;72(6):1752–9. https://doi.org/10.1093/jac/dkx053.
Isentress® [package insert]. Whitehouse Station, NJ. Merck & Co., Inc. January 2019.
Stribild® [package insert]. Foster City, CA. Gilead Sciences, Inc. January 2019.
Cabenuva® and Vocabria® [Product Monograph]. Laval, Quebec. Viiv Healthcare ULC. March 2020.
Dehority W, Abadi J, Wiznia A, Viani RM. Use of integrase inhibitors in HIV-infected children and adolescents. Drugs. 2015;75(13):1483–97. https://doi.org/10.1007/s40265-015-0446-2.
Biktarvy® [package insert]. Foster City, CA. Gilead Sciences, Inc. February 2018.
Tivicay® [package insert]. Research Triangle Park, NC. Viiv Healthcare. June 2020.
Genvoya® [package insert]. Foster City, CA. Gilead Sciences, Inc. February 2019.
Kobayashi M, Yoshinaga T, Seki T, Wakasa-Morimoto C, Brown KW, Ferris R, et al. In vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob Agents Chemother. 2011;55(2):813–21. https://doi.org/10.1128/AAC.01209-10.
Podany AT, Scarsi KK, Pham MM, Fletcher CV. Comparative clinical pharmacokinetics and pharmacodynamics of HIV-1 integrase strand transfer inhibitors: an updated review. Clin Pharmacokinet. 2020. https://doi.org/10.1007/s40262-020-00898-8.
Wainberg MA, Zaharatos GJ, Brenner BG. Development of antiretroviral drug resistance. N Engl J Med. 2011;365(7):637–46. https://doi.org/10.1056/NEJMra1004180.
Anstett K, Brenner B, Mesplede T, Wainberg MA. HIV drug resistance against strand transfer integrase inhibitors. Retrovirology. 2017;14(1):36. https://doi.org/10.1186/s12977-017-0360-7.
Tsiang M, Jones GS, Goldsmith J, Mulato A, Hansen D, Kan E, et al. Antiviral activity of bictegravir (GS-9883), a novel potent HIV-1 integrase strand transfer inhibitor with an improved resistance profile. Antimicrob Agents Chemother. 2016;60(12):7086–97. https://doi.org/10.1128/AAC.01474-16.
Orkin C, Arasteh K, Gorgolas Hernandez-Mora M, Pokrovsky V, Overton ET, Girard PM, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med. 2020;382(12):1124–35. https://doi.org/10.1056/NEJMoa1909512.
Swindells S, Andrade-Villanueva JF, Richmond GJ, Rizzardini G, Baumgarten A, Masia M, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med. 2020;382(12):1112–23. https://doi.org/10.1056/NEJMoa1904398.
Boffito M, Waters L, Cahn P, Paredes R, Koteff J, van WJ et al. Perspectives on the barrier to resistance for dolutegravir + lamivudine, a 2-drug antiretroviral therapy for HIV-1 infection. AIDS Res Hum Retrovirus. 2019. https://doi.org/10.1089/aid.2019.0171.
Spreen W, Min S, Ford S, Chen S, Lou Y, Bomar M, et al. Pharmacokinetics, safety, and monotherapy antiviral activity of GSK1265744, an HIV integrase strand transfer inhibitor. HIV Clin Trials. 2013;14(5):192–203. https://doi.org/10.1310/hct1405-192.
Min S, Sloan L, Dejesus E, Hawkins T, McCurdy L, Song I, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1-infected adults. AIDS. 2011;25(14):1737–45. https://doi.org/10.1097/QAD.0b013e32834a1dd9.
DeJesus E, Berger D, Markowitz M, Cohen C, Hawkins T, Ruane P, et al. Antiviral activity, pharmacokinetics, and dose response of the HIV-1 integrase inhibitor GS-9137 (JTK-303) in treatment-naive and treatment-experienced patients. J Acquir Immune Defic Syndr. 2006;43(1):1–5. https://doi.org/10.1097/01.qai.0000233308.82860.2f.
Gallant JE, Thompson M, DeJesus E, Voskuhl GW, Wei X, Zhang H, et al. Antiviral activity, safety, and pharmacokinetics of bictegravir as 10-day monotherapy in HIV-1-infected adults. J Acquir Immune Defic Syndr. 2017;75(1):61–6. https://doi.org/10.1097/QAI.0000000000001306.
Rizk ML, Hang Y, Luo WL, Su J, Zhao J, Campbell H, et al. Pharmacokinetics and pharmacodynamics of once-daily versus twice-daily raltegravir in treatment-naïve HIV-infected patients. Antimicrob Agents Chemother. 2012;56(6):3101–6. https://doi.org/10.1128/AAC.06417-11.
Food and Drug Administration (FDA). Guidance for industry, antiviral product development, conducting and submitting virology studies to the agency. 2006. https://www.fda.gov/media/71223/download Accessed 8 Mar 2020.
Cattaneo D, Gervasoni C. Pharmacokinetics and pharmacodynamics of cabotegravir, a long-acting HIV integrase strand transfer inhibitor. Eur J Drug Metab Pharmacokinet. 2019;44(3):319–27. https://doi.org/10.1007/s13318-018-0526-2.
Bowers GD, Culp A, Reese MJ, Tabolt G, Moss L, Piscitelli S, et al. Disposition and metabolism of cabotegravir: a comparison of biotransformation and excretion between different species and routes of administration in humans. 2016;46(2):147–62. https://doi.org/10.3109/00498254.2015.1060372.
Ford S, Crauwels H, Han K, Rossenu S, Zhang F, Huang JO, et al. Cabotegravir and rilpivirine PK following long-acting HIV treatment discontinuation. In: Conference on Retroviruses and Opportunistic Infections. March 8–11, 2020. Boston, MA. Abstract #466.
Spreen W, Ford SL, Chen S, Wilfret D, Margolis D, Gould E, et al. GSK1265744 pharmacokinetics in plasma and tissue following single-dose long-acting (la) injectable administration in healthy subjects. J Acquir Immune Defic Syndr. 2014. https://doi.org/10.1097/QAI.0000000000000301.
Landovitz RJ, Li S, Eron JJ Jr, Grinsztejn B, Dawood H, Liu AY, et al. Tail-phase safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in HIV-uninfected adults: a secondary analysis of the HPTN 077 trial. Lancet HIV. 2020;7(7):e472–81. https://doi.org/10.1016/S2352-3018(20)30106-5.
Vitekta® [package insert]. Foster City, CA. Gilead Sciences, Inc. September 2014.
Wohl DA, Dumond JB, Blevins S, Pittard D, Ragan D, Wang R, et al. Raltegravir pharmacokinetics in treatment-naive patients is not influenced by race: results from the raltegravir early therapy in african-americans living with HIV (REAL) Study. Antimicrob Agents Chemother. 2013;57(2):784–8. https://doi.org/10.1128/AAC.01826-12.
Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf. Accessed 19 Aug 2020.
Lu H, Rosenbaum S. Developmental pharmacokinetics in pediatric populations. J Pediatr Pharmacol Ther. 2014;19(4):262–76. https://doi.org/10.5863/1551-6776-19.4.262.
Elliot ER, Wang X, Singh S, Simmons B, Vera JH, Miller RF, et al. Increased dolutegravir peak concentrations in people living with human immunodeficiency virus aged 60 and over, and analysis of sleep quality and cognition. Clin Infect Dis. 2019;68(1):87–95. https://doi.org/10.1093/cid/ciy426.
Panel on Treatment of Pregnant Women with HIV Infection and Prevention of Perinatal Transmission. Recommendations for the use of antiretroviral drugs in pregnant women with HIV infection and interventions to reduce perinatal HIV transmission in the United States. http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed 19 Aug 2020.
Colbers A, De HM, Van CR, Kruijssen M, Duisenberg-Van EM, Abbink E et al. Pharmacokinetics of crushed elvitegravir combination tablet given with drip feed. Top Antiviral Med. 2016;24(-1):166.
Jeong H, Choi S, Song JW, Chen H, Fischer JH. Regulation of UDP-glucuronosyltransferase (UGT) 1A1 by progesterone and its impact on labetalol elimination. Xenobiotica. 2008;38(1):62–75. https://doi.org/10.1080/00498250701744633.
van der Galien R, Ter Heine R, Greupink R, Schalkwijk SJ, van Herwaarden AE, Colbers A, et al. Pharmacokinetics of HIV-integrase inhibitors during pregnancy: mechanisms, clinical implications and knowledge gaps. Clin Pharmacokinet. 2019;58(3):309–23. https://doi.org/10.1007/s40262-018-0684-z.
Mulligan N, Best BM, Wang J, Capparelli EV, Stek A, Barr E, et al. Dolutegravir pharmacokinetics in pregnant and postpartum women living with HIV. AIDS. 2018;32(6):729–37. https://doi.org/10.1097/QAD.0000000000001755.
Waitt C, Orrell C, Walimbwa S, Singh Y, Kintu K, Simmons B, et al. Safety and pharmacokinetics of dolutegravir in pregnant mothers with HIV infection and their neonates: a randomised trial (DolPHIN-1 study). PLoS Med. 2019;16(9):e1002895. https://doi.org/10.1371/journal.pmed.1002895.
Blonk MI, Colbers APH, Hidalgo-Tenorio C, Kabeya K, Weizsäcker K, Haberl AE, et al. Raltegravir in HIV-1-infected pregnant women: pharmacokinetics, safety, and efficacy. Clin Infect Dis. 2015;61(5):809–16. https://doi.org/10.1093/cid/civ366.
Watts DH, Stek A, Best BM, Wang J, Capparelli EV, Cressey TR, et al. Raltegravir pharmacokinetics during pregnancy. J Acquir Immune Defic Syndr. 2014. https://doi.org/10.1097/QAI.0000000000000318.
Momper JD, Best BM, Wang J, Capparelli EV, Stek A, Barr E, et al. Elvitegravir/cobicistat pharmacokinetics in pregnant and postpartum women with HIV. AIDS. 2018;32(17):2507–16. https://doi.org/10.1097/QAD.0000000000001992.
Patel P, Thiagarajah S, Ford S, Margolis DA, Romach BH, Baker M, Sutton K, Harrinton CM, Shaefer MS, Spreen W, Smith K, Vannappagari V. Cabotegravir pharmacokinetic tail in pregnancy and neonatal outcomes. In: Conference on retroviruses and opportunistic infections. March 8–11, 2020. Boston, MA. Abstract #775.
Schalkwijk S, Greupink R, Colbers AP, Wouterse AC, Verweij VG, van Drongelen J, et al. Placental transfer of the HIV integrase inhibitor dolutegravir in an ex vivo human cotyledon perfusion model. J Antimicrob Chemother. 2016;71(2):480–3. https://doi.org/10.1093/jac/dkv358.
Schalkwijk S, Colbers A, Konopnicki D, Greupink R, Russel FGM, Burger D. First reported use of elvitegravir and cobicistat during pregnancy. AIDS. 2016;30(5):807–8. https://doi.org/10.1097/QAD.0000000000000976.
McCormack SA, Best BM. Protecting the fetus against HIV infection: a systematic review of placental transfer of antiretrovirals. Clin Pharmacokinet. 2014;53(11):989–1004. https://doi.org/10.1007/s40262-014-0185-7.
World Health Organization, United Nations Children’s Fund. Guideline: updates on HIV and infant feeding: the duration of breastfeeding, and support from health services to improve feeding practices among mothers living with HIV. Geneva: World Health Organization; 2016.
Iwamoto M, Hanley WD, Petry AS, Friedman EJ, Kost JT, Breidinger SA, et al. Lack of a clinically important effect of moderate hepatic insufficiency and severe renal insufficiency on raltegravir pharmacokinetics; Merck and Co(United States). Antimicrob Agents Chemother. 2009;53(5):1747–52. https://doi.org/10.1128/AAC.01194-08.
Weller S, Borland J, Chen S, Johnson M, Savina P, Wynne B, et al. Pharmacokinetics of dolutegravir in HIV-seronegative subjects with severe renal impairment. Eur J Clin Pharmacol. 2014;70(1):29–35. https://doi.org/10.1007/s00228-013-1590-9.
Debinski HS, Lee CS, Danks JA, Mackenzie PI, Desmond PV. Localization of uridine 5′-diphosphate-glucuronosyltransferase in human liver injury. Gastroenterology. 1995;108(5):1464–9. https://doi.org/10.1016/0016-5085(95)90695-9.
Furlan V, Demirdjian S, Bourdon O, Magdalou J, Taburet AM. Glucuronidation of drugs by hepatic microsomes derived from healthy and cirrhotic human livers. J Pharmacol Exp Ther. 1999;289(2):1169–75.
George J, Murray M, Byth K, Farrell GC. Differential alterations of cytochrome P450 proteins in livers from patients with severe chronic liver disease. Hepatology. 1995;21(1):120–8.
Calza L, Danese I, Colangeli V, Manfredi R, Magistrelli E, Verucchi G, et al. Plasma concentrations of efavirenz, darunavir/ritonavir and raltegravir in HIV-HCV-coinfected patients without liver cirrhosis in comparison with HIV-monoinfected patients. Infect Dis. 2015;47(9):625–36. https://doi.org/10.3109/23744235.2015.1034169.
Custodio JM, Rhee M, Shen G, Ling KHJ, Kearney BP, Ramanathan S. Pharmacokinetics and safety of boosted elvitegravir in subjects with hepatic impairment. Antimicrob Agents Chemother. 2014;58(5):2564–9. https://doi.org/10.1128/AAC.02180-13.
Hernández-Novoa B, Moreno A, Pérez-Elías MJ, Quereda C, Dronda F, Casado JL, et al. Raltegravir pharmacokinetics in HIV/HCV-coinfected patients with advanced liver cirrhosis (Child-Pugh C); Merck Sharp and Dohme(United States). J Antimicrob Chemother. 2014;69(2):471–5. https://doi.org/10.1093/jac/dkt386.
Song IH, Borland J, Savina PM, Chen S, Patel P, Wajima T, et al. Pharmacokinetics of single-dose dolutegravir in HIV-seronegative subjects with moderate hepatic impairment compared to healthy matched controls. Clin Pharmacol Drug Dev. 2013;2(4):342–8. https://doi.org/10.1002/cpdd.55.
Shaik JSB, Ford SL, Lou Y, Zhang Z, Bakshi KK, Tenorio AR, et al. A phase 1 study to evaluate the pharmacokinetics and safety of cabotegravir in patients with hepatic impairment and healthy matched controls. Clin Pharmacol Drug Dev. 2019;8(5):664–73. https://doi.org/10.1002/cpdd.655.
Dooley KE, Sayre P, Borland J, Purdy E, Chen S, Song I, et al. Safety, tolerability, and pharmacokinetics of the HIV integrase inhibitor dolutegravir given twice daily with rifampin or once daily with rifabutin: results of a phase 1 study among healthy subjects. J Acquir Immune Defic Syndr. 2013;62(1):21–7. https://doi.org/10.1097/QAI.0b013e318276cda9.
Dooley KE, Kaplan R, Mwelase N, Grinsztejn B, Ticona E, Lacerda M, et al. Dolutegravir-based antiretroviral therapy for patients co-infected with tuberculosis and hiv: a multicenter, noncomparative, open-label, randomized trial. Clin Infect Dis. 2019. https://doi.org/10.1093/cid/ciz256.
Grinsztejn B, De CN, Arnold V, Veloso VG, Morgado M, Pilotto JH, et al. Raltegravir for the treatment of patients co-infected with HIV and tuberculosis (ANRS 12 180 Reflate TB): a multicentre, phase 2, non-comparative, open-label, randomised trial. Lancet Infect Dis. 2014;14(6):459–67. https://doi.org/10.1016/S1473-3099(14)70711-X.
Ford SL, Sutton K, Lou Y, Zhang Z, Tenorio A, Trezza C et al. Effect of rifampin on the single-dose pharmacokinetics of oral cabotegravir in healthy subjects. Antimicrob Agents Chemother. 2017. https://doi.org/10.1128/aac.00487-17.
Rajoli RKR, Curley P, Chiong J, Back D, Flexner C, Owen A, et al. Predicting drug-drug interactions between rifampicin and long-acting cabotegravir and rilpivirine using physiologically based pharmacokinetic modeling. J Infect Dis. 2019;219(11):1735–42. https://doi.org/10.1093/infdis/jiy726.
Reese MJ, Savina PM, Generaux GT, Tracey H, Humphreys JE, Kanaoka E, et al. In vitro investigations into the roles of drug transporters and metabolizing enzymes in the disposition and drug interactions of dolutegravir, a hiv integrase inhibitor. Drug Metab Dispos. 2013;41(2):353–61. https://doi.org/10.1124/dmd.112.048918.
Cattaneo D, Resnati C, Rizzardini G, Gervasoni C. Dolutegravir and metformin: a clinically relevant or just a pharmacokinetic interaction? AIDS. 2018;32(4):532–3. https://doi.org/10.1097/QAD.0000000000001720.
Acosta RK, Willkom M, Martin R, Chang S, Wei X, Garner W et al. Resistance analysis of bictegravir-emtricitabine-tenofovir alafenamide in HIV-1 treatment-naive patients through 48 weeks. Antimicrob Agents Chemother. 2019. https://doi.org/10.1128/aac.02533-18.
Kintu K, Malaba TR, Nakibuka J, Papamichael C, Colbers A, Byrne K, et al. Dolutegravir versus efavirenz in women starting HIV therapy in late pregnancy (DolPHIN-2): an open-label, randomised controlled trial. Lancet HIV. 2020;7(5):e332–9. https://doi.org/10.1016/S2352-3018(20)30050-3.
Jacobson K, Ogbuagu O. Integrase inhibitor-based regimens result in more rapid virologic suppression rates among treatment-naive human immunodeficiency virus-infected patients compared to non-nucleoside and protease inhibitor-based regimens in a real-world clinical setting: a retrospective cohort study. Medicine (Baltimore). 2018;97(43):e13016. https://doi.org/10.1097/MD.0000000000013016.
Lennox JL, DeJesus E, Lazzarin A, Pollard RB, Madruga JVR, Berger DS, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009;374(9692):796–806. https://doi.org/10.1016/S0140-6736(09)60918-1.
Rockstroh JK, DeJesus E, Lennox JL, Yazdanpanah Y, Saag MS, Wan H, et al. Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naive HIV-1-infected patients: final 5-year results from STARTMRK. J Acquir Immune Defic Syndr. 2013;63(1):77–85. https://doi.org/10.1097/QAI.0b013e31828ace69.
Eron JJ, Young B, Cooper DA, Youle M, DeJesus E, Andrade-Villanueva J, et al. Switch to a raltegravir-based regimen versus continuation of a lopinavir-ritonavir-based regimen in stable HIV-infected patients with suppressed viraemia (SWITCHMRK 1 and 2): two multicentre, double-blind, randomised controlled trials. Lancet. 2010;375(9712):396–407. https://doi.org/10.1016/S0140-6736(09)62041-9.
Steigbigel RT, Cooper DA, Kumar PN, Eron JE, Schechter M, Markowitz M, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med. 2008;359(4):339–54. https://doi.org/10.1056/NEJMoa0708975.
Steigbigel RT, Cooper DA, Teppler H, Eron JJ, Gatell JM, Kumar PN, et al. Long-term efficacy and safety of raltegravir combined with optimized background therapy in treatmentexperienced patients with drugresistant hiv infection: week 96 results of the benchmrk 1 and 2 phase III trials. Clin Infect Dis. 2010;50(4):605–12. https://doi.org/10.1086/650002.
Eron JJ, Cooper DA, Steigbigel RT, Clotet B, Gatell JM, Kumar PN, et al. Efficacy and safety of raltegravir for treatment of HIV for 5 years in the BENCHMRK studies: final results of two randomised, placebo-controlled trials. Lancet Infect Dis. 2013;13(7):587–96. https://doi.org/10.1016/S1473-3099(13)70093-8.
Eron JJ, Rockstroh JK, Reynes J, Andrade-Villanueva J, Ramalho-Madruga JV, Bekker LG, et al. Raltegravir once daily or twice daily in previously untreated patients with HIV-1: a randomised, active-controlled, phase 3 non-inferiority trial. Lancet Infect Dis. 2011;11(12):907–15. https://doi.org/10.1016/S1473-3099(11)70196-7.
Cahn P, Kaplan R, Sax PE, Squires K, Molina JM, Avihingsanon A, et al. Raltegravir 1200 mg once daily versus raltegravir 400 mg twice daily, with tenofovir disoproxil fumarate and emtricitabine, for previously untreated HIV-1 infection: a randomised, double-blind, parallel-group, phase 3, non-inferiority trial. Lancet HIV. 2017;4(11):e486–94. https://doi.org/10.1016/S2352-3018(17)30128-5.
Cahn P, Sax PE, Squires K, Molina JM, Ratanasuwan W, Rassool M, et al. Raltegravir 1200 mg once daily vs 400 mg twice daily, with emtricitabine and tenofovir disoproxil fumarate, for previously untreated HIV-1 infection: week 96 results from ONCEMRK, a randomized, double-blind, noninferiority trial. J Acquir Immune Defic Syndr. 2018;78(5):589–98. https://doi.org/10.1097/QAI.0000000000001723.
Sax PE, DeJesus E, Mills A, Zolopa A, Cohen C, Wohl D, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012;379(9835):2439–48. https://doi.org/10.1016/S0140-6736(12)60917-9.
Wohl DA, Cohen C, Gallant JE, Mills A, Sax PE, DeJesus E, et al. A randomized, double-blind comparison of single tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF versus single tablet regimen efavirenz/emtricitabine/tenofovir DF for initial treatment of HIV-1 infection: analysis of week 144 results. J Acquir Immune Defic Syndr. 2013. https://doi.org/10.1097/QAI.0000000000000057.
Zolopa A, Sax PE, Dejesus E, Mills A, Cohen C, Wohl D, et al. A randomized double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr. 2013;63(1):96–100. https://doi.org/10.1097/QAI.0b013e318289545c.
DeJesus E, Rockstroh JK, Henry K, Molina JM, Gathe J, Ramanathan S, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet. 2012;379(9835):2429–38. https://doi.org/10.1016/S0140-6736(12)60918-0.
Rockstroh JK, DeJesus E, Henry K, Molina JM, Gathe J, Ramanathan S, et al. A randomized, double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir DF vs ritonavir-boosted atazanavir plus coformulated emtricitabine and tenofovir DF for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr. 2013;62(5):483–6. https://doi.org/10.1097/QAI.0b013e318286415c.
Clumeck N, Molina JM, Henry K, Gathe J, Rockstroh JK, DeJesus E, et al. A randomized, double-blind comparison of single-tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF vs ritonavir-boosted atazanavir plus emtricitabine/tenofovir DF for initial treatment of HIV-1 infection: analysis of week 144 results. J Acquir Immune Defic Syndr. 2014;65(3):e121–4. https://doi.org/10.1097/QAI.0000000000000089.
Pozniak A, Markowitz M, Mills A, Stellbrink HJ, Antela A, Domingo P, et al. Switching to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus continuation of non-nucleoside reverse transcriptase inhibitor with emtricitabine and tenofovir in virologically suppressed adults with HIV (STRATEGY-NNRTI): 48 week results of a randomised, open-label, phase 3b non-inferiority trial. Lancet Infect Dis. 2014;14(7):590–9. https://doi.org/10.1016/S1473-3099(14)70796-0.
Pozniak A, Flamm J, Antinori A, Bloch M, Ward D, Berenguer J, et al. Switching to the single-tablet regimen of elvitegravir, cobicistat, emtricitabine, and tenofovir DF from non-nucleoside reverse transcriptase inhibitor plus coformulated emtricitabine and tenofovir DF regimens: week 96 results of STRATEGY-NNRTI. HIV Clin Trials. 2017;18(4):141–8. https://doi.org/10.1080/15284336.2017.1338844.
Arribas JR, DeJesus E, van Lunzen J, Zurawski C, Doroana M, Towner W, et al. Simplification to single-tablet regimen of elvitegravir, cobicistat, emtricitabine, tenofovir DF from multi-tablet ritonavir-boosted protease inhibitor plus coformulated emtricitabine and tenofovir DF regimens: week 96 results of STRATEGY-PI. HIV Clin Trials. 2017;18(3):118–25. https://doi.org/10.1080/15284336.2017.1330440.
Arribas JR, Pialoux G, Gathe J, Di Perri G, Reynes J, Tebas P, et al. Simplification to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus continuation of ritonavir-boosted protease inhibitor with emtricitabine and tenofovir in adults with virologically suppressed HIV (STRATEGY-PI): 48 week results of a randomised, open-label, phase 3b, non-inferiority trial. Lancet Infect Dis. 2014;14(7):581–9. https://doi.org/10.1016/S1473-3099(14)70782-0.
Mills A, Crofoot G, Ortiz R, Rashbaum B, Towner W, Ward D, et al. Switching from twice-daily raltegravir plus tenofovir disoproxil Fumarate/Emtricitabine to once-daily Elvitegravir/Cobicistat/Emtricitabine/Tenofovir disoproxil fumarate in virologically suppressed, HIV-1-infected subjects: 48 weeks data. HIV Clinical Trials. 2014;15(2):51–6. https://doi.org/10.1310/hct1502-51.
Sax PE, Wohl D, Yin MT, Post F, DeJesus E, Saag M, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385(9987):2606–15. https://doi.org/10.1016/S0140-6736(15)60616-X.
Wohl D, Oka S, Clumeck N, Clarke A, Brinson C, Stephens J, et al. A randomized, double-blind comparison of tenofovir alafenamide versus tenofovir disoproxil fumarate, each coformulated with elvitegravir, cobicistat, and emtricitabine for initial HIV-1 treatment: week 96 results. J Acquir Immune Defic Syndr. 2016;72(1):58–64. https://doi.org/10.1097/QAI.0000000000000940.
Arribas JR, Thompson M, Sax PE, Haas B, McDonald C, Wohl DA, et al. Brief report: randomized, double-blind comparison of tenofovir alafenamide (TAF) vs tenofovir disoproxil fumarate (TDF), each coformulated with elvitegravir, cobicistat, and emtricitabine (E/C/F) for initial HIV-1 treatment: week 144 results. J Acquir Immune Defic Syndr. 2017;75(2):211–8. https://doi.org/10.1097/QAI.0000000000001350.
Mills A, Arribas JR, Andrade-Villanueva J, DiPerri G, Van Lunzen J, Koenig E, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis. 2016;16(1):43–52. https://doi.org/10.1016/S1473-3099(15)00348-5.
DeJesus E, Haas B, Segal-Maurer S, Ramgopal MN, Mills A, Margot N, et al. Superior efficacy and improved renal and bone safety after switching from a tenofovir disoproxil fumarate- to a tenofovir alafenamide-based regimen through 96 weeks of treatment. AIDS Res Hum Retroviruses. 2018;34(4):337–42. https://doi.org/10.1089/AID.2017.0203.
Walmsley SL, Antela A, Clumeck N, Duiculescu D, Eberhard A, Gutieŕrez F, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369(19):1807–18. https://doi.org/10.1056/NEJMoa1215541.
Walmsley S, Baumgarten A, Berenguer J, Felizarta F, Florence E, Khuong-Josses MA, et al. Dolutegravir plus abacavir/lamivudine for the treatment of HIV-1 infection in antiretroviral therapy-naive patients: week 96 and week 144 results from the SINGLE randomized clinical trial. J Acquir Immune Defic Syndr. 2015;70(5):515–9. https://doi.org/10.1097/QAI.0000000000000790.
Clotet B, Feinberg J, Van LJ, Khuong-Josses MA, Antinori A, Dumitru I, et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 4 8 week results from the randomised open-label phase 3b study. Lancet. 2014;383(9936):2222–31. https://doi.org/10.1016/S0140-6736(14)60084-2.
Molina JM, Clotet B, van Lunzen J, Lazzarin A, Cavassini M, Henry K, et al. Once-daily dolutegravir versus darunavir plus ritonavir for treatment-naive adults with HIV-1 infection (FLAMINGO): 96 week results from a randomised, open-label, phase 3b study. Lancet HIV. 2015;2(4):e127–36. https://doi.org/10.1016/S2352-3018(15)00027-2.
Raffi F, Rachlis A, Stellbrink HJ, Hardy WD, Torti C, Orkin C, et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet. 2013;381(9868):735–43. https://doi.org/10.1016/S0140-6736(12)61853-4.
Raffi F, Jaeger H, Quiros-Roldan E, Albrecht H, Belonosova E, Gatell JM, et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2013;13(11):927–35. https://doi.org/10.1016/S1473-3099(13)70257-3.
Cahn P, Pozniak AL, Mingrone H, Shuldyakov A, Brites C, Andrade-Villanueva JF, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet. 2013;382(9893):700–8. https://doi.org/10.1016/S0140-6736(13)61221-0.
Eron JJ, Clotet B, Durant J, Katlama C, Kumar P, Lazzarin A, et al. Safety and efficacy of dolutegravir in treatment-experienced subjects with raltegravir-resistant HIV type 1 infection: 24-week results of the VIKING Study. J Infect Dis. 2013;207(5):740–8. https://doi.org/10.1093/infdis/jis750.
Castagna A, Maggiolo F, Penco G, Wright D, Mills A, Grossberg R, et al. Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir-resistant HIV-1: 24-week results of the phase III VIKING-3 study. J Infect Dis. 2014;210(3):354–62. https://doi.org/10.1093/infdis/jiu051.
Llibre JM, Hung CC, Brinson C, Castelli F, Girard PM, Kahl LP, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet. 2018;391(10123):839–49. https://doi.org/10.1016/S0140-6736(17)33095-7.
Aboud M, Orkin C, Podzamczer D, Bogner JR, Baker D, Khuong-Josses MA, et al. Efficacy and safety of dolutegravir-rilpivirine for maintenance of virological suppression in adults with HIV-1: 100-week data from the randomised, open-label, phase 3 SWORD-1 and SWORD-2 studies. Lancet HIV. 2019;6(9):e576–87. https://doi.org/10.1016/S2352-3018(19)30149-3.
Cahn P, Madero JS, Arribas JR, Antinori A, Ortiz R, Clarke AE, et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet. 2019;393(10167):143–55. https://doi.org/10.1016/S0140-6736(18)32462-0.
Dovato® [package insert]. Research Triangle Park, NC. Viiv Healthcare. March 2020.
Cahn P, Madero JS, Arribas JR, Antinori A, Ortiz R, Clarke AE, et al. Durable efficacy of dolutegravir plus lamivudine in antiretroviral treatment-naive adults with HIV-1 infection: 96-week results from the GEMINI-1 and GEMINI-2 randomized clinical trials. J Acquir Immune Defic Syndr. 2020;83(3):310–8. https://doi.org/10.1097/QAI.0000000000002275.
Gallant J, Lazzarin A, Mills A, Orkin C, Podzamczer D, Tebas P, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet. 2017;390(10107):2063–72. https://doi.org/10.1016/S0140-6736(17)32299-7.
Wohl DA, Yazdanpanah Y, Baumgarten A, Clarke A, Thompson MA, Brinson C, et al. Bictegravir combined with emtricitabine and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2019;6(6):e355–63. https://doi.org/10.1016/S2352-3018(19)30077-3.
Sax PE, Pozniak A, Montes ML, Koenig E, DeJesus E, Stellbrink HJ, et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380–1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet. 2017;390(10107):2073–82. https://doi.org/10.1016/S0140-6736(17)32340-1.
Stellbrink HJ, Arribas JR, Stephens JL, Albrecht H, Sax PE, Maggiolo F, et al. Co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2019;6(6):e364–72. https://doi.org/10.1016/S2352-3018(19)30080-3.
Molina JM, Ward D, Brar I, Mills A, Stellbrink HJ, López-Cortés L, et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from dolutegravir plus abacavir and lamivudine in virologically suppressed adults with HIV-1: 48 week results of a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet HIV. 2018;5(7):e357–65. https://doi.org/10.1016/S2352-3018(18)30092-4.
Daar ES, DeJesus E, Ruane P, Crofoot G, Oguchi G, Creticos C, et al. Efficacy and safety of switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from boosted protease inhibitor-based regimens in virologically suppressed adults with HIV-1: 48 week results of a randomised, open-label, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2018;5(7):e347–56. https://doi.org/10.1016/S2352-3018(18)30091-2.
Sax PE, Rockstroh JK, Luetkemeyer AF, Yazdanpanah Y, Ward D, Trottier B, et al. Switching to bictegravir, emtricitabine, and tenofovir alafenamide in virologically suppressed adults with HIV. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa988.
Overton ET, Richmond GJ, Rizzardini G, Jaeger H, Orrell C, Nagimova F et al. Cabotegravir + rilpivirine every 2 months is noninferior to monthly: ATLAS-2 M study. Conference on Retroviruses and Opportunistic Infections (CROI). March 8-11, 2020. Boston. Abstract 34.
Landovitz RJ, Li S, Grinsztejn B, Dawood H, Liu AY, Magnus M et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Medicine. 2018. https://doi.org/10.1371/journal.pmed.1002690.
Markowitz M, Frank I, Grant RM, Mayer KH, Elion R, Goldstein D, et al. Safety and tolerability of long-acting cabotegravir injections in HIV-uninfected men (ECLAIR): a multicentre, double-blind, randomised, placebo-controlled, phase 2a trial. Lancet HIV. 2017;4(8):e331–40. https://doi.org/10.1016/S2352-3018(17)30068-1.
Llibre JM, Montoliu A, Miró JM, Domingo P, Riera M, Tiraboschi J, et al. Discontinuation of dolutegravir, elvitegravir/cobicistat and raltegravir because of toxicity in a prospective cohort. HIV Med. 2019;20(3):237–47. https://doi.org/10.1111/hiv.12710.
Lennox JL, Dejesus E, Berger DS, Lazzarin A, Pollard RB, Ramalho Madruga JV, et al. Raltegravir versus Efavirenz regimens in treatment-naive HIV-1-infected patients: 96-week efficacy, durability, subgroup, safety, and metabolic analyses. J Acquir Immune Defic Syndr. 2010;55(1):39–48. https://doi.org/10.1097/QAI.0b013e3181da1287.
Teppler H, Brown DD, Leavitt RY, Sklar P, Wan H, Xu X, et al. Long-term safety from the raltegravir clinical development program. Curr HIV Res. 2011;9(1):40–53. https://doi.org/10.2174/157016211794582650.
Nguyen A, Calmy A, Delhumeau C, Mercier I, Cavassini M, Mello AF, et al. A randomized cross-over study to compare raltegravir and efavirenz (SWITCH-ER study). AIDS. 2011;25(12):1481–7. https://doi.org/10.1097/QAD.0b013e328348dab0.
Lennox JL, Landovitz RJ, Ribaudo HJ, Ofotokun I, Na LH, Godfrey C, et al. Efficacy and tolerability of 3 nonnucleoside reverse transcriptase inhibitor-sparing antiretroviral regimens for treatment-naïve volunteers infected with HIV-1: a randomized, controlled equivalence trial. Ann Internal Med. 2014;161(7):461–71. https://doi.org/10.7326/M14-1084.
Martinez E, Larrousse M, Llibre JM, Gutierrez F, Saumoy M, Antela A, et al. Substitution of raltegravir for ritonavir-boosted protease inhibitors in HIV-infected patients: the SPIRAL study. AIDS. 2010;24(11):1697–707. https://doi.org/10.1097/QAD.0b013e32833a608a.
Curtis L, Nichols G, Stainsby C, Lim J, Aylott A, Wynne B, et al. Dolutegravir: clinical and laboratory safety in integrase inhibitor-naive patients. HIV Clin Trials. 2014;15(5):199–208. https://doi.org/10.1310/hct1505-199.
Lee FJ, Amin J, Bloch M, Pett SL, Marriott D, Carr A. Skeletal muscle toxicity associated with raltegravir-based combination antiretroviral therapy in HIV-infected adults. J Acquir Immune Defic Syndr. 2013;62(5):525–33. https://doi.org/10.1097/QAI.0b013e3182832578.
Tsai WJ, Lee SS, Tsai HC, Sy CL, Chen JK, Wu KS, et al. Rapid onset of rhabdomyolysis after switching to a raltegravir-based antiretroviral regimen. J Microbiol Immunol Infect. 2016;49(2):286–8. https://doi.org/10.1016/j.jmii.2013.02.008.
Masia M, Enriquez R, Sirvent A, Gutierrez F. Severe acute renal failure associated with rhabdomyolysis during treatment with raltegravir. A call for caution. J Infect. 2010;61(2):189–90. https://doi.org/10.1016/j.jinf.2010.04.011.
Zembower TR, Gerzenshtein L, Coleman K, Palella FJ Jr. Severe rhabdomyolysis associated with raltegravir use. AIDS. 2008;22(11):1382–4. https://doi.org/10.1097/QAD.0b013e328303be40.
Croce F, Vitello P, Dalla Pria A, Riva A, Galli M, Antinori S. Severe raltegravir-associated rhabdomyolysis: a case report and review of the literature. Int J STD AIDS. 2010;21(11):783–5. https://doi.org/10.1258/ijsa.2010.010246.
Dori L, Buonomini AR, Viscione M, Sarmati L, Andreoni M. A case of rhabdomiolysis associated with raltegravir use. AIDS. 2010;24(3):473–5. https://doi.org/10.1097/QAD.0b013e328334cc4a.
Lepik KJ, Yip B, Ulloa AC, Wang L, Toy J, Akagi L, et al. Adverse drug reactions to integrase strand transfer inhibitors. AIDS. 2018;32(7):903–12. https://doi.org/10.1097/QAD.0000000000001781.
Lepist EI, Zhang X, Hao J, Huang J, Kosaka A, Birkus G, et al. Contribution of the organic anion transporter OAT2 to the renal active tubular secretion of creatinine and mechanism for serum creatinine elevations caused by cobicistat. Kidney Int. 2014;86(2):350–7. https://doi.org/10.1038/ki.2014.66.
Eron J, Kalayjian R, Wurapa A, Stephens J, McDonald C, Wilkin A, et al. Safety and efficacy of elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (E/C/F/TAF) in HIV-infected adults on chronic haemodialysis. HIV Med. 2018;19:S16.
Sax PE, DeJesus E, Crofoot G, Ward D, Benson P, Dretler R, et al. Bictegravir versus dolutegravir, each with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection: a randomised, double-blind, phase 2 trial. Lancet HIV. 2017;4(4):e154–60. https://doi.org/10.1016/S2352-3018(17)30016-4.
Hill AM, Mitchell N, Hughes S, Pozniak AL. Risks of cardiovascular or central nervous system adverse events and immune reconstitution inflammatory syndrome, for dolutegravir versus other antiretrovirals: meta-analysis of randomized trials. Curr Opin HIV AIDS. 2018;13(2):102–11. https://doi.org/10.1097/COH.0000000000000445.
Hayes E, Derrick C, Smalls D, Smith H, Kremer N, Weissman S. Adverse events with Biktarvy: post-marketing study. ID Week. October 2–6, 2019. Washington, DC. Abstract #2489.
Brehm TT, Franz M, Hufner A, Hertling S, Schmiedel S, Degen O, et al. Safety and efficacy of elvitegravir, dolutegravir, and raltegravir in a real-world cohort of treatment-naive and -experienced patients. Medicine (Baltimore). 2019;98(32):e16721. https://doi.org/10.1097/MD.0000000000016721.
Cuzin L, Pugliese P, Katlama C, Bani-Sadr F, Ferry T, Rey D, et al. Integrase strand transfer inhibitors and neuropsychiatric adverse events in a large prospective cohort. J Antimicrob Chemother. 2019;74(3):754–60. https://doi.org/10.1093/jac/dky497.
Hoffmann C, Llibre JM. Neuropsychiatric adverse events with dolutegravir and other integrase strand transfer inhibitors. AIDS Rev. 2019;21(1):4–10. https://doi.org/10.24875/AIDSRev.19000023.
Hoffmann C, Welz T, Sabranski M, Kolb M, Wolf E, Stellbrink HJ, et al. Higher rates of neuropsychiatric adverse events leading to dolutegravir discontinuation in women and older patients. HIV Med. 2017;18(1):56–63. https://doi.org/10.1111/hiv.12468.
Cid-Silva P, Llibre JM, Fernández-Bargiela N, Margusino-Framiñán L, Balboa-Barreiro V, Pernas-Souto B, et al. Clinical experience with the integrase inhibitors dolutegravir and elvitegravir in HIV-infected patients: efficacy, safety and tolerance. Basic Clin Pharmacol Toxicol. 2017;121(5):442–6. https://doi.org/10.1111/bcpt.12828.
Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral Pregnancy Registry interim report for 1 January 1989–31 January 2019. Wilmington, NC: Registry Coordinating Center. 2019. Available at http://apregistry.com/forms/exec-summary.pdf. Accessed 19 Aug 2020.
Centers for Disease Control and Prevention. Update on overall prevalence of major birth defects–Atlanta, Georgia, 1978-2005. MMWR Morb Mortal Wkly Rep. 2008;57(1):1–5.
Sibiude J, Warszawski J, Blanche S, Dialla O, Faye A, Dollfus C, et al. Evaluation of the risk of birth defects among children exposed to raltegravir in utero in the ANRS-French perinatal cohort EPF. Presented at: 9th IAS Conference on HIV Science; July 23-26, 2017; Paris, France. Abstract MOAB0204.
Zash R, Makhema J, Shapiro RL. Neural-tube defects with dolutegravir treatment from the time of conception. N Engl J Med. 2018;379(10):979–81. https://doi.org/10.1056/NEJMc1807653.
Zash R, Holmes L, Diseko M, Jacobson DL, Brummel S, Mayondi G, et al. Neural-tube defects and antiretroviral treatment regimens in Botswana. N Engl J Med. 2019;381(9):827–40. https://doi.org/10.1056/NEJMoa1905230.
Bakal DR, Coelho LE, Luz PM, Clark JL, De BR, Cardoso SW, et al. Obesity following ART initiation is common and influenced by both traditional and HIV-/ART-specific risk factors. J Antimicrob Chemother. 2018;73(8):2177–85. https://doi.org/10.1093/jac/dky145.
Menard A, Meddeb L, Tissot-Dupont H, Ravaux I, Dhiver C, Mokhtari S, et al. Dolutegravir and weight gain: an unexpected bothering side effect? AIDS. 2017;31(10):1499–500. https://doi.org/10.1097/QAD.0000000000001495.
Sax PE, Erlandson KM, Lake JE, McComsey GA, Orkin C, Esser S, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis. 2019. https://doi.org/10.1093/cid/ciz999.
Kouanfack C, Mpoudi-Etame M, Bassega PO, Eymard-Duvernay S, Leroy S, Boyer S, et al. Dolutegravir-based or low-dose efavirenz-based regimen for the treatment of HIV-1. N Engl J Med. 2019;381(9):816–26. https://doi.org/10.1056/NEJMoa1904340.
Venter WDF, Moorhouse M, Sokhela S, Fairlie L, Mashabane N, Masenya M, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV; Gilead; Macleods; Mylan. N Engl J Med. 2019;381(9):803–15. https://doi.org/10.1056/NEJMoa1902824.
Bourgi K, Rebeiro PF, Turner M, Castilho JL, Hulgan T, Raffanti SP, et al. Greater weight gain in treatment naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis. 2019. https://doi.org/10.1093/cid/ciz407.
Norwood J, Turner M, Bofill C, Rebeiro P, Shepherd B, Bebawy S, et al. Brief report: weight gain in persons with HIV switched from efavirenz-based to integrase strand transfer inhibitor-based regimens. J Acquir Immune Defic Syndr. 2017;76(5):527–31. https://doi.org/10.1097/QAI.0000000000001525.
Landovitz RJ, Zangeneh SZ, Chau G, Grinsztejn B, Eron JJ, Dawood H, et al. Cabotegravir is not associated with weight gain in human immunodeficiency virus-uninfected individuals in HPTN 077. Clin Infect Dis. 2020;70(2):319–22. https://doi.org/10.1093/cid/ciz439.
Landovitz RJ, Donnell D, Clement M, Hanscom B, Cottle L, Coelho L et al. HPTN083 interim results: Pre-exposure prophylaxis (PrEP) containing long-acting injectable cabotegravir (CAB-LA) is safe and highly effective for cisgender men and transgender women who have sex with men (MSM,TGW). 23rd International HIV Conference (AIDS 2020: Virtual), abstract OAXLB0101, 2020.
Yuh B, Tate J, Butt AA, Crothers K, Freiberg M, Leaf D, et al. Weight change after antiretroviral therapy and mortality. Clin Infect Dis. 2015;60(12):1852–9. https://doi.org/10.1093/cid/civ192.
Achhra AC, Mocroft A, Reiss P, Sabin C, Ryom L, de Wit S, et al. Short-term weight gain after antiretroviral therapy initiation and subsequent risk of cardiovascular disease and diabetes: the D:A: D study. HIV Med. 2016;17(4):255–68. https://doi.org/10.1111/hiv.12294.
Herrin M, Tate JP, Akgun KM, Butt AA, Crothers K, Freiberg MS, et al. Weight gain and incident diabetes among HIV-infected veterans initiating antiretroviral therapy compared with uninfected individuals. J Acquir Immune Defic Syndr. 2016;73(2):228–36. https://doi.org/10.1097/QAI.0000000000001071.
Hill A, McCann KM, Pilkington V, Moorhouse MA, Sokhela S, Serenata CM, et al. Risks of metabolic syndrome, diabetes, and cardiovascular disease in ADVANCE Trial. Conference on Retroviruses and Opportunistic Infections (CROI). March 8–11, 2020. Boston. Abstract 81.
Fong PS, Flynn DM, Evans CD, Korthuis PT. Integrase strand transfer inhibitor-associated diabetes mellitus: a case report. Int J STD AIDS. 2017;28(6):626–8. https://doi.org/10.1177/0956462416675107.
McLaughlin M, Walsh S, Galvin S. Dolutegravir-induced hyperglycaemia in a patient living with HIV. J Antimicrob Chemother. 2018;73(1):258–60. https://doi.org/10.1093/jac/dkx365.
Lamorde M, Atwiine M, Owarwo NC, Ddungu A, Laker EO, Mubiru F, et al. Dolutegravir-associated hyperglycaemia in patients with HIV. Lancet HIV. 2020. https://doi.org/10.1016/S2352-3018(20)30042-4.
Bhagwat P, Ofotokun I, McComsey GA, Brown TT, Moser C, Sugar CA et al. Changes in waist circumference in HIV-infected individuals initiating a raltegravir or protease inhibitor regimen: effects of sex and race. Open Forum Infect Dis. 2018;5(11):ofy201. https://doi.org/10.1093/ofid/ofy201.
Bernardino JI, Mocroft A, Wallet C, de Wit S, Katlama C, Reiss P, et al. Body composition and adipokines changes after initial treatment with darunavir-ritonavir plus either raltegravir or tenofovir disoproxil fumarate-emtricitabine: a substudy of the NEAT001/ANRS143 randomised trial. PLoS One. 2019;14(1):e0209911. https://doi.org/10.1371/journal.pone.0209911.
Triumeq® [package insert]. Research Triangle Park, NC. Viiv Healthcare. March 2020.
New York Department of Health AIDS Insitite. Clinical Guidelines Program. When to initiate ART, with protocol for rapid initiation. Available at https://www.hivguidelines.org/antiretroviral-therapy/when-to-start-plus-rapid-start/ Accessed 5 Apr 2020.
Juluca® [package insert]. Research Triangle Park, NC. Viiv Healthcare. October 2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
We acknowledge support from the following grants from the National Institutes of Health: 1R01HD085887-01A1 (to KS), 1K23AI134307 (to ATP), RO1 AI124965-01 and UM1AI06701 (to CVF). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest
KKS, ATP, SNA, CVF declare no conflict of interest related to this article. JPH reports research grants paid to his institution from Gilead Sciences.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Rights and permissions
About this article
Cite this article
Scarsi, K.K., Havens, J.P., Podany, A.T. et al. HIV-1 Integrase Inhibitors: A Comparative Review of Efficacy and Safety. Drugs 80, 1649–1676 (2020). https://doi.org/10.1007/s40265-020-01379-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01379-9